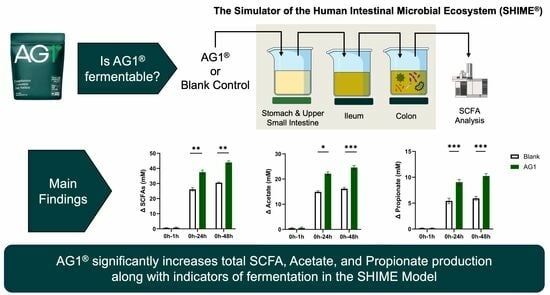
The Novel Synbiotic, AG1®, Increases Short-Chained Fatty Acid Production in the Simulator of Human Intestinal Microbial Ecosystem (SHIME) Model®
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Products
2.2. Test Gastrointestinal Tract System
2.3. Gastric Phase
2.4. Small Intestine Phase
2.5. Short-Term Colonic Batch Simulations
2.6. Statistics
3. Results
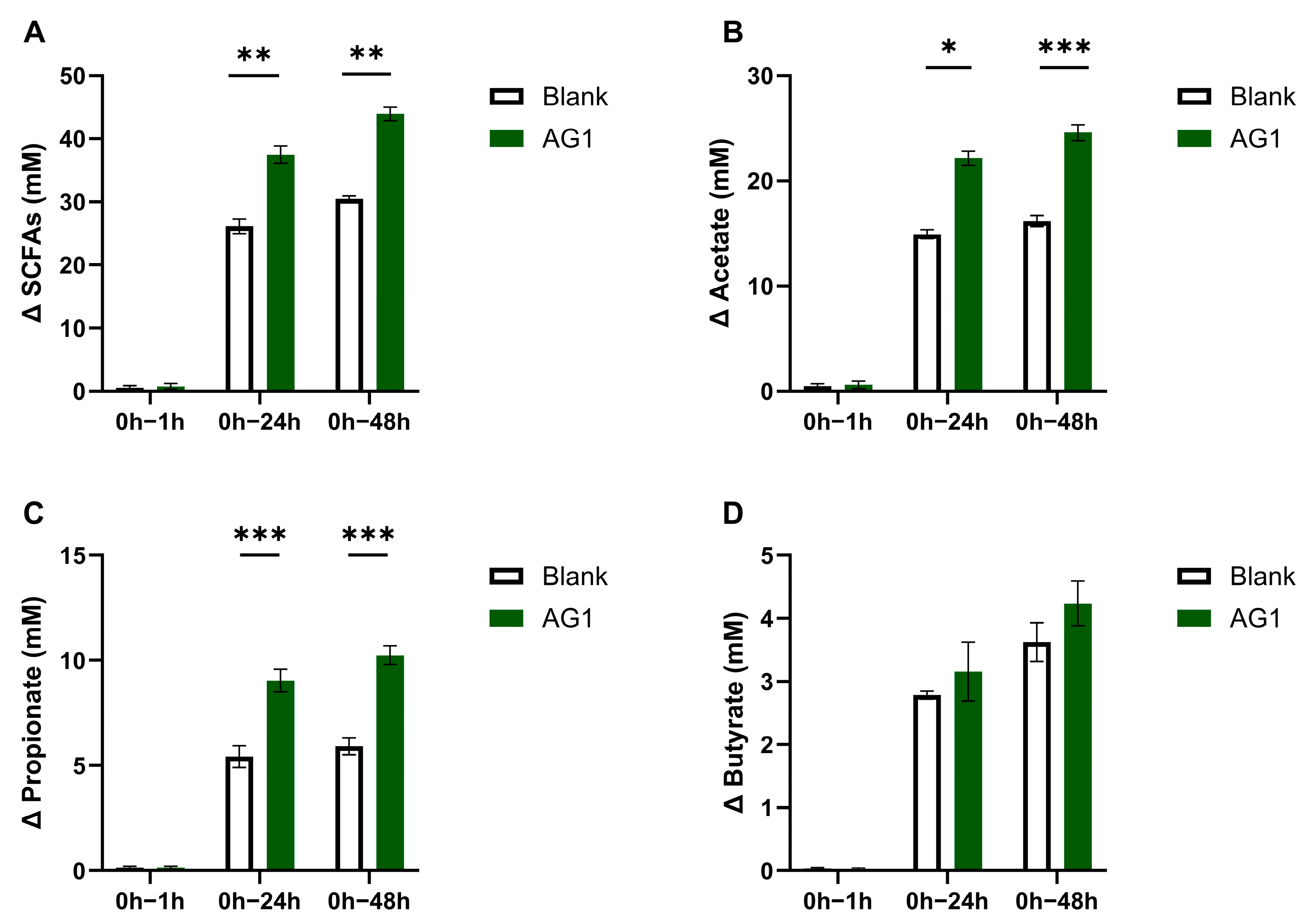
3.1. Physical Evidence of Microbial Fermentation
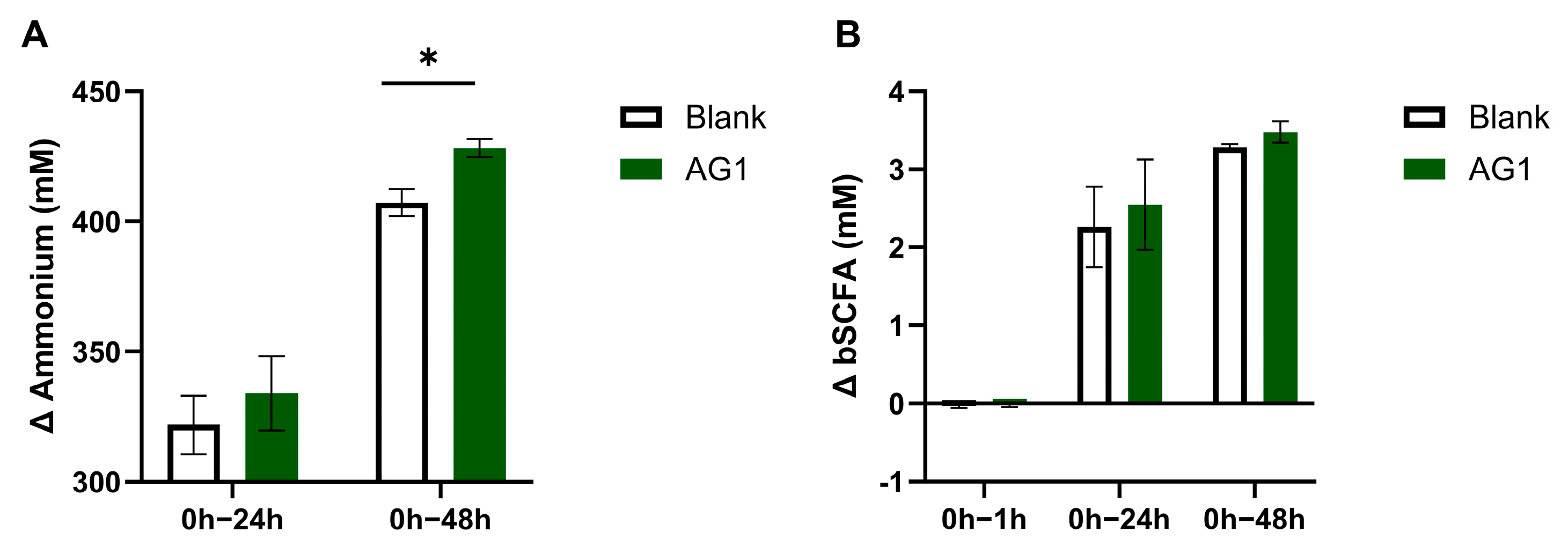
3.2. Byproducts of Fermentation
3.3. Byproducts from Protein Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, Y.; Roberfroid, M.B. Dietary Modulation of the Human Colonie Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Roa, R.I.L.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, Bioavailability, Interaction with Gut Microbiota, and Their Impacts on Human Health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as Modifiers of Gut Microbial Communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Peterson, C.T.; Sharma, V.; Uchitel, S.; Denniston, K.; Chopra, D.; Mills, P.J.; Peterson, S.N. Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. J. Altern. Complement. Med. 2018, 24, 656–665. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Salminen, S.J.; Gueimonde, M.; Isolauri, E. Symposium: Innate Immunity and Human Milk Probiotics That Modify Disease Risk 1. J. Nutr. 2005, 135, 1294–1298. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of Prebiotics, Probiotics, and Synbiotics in Management of Inflammatory Bowel Disease: Current Perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Gahche, J.J.; Herrick, K.A.; Davis, C.D.; Potischman, N.; Vargas, A.J. Nonfood Prebiotic, Probiotic, and Synbiotic Use Has Increased in US Adults and Children From 1999 to 2018. Gastroenterology 2021, 161, 476–486.e3. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut Microbiome–Micronutrient Interaction: The Key to Controlling the Bioavailability of Minerals and Vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal Microbiota as a Route for Micronutrient Bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef]
- Dey, A.; Chatterjee, S.; Kumar, V. Triethylene Glycol-like Effects of Ashwagandha (Withania Somnifera (L.) Dunal) Root Extract Devoid of Withanolides in Stressed Mice. AYU (Int. Q. J. Res. Ayurveda) 2018, 39, 230. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Qian, C.; Hussain, M.; Liu, S.; Zhang, A.; He, R.; Sun, P. The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review. Biology 2023, 12, 122. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R.M. Potential of Probiotics, Prebiotics and Synbiotics for Management of Colorectal Cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Athletic Greens International Ingredients. Available online: https://drinkag1.com/en (accessed on 18 August 2023).
- Travis, J.; Lattimore, L.G.; Harvey, M.; Frey, T. NSF International’s Role in the Dietary Supplements and Nutraceuticals Industries. In Nutraceutical and Functional Food Regulations in the United States and around the World; Academic Press: Cambridge, MA, USA, 2019; pp. 147–158. [Google Scholar] [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Applied AFwrobiology Biotechnology Development of a S-Step Multi-Chamber Reactor as a Simulation of the Human Intestinal Microbial Ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef]
- Bell, L.; Whyte, A.; Duysburgh, C.; Marzorati, M.; Van den Abbeele, P.; Le Cozannet, R.; Fança-Berthon, P.; Fromentin, E.; Williams, C. A Randomized, Placebo-Controlled Trial Investigating the Acute and Chronic Benefits of American Ginseng (Cereboost®) on Mood and Cognition in Healthy Young Adults, Including in Vitro Investigation of Gut Microbiota Changes as a Possible Mechanism of Action. Eur. J. Nutr. 2022, 61, 413–428. [Google Scholar] [CrossRef]
- Duysburgh, C.; Verstrepen, L.; Broeck, M.V.D.; Righetto, Z.; Perez, M. Investigation of Enterogermina’s Protective and Restorative Mechanisms on the Gut Microbiota with PPI, Using SHIME Technology. Nutrients 2023, 15, 653. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: New York, NY, USA, 2015; pp. 13–22. ISBN 9783319161044. [Google Scholar]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef]
- De Boever, P.; Deplancke, B.; Verstraete, W. Nutritional Methodology Fermentation by Gut Microbiota Cultured in a Simulator of the Human Intestinal Microbial Ecosystem Is Improved by Supplementing a Soygerm Powder 1. J. Nutr. 2000, 130, 2599–2606. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.C.D.; Albenberg, L.; Bittinger, K.; Chehoud, C.; Chen, Y.Y.; Judge, C.A.; Chau, L.; Ni, J.; Sheng, M.; Lin, A.; et al. Engineering the Gut Microbiota to Treat Hyperammonemia. J. Clin. Investig. 2015, 125, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Steinert, R.E.; Duysburgh, C.; Ghyselinck, J.; Marzorati, M.; Dekker, P.J.T. In Vitro Effect of Enzymes and Human Milk Oligosaccharides on FODMAP Digestion and Fecal Microbiota Composition. Nutrients 2023, 15, 1637. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and Their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched Short-Chain Fatty Acids Modulate Glucose and Lipid Metabolism in Primary Adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut Microbiota Role in Dietary Protein Metabolism and Health-Related Outcomes: The Two Sides of the Coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.D.A. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirby, T.O.; Townsend, J.R.; Sapp, P.A.; Govaert, M.; Duysburgh, C.; Marshall, T.M.; Marzorati, M.; Esposito, R. The Novel Synbiotic, AG1®, Increases Short-Chained Fatty Acid Production in the Simulator of Human Intestinal Microbial Ecosystem (SHIME) Model®. Nutraceuticals 2023, 3, 489-498. https://doi.org/10.3390/nutraceuticals3040035
Kirby TO, Townsend JR, Sapp PA, Govaert M, Duysburgh C, Marshall TM, Marzorati M, Esposito R. The Novel Synbiotic, AG1®, Increases Short-Chained Fatty Acid Production in the Simulator of Human Intestinal Microbial Ecosystem (SHIME) Model®. Nutraceuticals. 2023; 3(4):489-498. https://doi.org/10.3390/nutraceuticals3040035
Chicago/Turabian StyleKirby, Trevor O., Jeremy R. Townsend, Philip A. Sapp, Marlies Govaert, Cindy Duysburgh, Tess M. Marshall, Massimo Marzorati, and Ralph Esposito. 2023. "The Novel Synbiotic, AG1®, Increases Short-Chained Fatty Acid Production in the Simulator of Human Intestinal Microbial Ecosystem (SHIME) Model®" Nutraceuticals 3, no. 4: 489-498. https://doi.org/10.3390/nutraceuticals3040035
APA StyleKirby, T. O., Townsend, J. R., Sapp, P. A., Govaert, M., Duysburgh, C., Marshall, T. M., Marzorati, M., & Esposito, R. (2023). The Novel Synbiotic, AG1®, Increases Short-Chained Fatty Acid Production in the Simulator of Human Intestinal Microbial Ecosystem (SHIME) Model®. Nutraceuticals, 3(4), 489-498. https://doi.org/10.3390/nutraceuticals3040035