Analysis of Tianeptine in Dietary Supplements
Abstract
:1. Introduction
2. Materials and Methods
3. Results
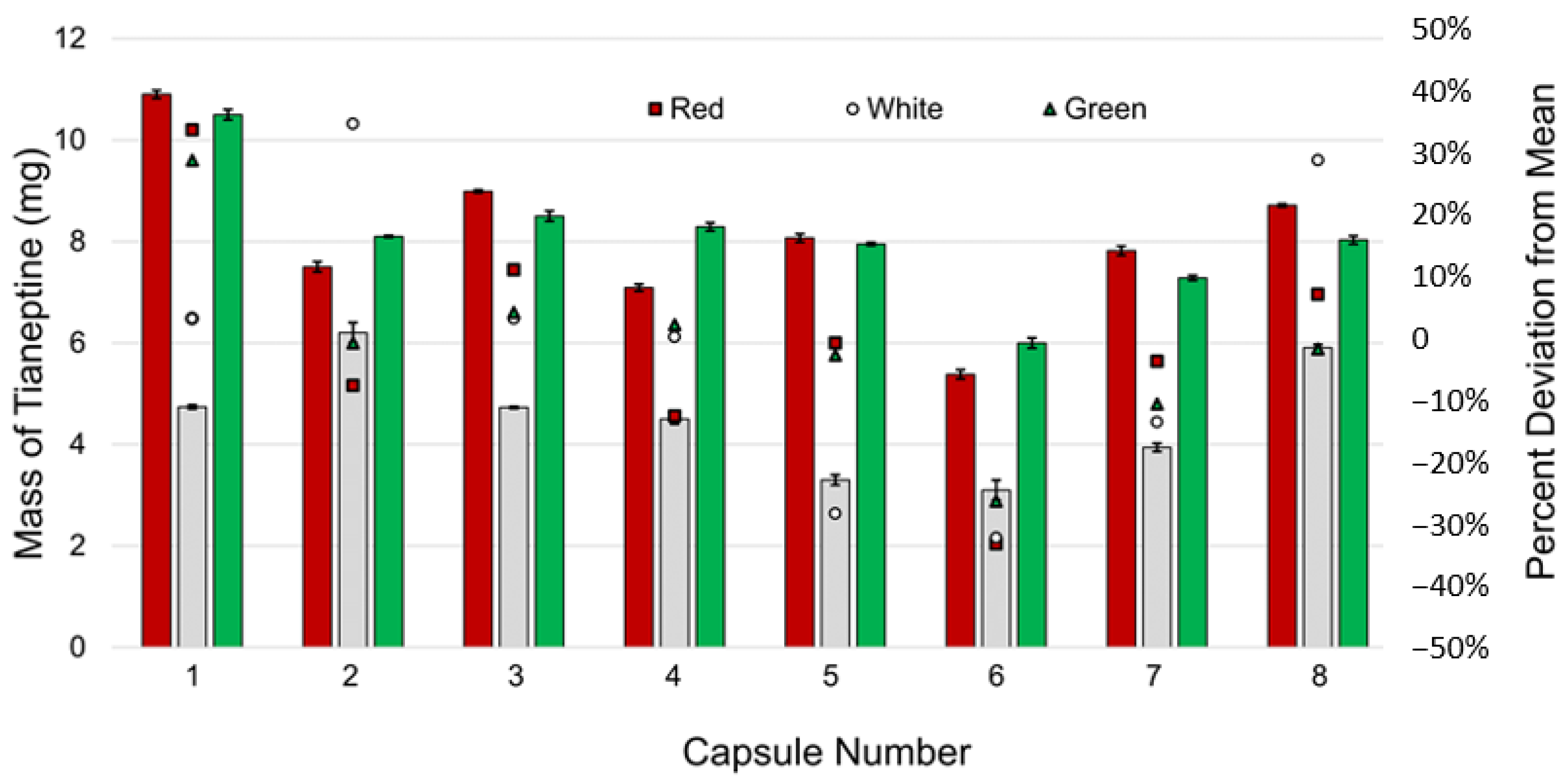
3.1. TianaaTM Product Comparison—Red, Green, and White
3.1.1. Tianeptine and Kavapyrone Analysis
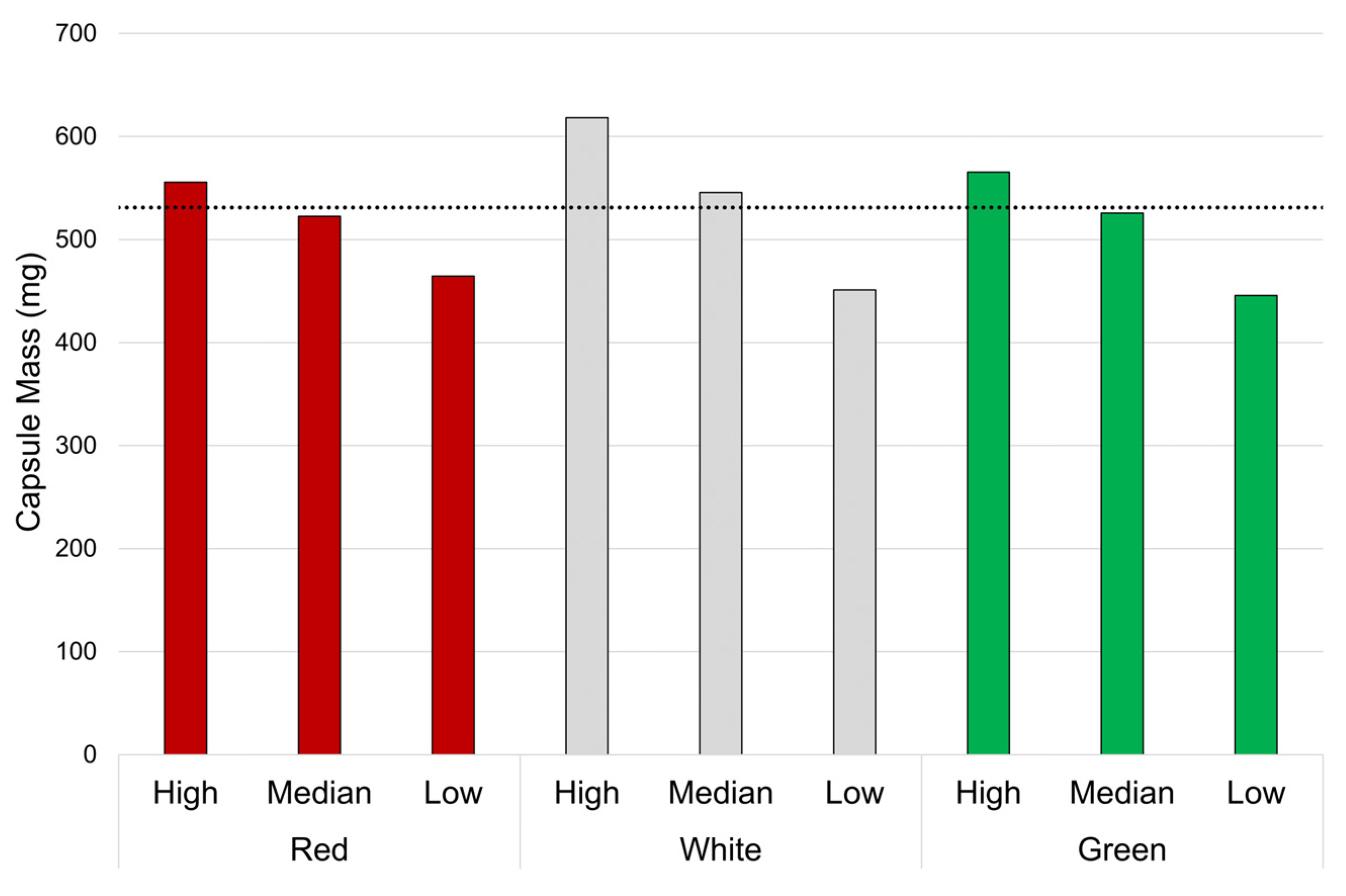
3.1.2. Capsule-to-Capsule Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medicines and Healthcare products Regulatory Agency (MHRA). The British Pharmacopeia, 2022. Monographs: Tianeptine Sodium; The Stationary Office: London, UK, 2022.
- Malen, C.; Danree, B.; en Laye, S.G.; Poignant, J.-C. Tricyclic Compounds. U.S. Patent #3758528; United States Patent and Trademark Office, 11 September 1973. Available online: https://patentimages.storage.googleapis.com/94/81/93/24ca0b63a45d8a/US3758528.pdf (accessed on 18 September 2023).
- Rangisetty, J.B.; Pallagurla, M.R.; Bhudeti, R. Novel Process for the Preparation of 7-((3-Chloro-6-methyI-5,5-dioxo-6,11-dihydro dibenzo(c,f) (1,2) thiazepin-11-yl)amino) heptanoate. International Patent WO 2010/070667A2, 24 June 2010. [Google Scholar]
- Banasr, M.; Dwyer, J.; Duman, R. Cell atrophy and loss in depression: Reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011, 23, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Ormrod, D.; Spencer, C.M. Tianeptine: A review of its use in depressive disorders. CNS Drugs 2001, 15, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.A.; Nautiyal, K.M.; Kruegel, A.C.; Levinstein, M.R.; Magalong, V.M.; Gassaway, M.M.; Grinnell, S.G.; Han, J.; Ansonoff, M.A.; Pintar, J.E.; et al. The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology 2017, 42, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Chattarji, S.; Diamond, D.M.; Jay, T.M.; Reagan, L.P.; Svenningsson, P.; Fuchs, E. The neurobiological properties of tianeptine (Stablon): From monoamine hypothesis to glutamatergic modulation. Mol. Psychiatry. 2010, 15, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gassaway, M.M.; Rives, M.; Kruegel, A.C.; Javitch, J.; Sames, D. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl. Psychiatry 2014, 4, e411. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.J.; Volkow, N. Untangling the complexity of opioid receptor function. Neuropsychopharmacology 2018, 43, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.; Ozbilen, G.; Kasap, Y.; Koyuncu, O.; Yildirim, O.; Artiran, G.; Kerman, S.; Aydinkarahaliloglu, D. Risk Management in Tianeptine Abuse in Turkey: A National Experience. Klinik Psikofarmakoloji Bulteni 2013, 23, 149–154. [Google Scholar] [CrossRef]
- Bakota, E.; Samms, W.; Gray, T.; Oleske, D.; O Hines, M. Case reports of fatalities involving tianeptine in the United States. J. Anal. Toxicol. 2018, 42, 503–509. [Google Scholar] [CrossRef] [PubMed]
- El Zahran, T.; Schier, J.; Glidden, E.; Kieszak, S.; Law, R.; Bottei, E.; Aaron, C.; King, A.; Chang, A. Characteristics of Tianeptine Exposures Reported to the National Poison Data System—United States 2000–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; Cubała, W.J. Tianeptine Abuse and Dependence in Psychiatric Patients: A Review of 18 Case Reports in the Literature. J. Psychoact. Drugs 2018, 50, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Marraffa, J.M.; Stork, C.M.; Hoffman, R.S.; Su, M.K. Poison control center experience with tianeptine: An unregulated pharmaceutical product with potential for abuse. Clin. Toxicol. 2018, 56, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. Tianeptine; Drug Enforcement Administration: Springfield, VA, USA, 2023.
- Felton, R. An Illegal Dietary Supplement Named Tianeptine Is Being Sold to Americans—And the FDA Knows It. Consumer Reports. 4 February 2021. [Google Scholar]
- Food and Drug Administration. Tianeptine in Dietary Supplements. Food and Drug Administration. 2018. Available online: https://www.fda.gov/food/dietary-supplement-products-ingredients/tianeptine-dietary-supplements (accessed on 18 September 2023).
- Parker, A.; Byars, A.; Purpura, M.; Jäger, R. The effects of alpha-glycerylphosphorylcholine, caffeine or placebo on markers of mood, cognitive function, power, speed, and agility. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef]
- Fioravanti, M.; Buckley, A. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin. Interv. Aging 2006, 1, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lauhan, R.; Hsu, A.; Alam, A.; Beizai, K. Tianeptine Abuse and Dependence: Case Report and Literature Review. Psychosomatics. 2018, 59, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Tianaa Supplements. Available online: https://mtbrands.com/tianaa/ (accessed on 7 September 2023).
- Cayman Chemical. Tianeptine (Sodium Salt). Item No. 17561 2022. Available online: https://cdn.caymanchem.com/cdn/insert/17561.pdf (accessed on 18 September 2023).
- Shao, Y.; He, K.; Zheng, B.; Zheng, Q. Reversed-phase high-performance liquid chromatographic method for quantitative analysis of the six major kavalactones in Piper methysticum. J. Chromatogr. A 1998, 825, 1–8. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services, Food & Drug Administration. CFR—Code of Federal Regulations Title 21 [21CFR210.1], Last Revised July 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=210.1 (accessed on 18 September 2023).
- Smith, K.E.; Rogers, J.M.; Strickland, J.C.; Epstein, D.H. When an obscurity becomes trend: Social-media descriptions of tianeptine use and associated atypical drug use. Am. J. Drug Alcohol Abus. 2021, 47, 455–466. [Google Scholar] [CrossRef]
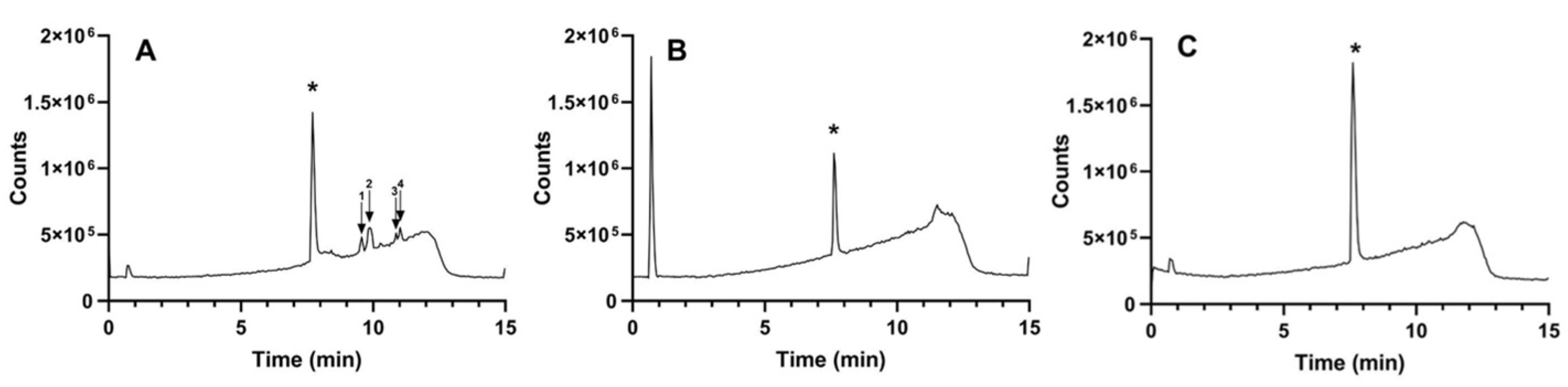
| Compound Name | Structure | Retention Time (min) | [M + H]+ (m/z) |
|---|---|---|---|
| Dihydromethysticin (1) | 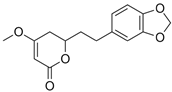 | 9.570 | 277.1 |
| Kavain (2) | 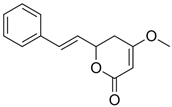 | 9.820 | 231.1 |
| Desmethoxyyangonin (3) | 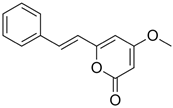 | 10.872 | 229.1 |
| Yangonin (4) | 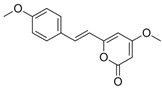 | 11.022 | 259.1 |
| Capsule | Red | Green | White |
| % Mass | % Mass | % Mass | |
| 1 | 2.35 | 1.05 | 2.36 |
| 2 | 1.36 | 1.04 | 1.44 |
| 3 | 1.81 | 0.85 | 1.67 |
| 4 | 1.39 | 0.82 | 1.50 |
| 5 | 1.47 | 0.59 | 1.55 |
| 6 | 1.07 | 0.66 | 1.06 |
| 7 | 1.51 | 0.69 | 1.40 |
| 8 | 1.66 | 1.00 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seale, J.T.; Garden, E.A.; French, J.M.T.; McDougal, O.M. Analysis of Tianeptine in Dietary Supplements. Nutraceuticals 2023, 3, 481-488. https://doi.org/10.3390/nutraceuticals3030034
Seale JT, Garden EA, French JMT, McDougal OM. Analysis of Tianeptine in Dietary Supplements. Nutraceuticals. 2023; 3(3):481-488. https://doi.org/10.3390/nutraceuticals3030034
Chicago/Turabian StyleSeale, Jared T., Emily A. Garden, John M. T. French, and Owen M. McDougal. 2023. "Analysis of Tianeptine in Dietary Supplements" Nutraceuticals 3, no. 3: 481-488. https://doi.org/10.3390/nutraceuticals3030034
APA StyleSeale, J. T., Garden, E. A., French, J. M. T., & McDougal, O. M. (2023). Analysis of Tianeptine in Dietary Supplements. Nutraceuticals, 3(3), 481-488. https://doi.org/10.3390/nutraceuticals3030034







