Nutritional Properties, Antioxidant Activity, and Heavy Metal Accumulation in Selected Marine Macro-Algae Species of Sri Lanka
Abstract
1. Introduction
2. Materials and Methods
2.1. Seaweed Sample Collection
2.2. Proximate Analysis
2.2.1. Determination of Moisture Content
2.2.2. Determination Dry Matter and Ash
2.2.3. Determination of Crude Protein
2.2.4. Determination of Crude Fat
2.3. Determination of Crude Fiber
2.4. Preparation of Crude Ethanol Extract of the Samples
2.5. Antioxidant Assays
2.5.1. Total Phenolic Content
2.5.2. DPPH Assay
2.5.3. ABTS Assay
2.5.4. FRAP Assay
2.6. Trace Elements and Heavy Metal Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
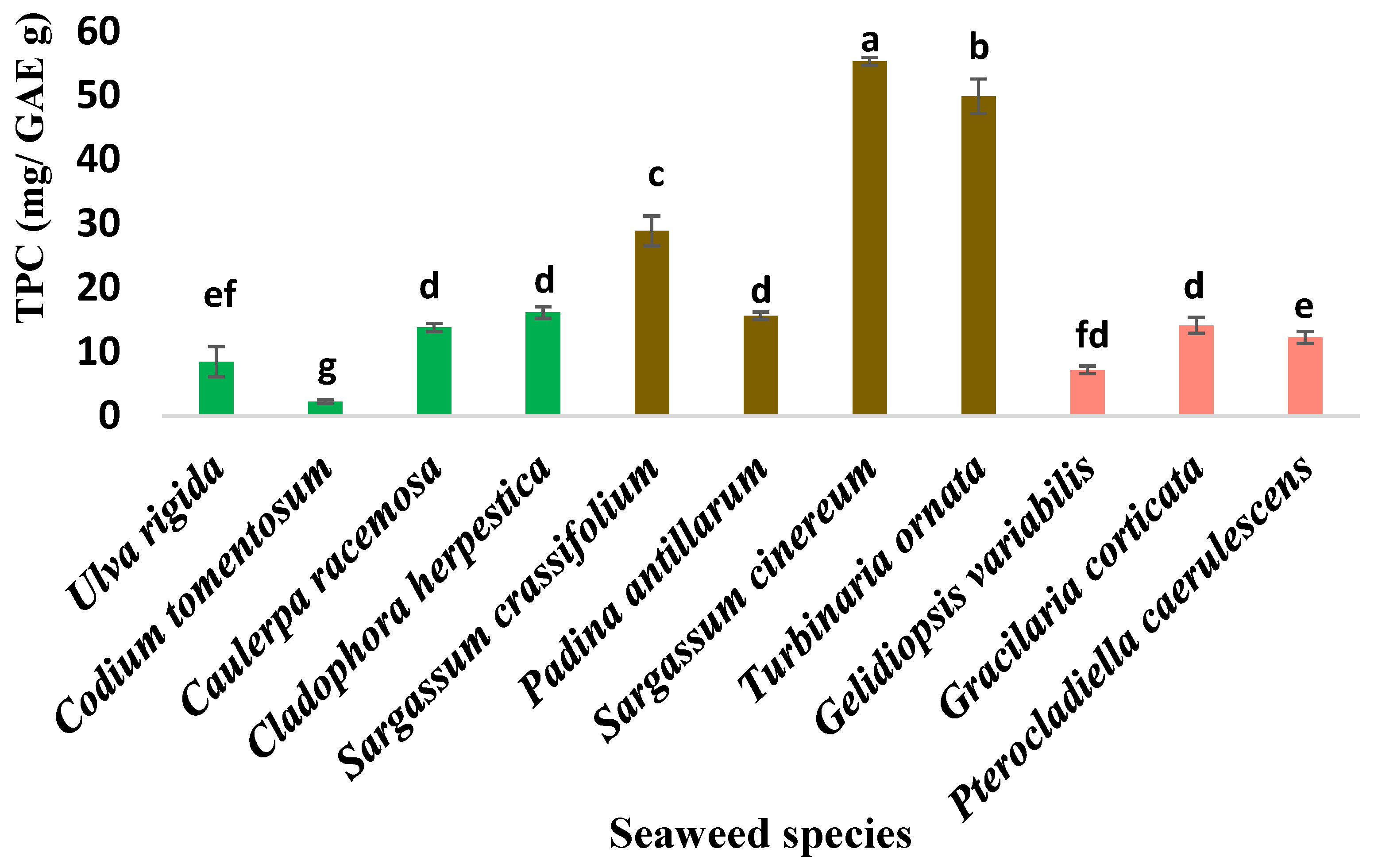
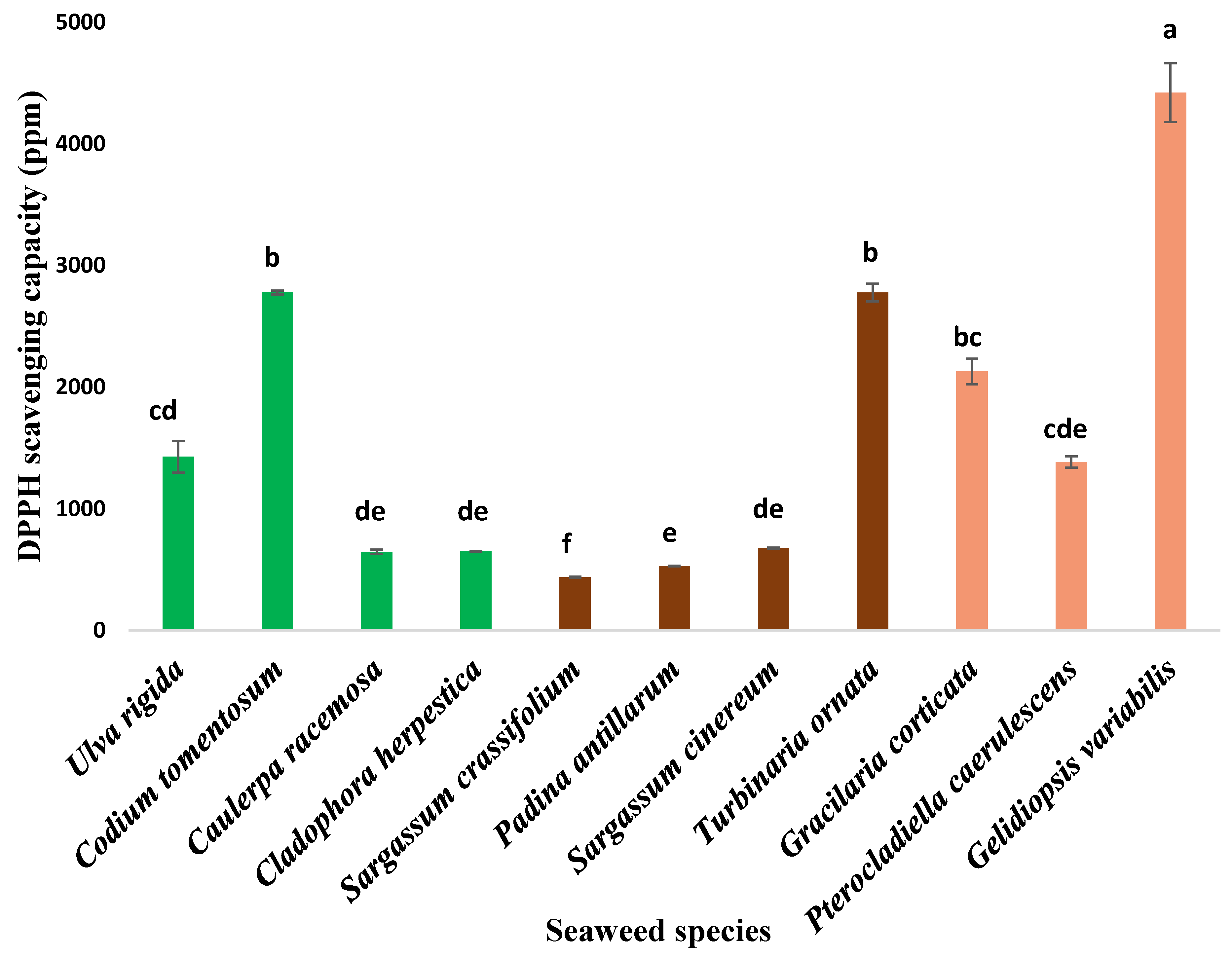
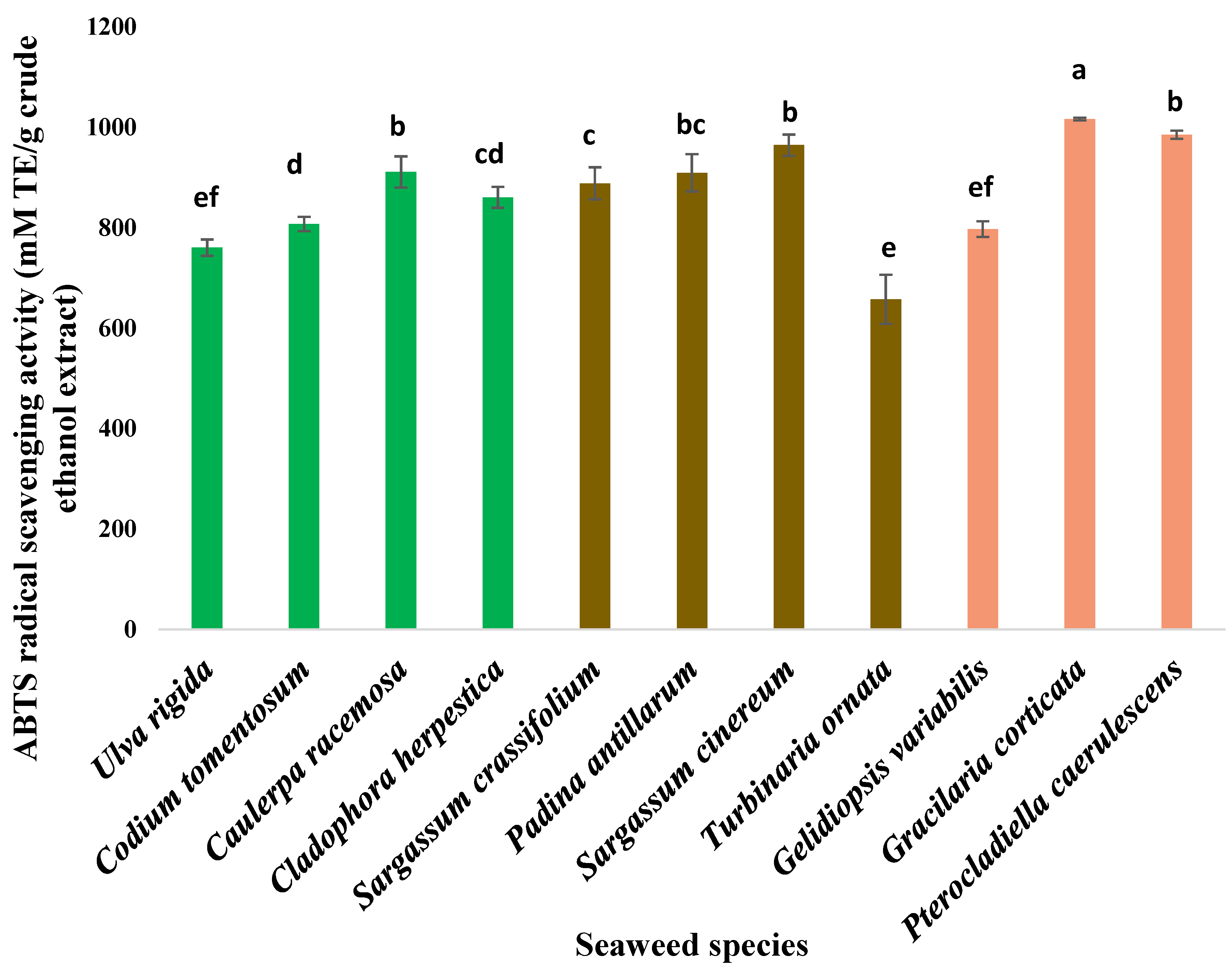
3.2. Antioxidant Activity
3.3. Trace Elements and Heavy Metal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Guedes, C.; Gomes-Dias, J.S.; Cunha, S.A.; Pintado, M.E.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Enzymatic Approach for the Extraction of Bioactive Fractions from Red, Green and Brown Seaweeds. Food Bioprod. Process. 2023, 138, 25–39. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.; Bin Dhama, K. Antioxidant Effects of Seaweeds and Their Active Compounds on Animal Health and Production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Pirian, K.; Jeliani, Z.Z.; Sohrabipour, J.; Arman, M.; Faghihi, M.M.; Yousefzadi, M. Nutritional and Bioactivity Evaluation of Common Seaweed Species from the Persian Gulf. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 1795–1804. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as Nutraceuticals for Health and Nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.-S. Seaweed Exhibits Therapeutic Properties against Chronic Diseases: An Overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Fayzi, L.; Askarne, L.; Cherifi, O.; Boufous, E.H.; Cherifi, K. Comparative Antibacterial Activity of Some Selected Seaweed Extracts from Agadir Coastal Regions in Morocco. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 390–399. [Google Scholar] [CrossRef]
- Nurkolis, F.; Taslim, N.A.; Qhabibi, F.R.; Kang, S.; Moon, M.; Choi, J.; Choi, M.; Park, M.N.; Mayulu, N.; Kim, B. Ulvophyte Green Algae Caulerpa lentillifera: Metabolites Profile and Antioxidant, Anticancer, Anti-Obesity, and In Vitro Cytotoxicity Properties. Molecules 2023, 28, 1365. [Google Scholar] [CrossRef]
- Pourakbar, L.; Moghaddam, S.S.; El Enshasy, H.A.; Sayyed, R.Z. Antifungal Activity of the Extract of a Macroalgae, Gracilariopsis Persica, against Four Plant Pathogenic Fungi. Plants 2021, 10, 1781. [Google Scholar] [CrossRef]
- Malhão, F.; Ramos, A.; Macedo, A.; Rocha, E. Cytotoxicity of Seaweed Compounds, Alone or Combined to Reference Drugs, against Breast Cell Lines Cultured in 2D and 3D. Toxics 2021, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- El Shafay, S.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, Antidiabetic, Anti-Inflammatory and Anticancer Potential of Some Seaweed Extracts. Food Sci. Technol. 2022, 42, e20521. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–Bioactivity and Extraction Perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive Phenolic Compounds from Algae. In Bioactive Compounds from Marine Foods; Wiley: Hoboken, NJ, USA, 2013; pp. 113–129. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed Carotenoid, Fucoxanthin, as a Multi-Functional Nutrient. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 196–199. [Google Scholar]
- Rupérez, P. Mineral Content of Edible Marine Seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Rubio, C.; Napoleone, G.; Luis-González, G.; Gutiérrez, A.J.; González-Weller, D.; Hardisson, A.; Revert, C. Metals in Edible Seaweed. Chemosphere 2017, 173, 572–579. [Google Scholar] [CrossRef]
- Coppejans, E.; Leliaert, F.; Dargent, O.; Gunasekara, R.; De Clerck, O. Sri Lankan Seaweeds: Methodologies and Field Guide to the Dominant Species; Belgian National Focal Point to the Global Taxonomy Initiative: Brussels, Belgium, 2009. [Google Scholar]
- Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Suriya, M.S.; Ridma, L.; Ruksheela, B.; Isuri, R.; Afka, D.; Barana, J.; Ruvini, L. Morphological, physicochemical, and functional properties of fifteen different dietary carbohydrate sources in Sri Lanka. JSFA Rep. 2023, 3, 463–472. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Tuvikene, R.; Fernando, P.H.P.; Adhikari, R.; Perera, M.C.N.; Ranahewa, T.H.; Howlader, M.M.; Wangchuk, P.; Jayasooriya, A.P.; Rajapakse, R.P.V.J. Comparative Analysis of Proximate Compositions, Mineral and Functional Chemical Groups of 15 Different Seaweed Species. Sci. Rep. 2022, 12, 19610. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty Acids, Total Lipid, Protein and Ash Contents of Processed Edible Seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Nafiqoh, N.; Suryaningrum, L.H.; Novita, H.; Andriyanto, S. Nutrient Content of Seaweed and Its Digestibility in Osteochilus Hasseltii. IOP Conf. Ser. Earth Environ. Sci. 2021, 695, 012015. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of Using Algae as Natural Sources of Functional Ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]
- Taboada, C.; MillÃn, R.; MÃ guez, I. Composition, Nutritional Aspects and Effect on Serum Parameters of Marine Algae Ulva rigida. J. Sci. Food Agric. 2009, 90, 445–449. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.-K. Nutritional and Digestive Health Benefits of Seaweed. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 64, pp. 17–28. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty Acids, Amino Acids, Mineral Contents, and Proximate Composition of Some Brown Seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B. Nutritional Composition of Edible Hawaiian Seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Ivanova, V.; Stancheva, M.; Petrova, D.; Ivanova, V.; Stancheva, M.; Petrova, D. Fatty Acid Composition of Black Sea Ulva rigida and Cystoseira crinita. Bulg. J. Agric. Sci. 2013, 19 (Suppl. S1), 42–47. [Google Scholar]
- Grant, T.; Ingegerd, S.; Federico, G.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Grace, S.; Djuhria, W.; Nurmelita, T.; Verly, D.; Aurielle, A.S.; Happy, K.P.; Sidik, M.; Fahrul, N.; Apollinaire, T.; Bonglee, K. Green seaweed Caulerpa racemosa—Chemical constituents, cytotoxicity in breast cancer cells and molecular docking simulation. J. Agric. Food Res. 2023, 12, 100621. [Google Scholar] [CrossRef]
- Petra, T.; Barbara, Č.; Nataša, P.U.; Helena, A. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops. Prod. 2012, 39, 210–221, ISSN 0926-6690. [Google Scholar]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant Activity of Different Molecular Weight Sulfated Polysaccharides from Ulva Pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total Phenolic Compounds, Radical Scavenging and Metal Chelation of Extracts from Icelandic Seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Smith, J.; Summers, G.; Wong, R. Nutrient and Heavy Metal Content of Edible Seaweeds in New Zealand. New Zeal. J. Crop Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Marquès, M.; Correig, E.; Capdevila, E.; Gargallo, E.; González, N.; Nadal, M.; Domingo, J.L. Essential and Non-Essential Trace Elements in Milks and Plant-Based Drinks. Biol. Trace Elem. Res. 2022, 200, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Maria, F.; Anna, B.; Simonetta, M.; Giorgio, F.; Silva, R.; Domenico, G.; Giorgia, V.; Raissa, B.; Stefano, M.; Silvia, V. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2020, 263, 127983. [Google Scholar] [CrossRef]
- Muse, J.O.; Tudino, M.B.; D’Huicque, L.; Troccoli, O.E.; Carducci, C.N. A Survey of Some Trace Elements in Seaweeds from Patagonia, Argentina. Environ. Pollut. 1995, 87, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, B.C.; Warnasooriya, S.G.V.B.; Vithange, M.; Thilakarathna, H.M.E.; Jayawardana, B.M.Y.B.; Liyanage, R. Nutritional and Bioactive Properties of Selected Marine Microalge Species in Sri Lanka. In International Conference of Food Security and Roles of Fisheries; Korean Federation of Fisheries Science and technology Societies: Busan, Republic of Korea, 2017; p. 464. [Google Scholar]


| Seaweed Species | Voucher No. | Type | Collected Location | Location Coordinates | Collected Date |
|---|---|---|---|---|---|
| Gelidiopsis variabilis | SW1 | R | South Bar, Mannar | N 8°97′13″, E 79°88′06″ | 31 August 2016 |
| Pterocladiella caerulescens | SW2 | R | Madiha, Matara | N 5°56′11″, E 80°30′56″ | 25 September 2016 |
| Gracilaria corticata | SW3 | R | Wellamadama, Matara | N 5°94′26″, E 80°56′74″ | 26 September 2016 |
| Caulerpa racemosa | SW4 | G | |||
| Ulva rigida | SW5 | G | Madiha, Matara | N 5°56′11″, E 80°30′56″ | 25 September 2016 |
| Codium tomentosum | SW6 | G | Madiha, Matara | N 5°56′11″, E 80°30′56″ | 25 September 2016 |
| Cladophora herpestica | SW7 | G | Madiha, Matara | N 5°56′11″, E 80°30′56″ | 25 September 2016 |
| Sargassum crassifolium | SW8 | B | Madiha, Matara | N 5°56′11″, E 80°30′56″ | 25 September 2016 |
| Sargassum cinereum | SW9 | B | Erukkalampiddy, Mannar | N 9°2′27″, E 79°52′43″ | 30 August 2016 |
| Turbinaria ornata | SW10 | B | South Bar, Mannar | N 8°97′13″, E 79°88′06″ | 31 August 2016 |
| Padina antillarum | SW11 | B | Wellamadama, Matara | N 5°94′26″, E 80°56′74″ | 25 September 2016 |
| Types of Seaweed | Sample No. | MC% (FW) | Ash% (DW) | Crude Protein% (DW) | Crude Fat% (DW) | Crude Fiber% (DW) |
|---|---|---|---|---|---|---|
| Gelidiopsis variabilis | R1 | 91.31 | 16.69 ± 0.00 g | 13.66 ± 0.04 d | 1.46 ± 0.09 cd | 9.94 ± 0.13 c |
| Pterocladiella caerulescens | R2 | 87.36 | 22.58 ± 0.19 fc | 22.58 ± 0.39 ab | 2.09 ± 0.14 bc | 10.49 ± 0.04 d |
| Gracilaria corticata | R3 | 85.68 | 35.20 ± 0.17 a | 19.43 ± 0.27 b | 2.68 ± 0.09 b | 18.73 ± 0.39 a |
| Caulerpa racemosa | G1 | 92.89 | 26.78 ± 0.04 dc | 18.61 ± 0.82 bc | 0.54 ± 0.66 c | 12.33 ± 0.08 c |
| Ulva rigida | G2 | 80.84 | 20.26 ± 0.11 cf | 23.67 ± 0.73 a | 0.09 ± 0.02 c | 11.64 ± 0.31 cd |
| Codium tomentosum | G3 | 91.80 | 21.43 ± 0.73 c | 13.85 ± 0.48 d | 1.31 ± 0.23 cd | 10.66 ± 0.39 d |
| Cladophora herpestica | G4 | 93.76 | 28.18 ± 0.61 d | 17.53 ± 1.90 bc | 3.89 ± 0.40 a | 15.42 ± 0.26 b |
| Sargassum crassifolium | B1 | 89.02 | 31.18 ± 0.05 bc | 10.44 ± 0.51 c | 2.65 ± 0.01 b | 15.78 ± 0.11 b |
| Sargassum cinereum | B2 | 88.02 | 12.96 ± 0.37 h | 8.30 ± 0.24 f | 2.47 ± 0.22 b | 17.46 ± 0.38 a |
| Turbinaria ornata | B3 | 87.02 | 21.11 ± 0.12 c | 4.87 ± 0.85 g | 4.13 ± 0.12 a | 15.33 ± 0.56 b |
| Padina antillarum | B4 | 87.97 | 33.39 ± 0.11 bc | 18.44 ± 0.51 bc | 2.33 ± 0.00 b | 16.32 ± 0.04 b |
| Seaweed Type | Trace Metals | Heavy Metals | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Co | Ni | Mn | Cr | Al | Pb | As | Cd | |
| P. caerulescens | 0.48 ± 0.15 c | 0.30 ± 0.00 e | 0.95 ± 0.02 c,d | 14.29 ± 0.1 d | 0.55 ± 0.02 e | 123.74 ± 3.87 h | 0.19 ± 0.03 d | 6.08 ± 0.04 f | 2.76 ± 0.03 b |
| G. corticata | 1.85 ± 0.19 c | 0.37 ± 0.02 d | 0.95 ± 0.05 c,d | 53.41 ± 1.32 a | 1.17 ± 0.12 d | 246.69 ± 5.74 d | 0.32 ± 0.04 c | 7.33 ± 0.2 e | 3.67 ± 0.08 a |
| C. racemosa | 1.95 ± 0.07 c | 0.66 ± 0.01 b | 5.88 ± 0.79 a | 34.58 ± 0.18 c | 4.17 ± 0.12 a | 453.29 ± 9.15 c | 0.58 ± 0.03 b | 5.90 ± 0.01 f | 3.66 ± 0.02 a |
| U. rigida | 1.574 ± 0.05 c | 0.23 ± 0.01 f | 2.76 ± 0.048 b | 9.74 ± 0.176 e | 2.23 ± 0.159 b | 180.88 ± 2.28 f | 0.374 ± 0.025 c | 2.35 ± 0.027 g | 0.99 ± 0.006 e |
| C. tomentosum | 0.50 ± 0.08 c | 0.16 ± 0.00 g | 1.43 ± 0.02 c,d | 9.70 ± 0.20 e | 1.93 ± 0.09 c | 214.59 ± 7.06 e | 0.35 ± 0.02 c | 18.50 ± 0.34 d | 0.59 ± 0.01 f |
| S. crassifolium | 0.25 ± 0.04 c | 0.25 ± 0.00 f | 0.70 ± 0.01 d | 7.54 ± 0.07 f | 0.68 ± 0.02 e | 152.93 ± 2.98 g | 0.14 ± 0.01 d | 36.91 ± 0.13 a | 2.13 ± 0.01 c |
| S. cinereum | 442.34 ± 20.94 a | 1.57 ± 0.04 a | 1.6 ± 0.03 c | 49.3 ± 0.58 b | 1.9 ± 0.04 c | 562.13 ± 6.47 a | 1.21 ± 0.07 a | 30.99 ± 0.3 c | 1.34 ± 0.02 d |
| T. ornata | 37.77 ± 2.47 b | 0.54 ± 0.01 c | 0.99 ± 0.06 c,d | 9.14 ± 0.09 e | 1.68 ± 0.13 c | 477.43 ± 4.49 b | 0.33 ± 0.01 c | 33.66 ± 0.07 b | 1.04 ± 0.01 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warnasooriya, V.; Gunawardena, S.; Weththasinghe, P.; Jayawardana, B.; Qader, M.; Liyanage, R. Nutritional Properties, Antioxidant Activity, and Heavy Metal Accumulation in Selected Marine Macro-Algae Species of Sri Lanka. Nutraceuticals 2024, 4, 50-64. https://doi.org/10.3390/nutraceuticals4010004
Warnasooriya V, Gunawardena S, Weththasinghe P, Jayawardana B, Qader M, Liyanage R. Nutritional Properties, Antioxidant Activity, and Heavy Metal Accumulation in Selected Marine Macro-Algae Species of Sri Lanka. Nutraceuticals. 2024; 4(1):50-64. https://doi.org/10.3390/nutraceuticals4010004
Chicago/Turabian StyleWarnasooriya, Viraji, Sadhisha Gunawardena, Pabodha Weththasinghe, Barana Jayawardana, Mallique Qader, and Ruvini Liyanage. 2024. "Nutritional Properties, Antioxidant Activity, and Heavy Metal Accumulation in Selected Marine Macro-Algae Species of Sri Lanka" Nutraceuticals 4, no. 1: 50-64. https://doi.org/10.3390/nutraceuticals4010004
APA StyleWarnasooriya, V., Gunawardena, S., Weththasinghe, P., Jayawardana, B., Qader, M., & Liyanage, R. (2024). Nutritional Properties, Antioxidant Activity, and Heavy Metal Accumulation in Selected Marine Macro-Algae Species of Sri Lanka. Nutraceuticals, 4(1), 50-64. https://doi.org/10.3390/nutraceuticals4010004







