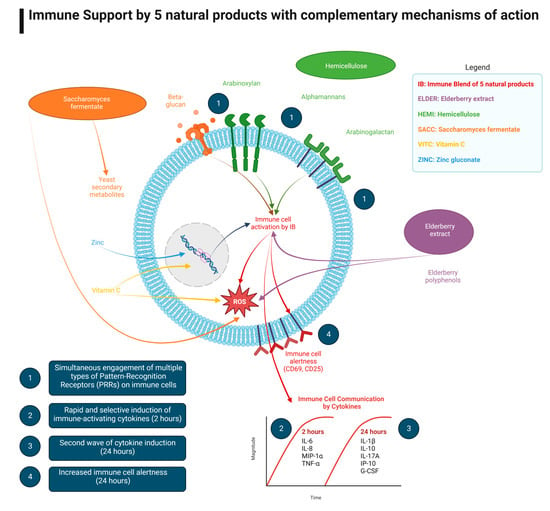
Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Test Products
2.3. Immune Cell Activation
2.4. Production of Cytokines, Chemokines, and Growth Factors
2.5. Statistical Analysis
3. Results
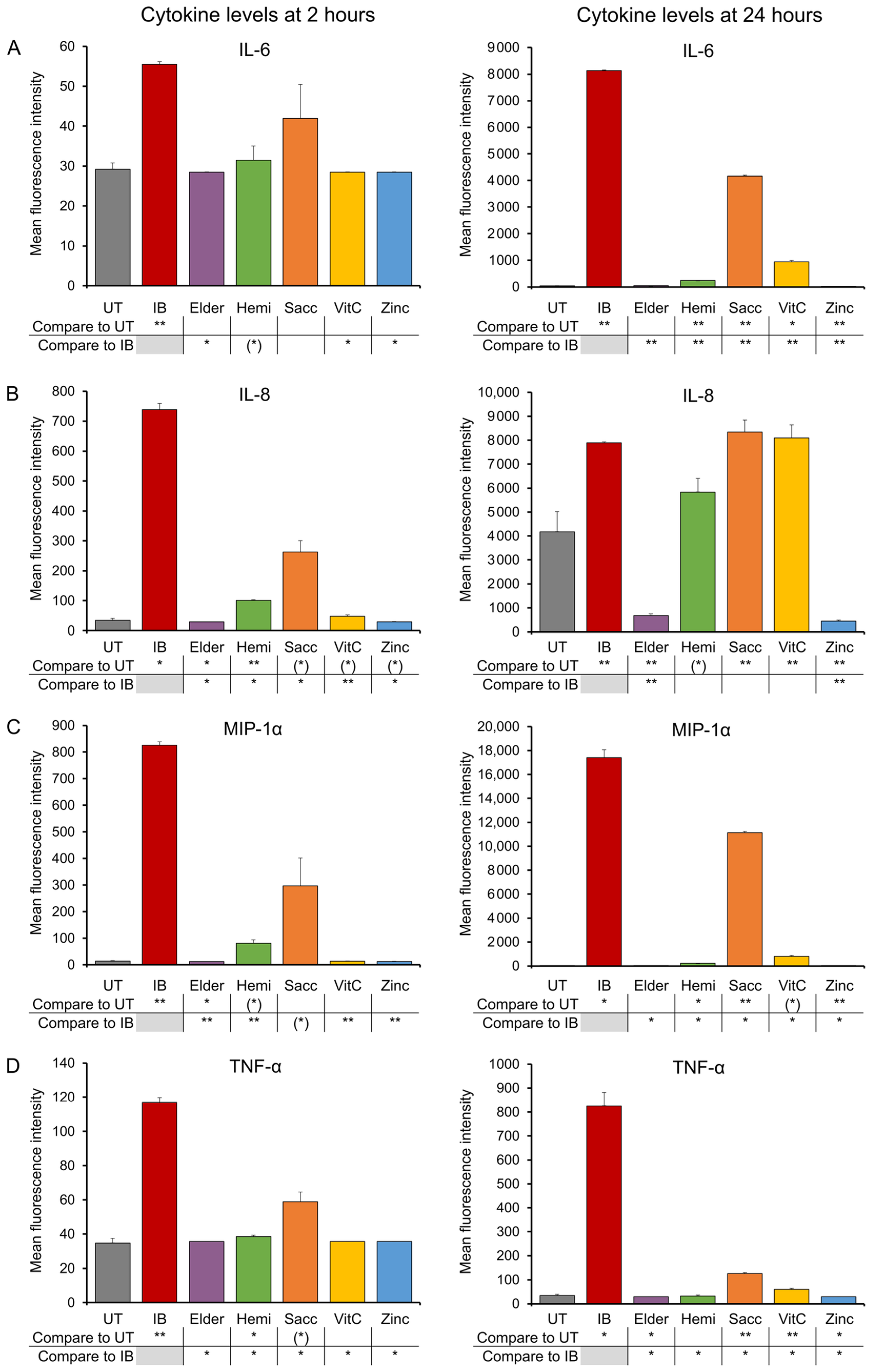
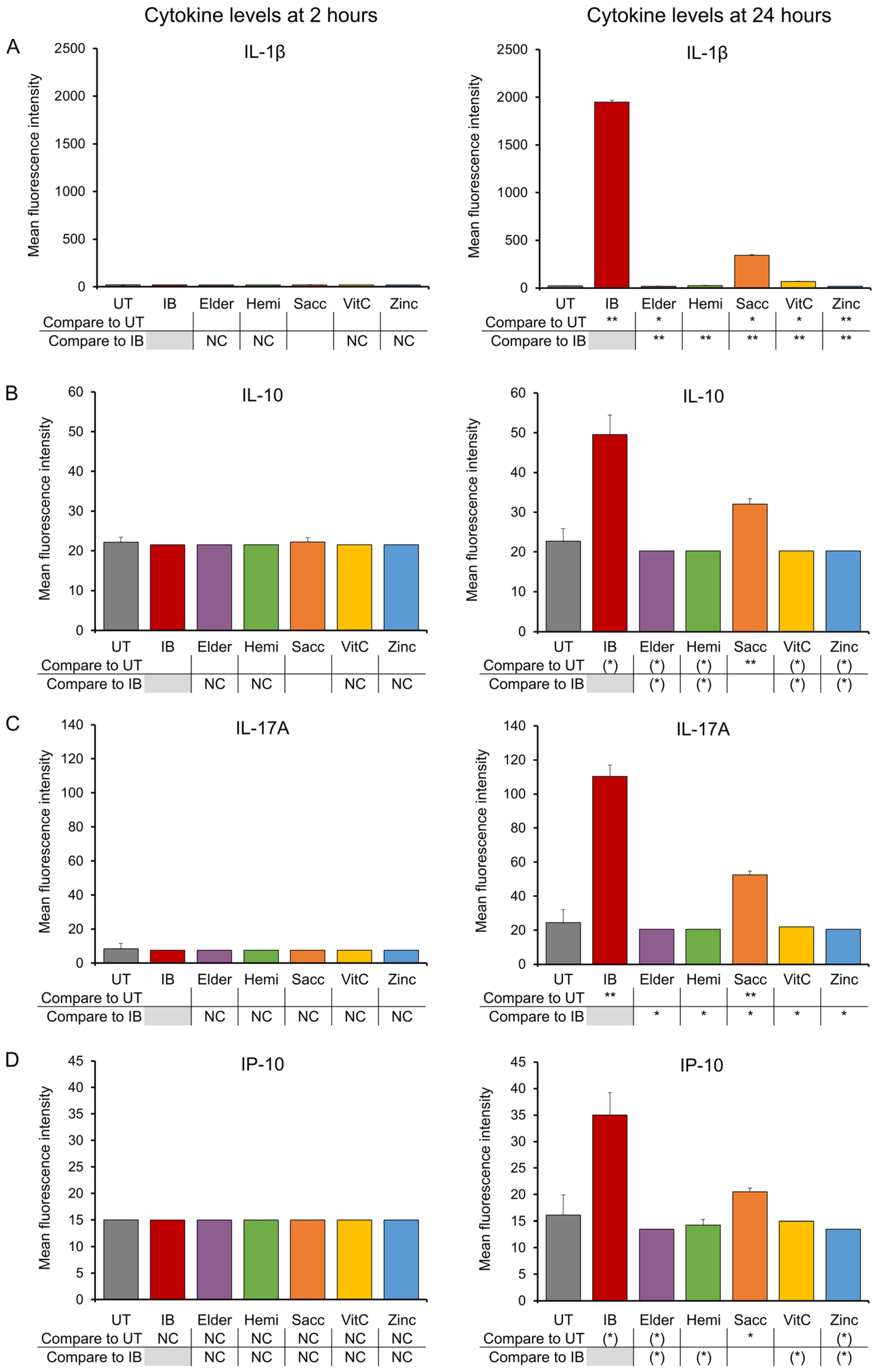
3.1. Early-Responding Cytokines
3.2. Late-Responding Cytokines
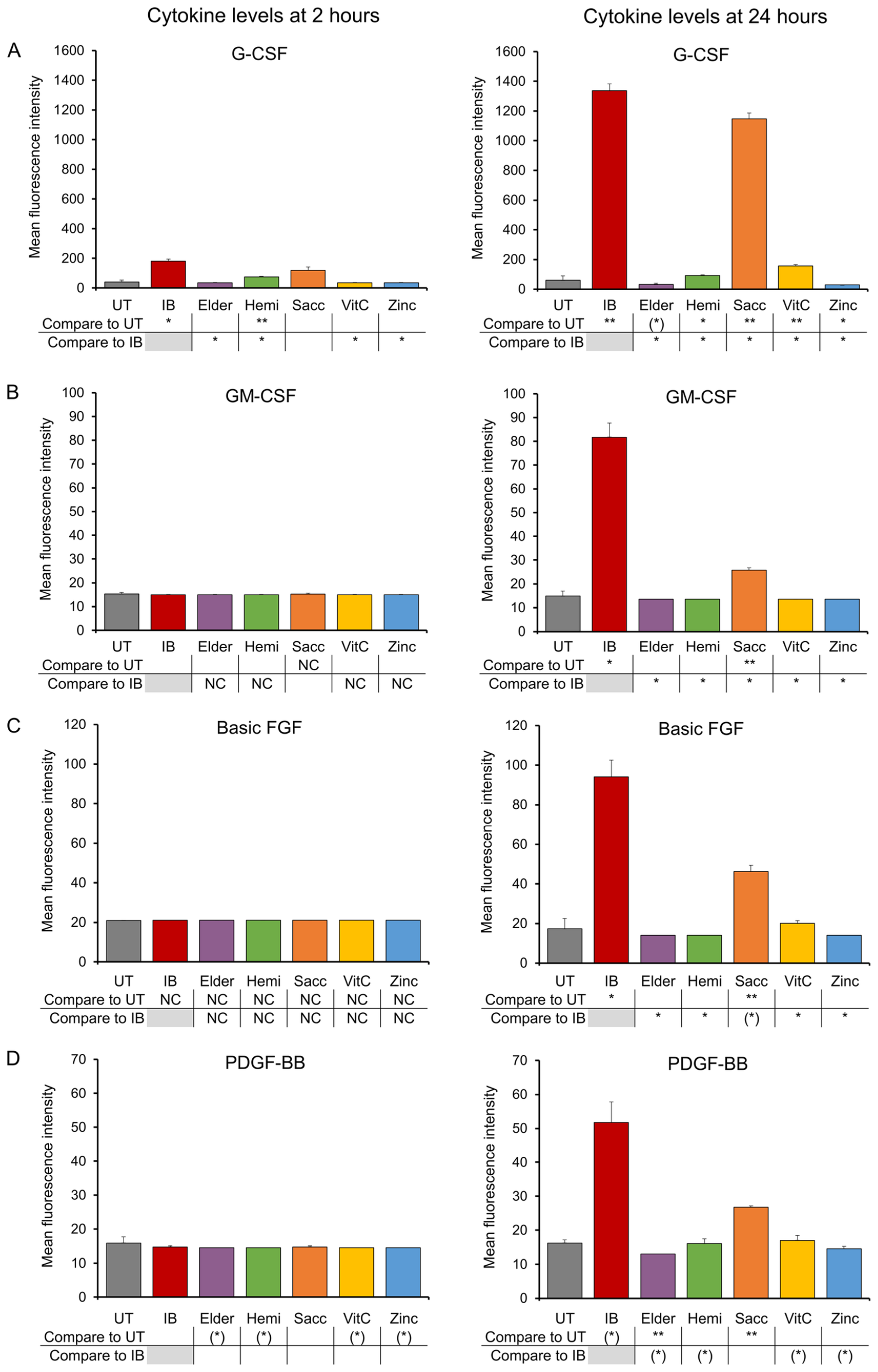
3.3. Growth Factors
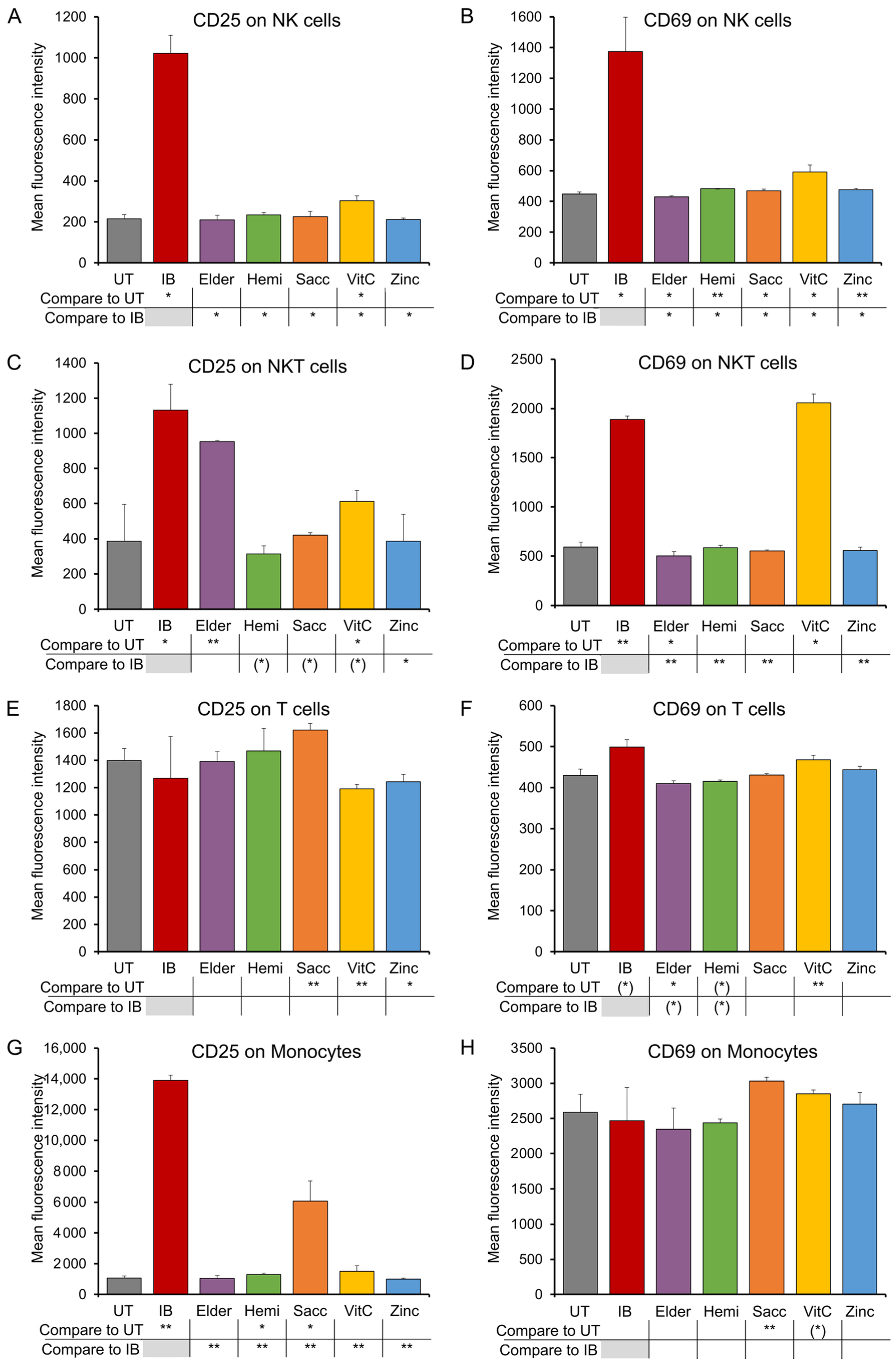
3.4. Expression of CD25 and CD69 Activation Markers on Immune Cell Subsets
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Plato, A.; Willment, J.A.; Brown, G.D. C-type lectin-like receptors of the dectin-1 cluster: Ligands and signaling pathways. Int. Rev. Immunol. 2013, 32, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Dion, C.; Chappuis, E.; Ripoll, C. Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutr. Metab. 2016, 12, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Guidato, P.M.; Peters, K.; Megger, D.A.; Sitek, B.; Classen, B.; Heise, E.M.; Bufe, A. Allergy-Protective Arabinogalactan Modulates Human Dendritic Cells via C-Type Lectins and Inhibition of NF-κB. J. Immunol. 2016, 96, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Moerings, B.G.J.; van Bergenhenegouwen, J.; Furber, M.; Abbring, S.; Schols, H.A.; Witkamp, R.F.; Govers, C.; Mes, J.J. Dectin-1b activation by arabinoxylans induces trained immunity in human monocyte-derived macrophages. Int. J. Biol. Macromol. 2022, 209 (Pt. A), 942–950. [Google Scholar] [CrossRef]
- Saijo, S.; Iwakura, Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int. Immunol. 2011, 23, 467–472. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dzharullaeva, A.S.; Erokhova, A.S.; Scheblyakov, D.V.; Naroditsky, B.S.; Gintsburg, A.L.; Logunov, D.Y. NOD1/2 and the C-Type Lectin Receptors Dectin-1 and Mincle Synergistically Enhance Proinflammatory Reactions Both In Vitro and In Vivo. J. Inflamm. Res. 2020, 13, 357–368. [Google Scholar] [CrossRef]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA 2011, 108, 4607–4614. [Google Scholar] [CrossRef]
- Jensen, G.S.; Hart, A.N.; Schauss, A.G. An antiinflammatory immunogen from yeast culture induces activation and alters chemokine receptor expression on human natural killer cells and B lymphocytes in vitro. Nutr. Res. 2007, 27, 327–335. [Google Scholar] [CrossRef]
- Jensen, G.S.; Redman, K.A.; Benson, K.F.; Carter, S.G.; Mitzner, M.A.; Reeves, S.; Robinson, L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: Results of a placebo-controlled double-blinded crossover pilot study. J. Med. Food 2011, 14, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Moyad, A.; Robinson, L.E.; Zawada, E.T.; Kittelsrud, J.; Chen, D.G.; Reeves, S.G.; Weaver, S. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J. Altern. Complement. Med. 2010, 16, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Robinson, L.E.; Kittelsrud, J.M.; Reeves, S.G.; Weaver, S.E.; Guzman, A.I.; Bubak, M.E. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: A randomized, double-blind, placebo-controlled trial. Adv. Ther. 2009, 26, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Carter, S.G.; Reeves, S.G.; Robinson, L.E.; Benson, K.F. Anti-inflammatory properties of a dried fermentate in vitro and in vivo. J. Med. Food 2015, 18, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, T.; Lai, C.; Ling, Z.; Zhou, Y.; Yong, Q. The in vitro and in vivo Antioxidant and Immunomodulatory Activity of Incomplete Degradation Products of Hemicellulosic Polysaccharide (Galactomannan) From Sesbania cannabina. Front. Bioeng. Biotechnol. 2021, 9, 679558. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Schols, H.A.; Faas, M.M.; de Vos, P. Arabinoxylan activates Dectin-1 and modulates particulate β-glucan-induced Dectin-1 activation. Mol. Nutr. Food Res. 2016, 60, 458–467. [Google Scholar] [CrossRef]
- Chavoustie, S.E.; Perez, P.P.; Fletcher, M.A.; Maher, K.J.; Mitrani, A.A.; Thomas, R.H. Pilot Study: Effect of PDS-2865 on Natural Killer Cell Cytotoxicity. J. Am. Nutraceutical Assoc. 2003, 6, 39–42. [Google Scholar]
- Weeks, B.S.; Perez, P.P. The hemicellulose preparation, Natramune (PDS-2865), increases macrophage phagocytosis and nitric oxide production and increases circulating human lymphocytes levels. Med. Sci. Monit. 2009, 15, BR43–BR46. [Google Scholar]
- Weeks, B.S.; Lee, S.; Perez, P.P.; Brown, K.; Chauhan, H.; Tsaava, T. Natramune and PureWay-C reduce xenobiotic-induced human T-cell alpha5beta1 integrin-mediated adhesion to fibronectin. Med. Sci. Monit. 2008, 14, BR279–BR285. [Google Scholar]
- Schön, C.; Mödinger, Y.; Krüger, F.; Doebis, C.; Pischel, I.; Bonnländer, B. A new high-quality elderberry plant extract exerts antiviral and immunomodulatory effects in vitro and ex vivo. Food Agric. Immunol. 2021, 32, 650–662. [Google Scholar] [CrossRef]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Kellens, J.T.; Allen, A.K.; Van Damme, E.J. Isolation and characterization of a seed lectin from elderberry (Sambucus nigra L.) and its relationship to the bark lectins. Carbohydr. Res. 1991, 213, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Strass, G.; Herbst, M.; Dietrich, H.; Bitsch, R.; Bitsch, I.; Frank, T. The excretion and biological antioxidant activity of elderberry antioxidants in healthy humans. Food Res. Int. 2005, 38, 905–910. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000, 29, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Dominika, T.; Valachová, K.; Rapta, P.; Šilhár, S.; Panghyová, E.; Horváth, A.; Šoltés, L. Antioxidative properties of Sambucus nigra extracts. Chem. Pap. 2015, 69, 1202–1210. [Google Scholar]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Stich, L.; Plattner, S.; McDougall, G.; Austin, C.; Steinkasserer, A. Polysaccharides from European Black Elderberry Extract Enhance Dendritic Cell Mediated T Cell Immune Responses. Int. J. Mol. Sci. 2022, 23, 3949. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef]
- Lindblad, B.; Lindstedt, G.; Lindstedt, S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J. Am. Chem. Soc. 1970, 92, 7446–7449. [Google Scholar] [CrossRef]
- Lachapelle, M.Y.; Drouin, G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica 2011, 139, 199–207. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M. Vitamins C and E donate single hydrogen atoms in vivo. FEBS Lett. 1991, 284, 147–151. [Google Scholar] [CrossRef]
- Parker, A.; Cuddihy, S.L.; Son, T.G.; Vissers, M.C.; Winterbourn, C.C. Roles of superoxide and myeloperoxidase in ascorbate oxidation in stimulated neutrophils and H2O2-treated HL60 cells. Free Radic. Biol. Med. 2011, 51, 1399–1405. [Google Scholar] [CrossRef]
- Brabson, J.P.; Leesang, T.; Mohammad, S.; Cimmino, L. Epigenetic Regulation of Genomic Stability by Vitamin C. Front. Genet. 2021, 12, 675780. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Apostolou, E.; Ferrari, F.; Choi, J.; Walsh, R.M.; Chen, T.; Ooi, S.S.; Kim, S.Y.; Bestor, T.H.; Shioda, T.; et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all–iPS cell mice from terminally differentiated B cells. Nat. Genet. 2012, 44, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Godoy-Tena, G.; Calafell-Segura, J.; Ciudad, L.; Martínez-Cáceres, E.M.; Sardina, J.L.; Ballestar, E. Vitamin C enhances NF-κB-driven epigenomic reprogramming and boosts the immunogenic properties of dendritic cells. Nucleic Acids Res. 2022, 50, 10981–10994. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W. Dietary Sources of Zinc and Factors Affecting its Bioavailability. Food Nutr. Bull. 2001, 22, 138–154. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.S.; Majumdar, I.; Grishin, N.V. Structural classification of zinc fingers: Survey and summary. Nucleic Acids Res. 2003, 31, 532–550. [Google Scholar] [CrossRef]
- Merkenschlager, M.; Nora, E.P. CTCF and Cohesin in Genome Folding and Transcriptional Gene Regulation. Annu. Rev. Genomics Hum. Genet. 2016, 17, 17–43. [Google Scholar] [CrossRef]
- Allen, J.I.; Perri, R.T.; McClain, C.J.; Kay, N.E. Alterations in human natural killer cell activity and monocyte cytotoxicity induced by zinc deficiency. J. Lab. Clin. Med. 1983, 102, 577–589. [Google Scholar]
- Mayer, L.S.; Uciechowski, P.; Meyer, S.; Schwerdtle, T.; Rink, L.; Haase, H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics 2014, 6, 1288–1295. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. Signal transduction in monocytes: The role of zinc ions. Biometals 2007, 20, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 6762343, 21. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Kalia, N.; Singh, J.; Kaur, M. The role of dectin-1 in health and disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, C.; Krüger, C.; Plaas, C.; Kirsch, F.; Dittgen, T.; Müller, R.; Laage, R.; Kastner, S.; Suess, S.; Spoelgen, R.; et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain 2008, 131, 3335–3347. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M. Intestinal dendritic cells. Adv. Immunol. 2010, 107, 109–138. [Google Scholar] [CrossRef]
- Chieppa, M.; Rescigno, M.; Huang, A.Y.; Germain, R.N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006, 203, 2841–2852. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr. Pharm. Des. 2013, 19, 1329–1342. [Google Scholar] [CrossRef]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Wang, J.P.; Specht, C.A.; Levitz, S.M. Distinct patterns of dendritic cell cytokine release stim-ulated by fungal beta-glucans and toll-like receptor agonists. Infect. Immun. 2009, 77, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.B.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; McGarry, S.; Cruickshank, D.; Jensen, G.S. Rapid increase in immune surveillance and expression of NKT and γδT cell activation markers after consuming a nutraceutical supplement containing Aloe vera gel, extracts of Poria cocos and rosemary. A randomized placebo-controlled cross-over trial. PLoS ONE 2023, 18, e0291254. [Google Scholar] [CrossRef]
| Receptor Class | Receptor Type | Ligands |
|---|---|---|
| Toll-Like Receptors | TLR-1 | Lipoprotein B |
| TLR-2 | Peptidoglycans B, Beta-glucans F | |
| TLR-4 | Lipopolysaccharides B(GN) | |
| TLR-2/TLR-6 | Beta-Glucans F | |
| C-Type Lectin Receptors | Dectin-1 | Beta-Glucans F Arabinoxylans P |
| Dectin-2 | Alpha-mannans F,P |
| Name | Abbreviation | % in Immune Blend * | Dose in Assays (mg/mL) ** |
|---|---|---|---|
| Immune Blend | IB | 100% | 1.000 |
| ElderMune | Elder | 3.36% | 0.033 |
| Natramune hemicellulose extract | Hemi | 16.8% | 0.168 |
| Saccharomyces cerevisiae | Sacc | 16.8% | 0.168 |
| PureWay Vitamin C | VitC | 33.6% | 0.338 |
| Zinc gluconate | Zinc | 0.50% | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGarry, S.V.; Yu, L.; Cruickshank, D.; Iloba, I.; Jensen, G.S. Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers. Nutraceuticals 2024, 4, 35-49. https://doi.org/10.3390/nutraceuticals4010003
McGarry SV, Yu L, Cruickshank D, Iloba I, Jensen GS. Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers. Nutraceuticals. 2024; 4(1):35-49. https://doi.org/10.3390/nutraceuticals4010003
Chicago/Turabian StyleMcGarry, Sage V., Liu Yu, Dina Cruickshank, Ifeanyi Iloba, and Gitte S. Jensen. 2024. "Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers" Nutraceuticals 4, no. 1: 35-49. https://doi.org/10.3390/nutraceuticals4010003
APA StyleMcGarry, S. V., Yu, L., Cruickshank, D., Iloba, I., & Jensen, G. S. (2024). Immune Activation by a Nutraceutical Blend: Rapid Increase in Immune-Modulating Cytokines, Followed by Induction of Anti-Inflammatory and Restorative Biomarkers. Nutraceuticals, 4(1), 35-49. https://doi.org/10.3390/nutraceuticals4010003