Effects of a Standardized Hydrogenated Extract of Curcumin (Curowhite™) on Melanogenesis: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DPPH Antioxidant Assay
2.3. Cell Culture
2.4. MTS Cytotoxicity Assay
2.4.1. B16F10 Cells
2.4.2. MNT-1 Human Melanoma Cells
2.4.3. HaCaT Cells
2.5. Intracellular Melanin Assay
2.5.1. B16F10 Cells
2.5.2. MNT-1 Human Melanoma Cells
2.6. Intracellular Tyrosinase Enzyme Activity
2.6.1. B16F10 Cells
2.6.2. MNT-1 Cells
2.7. Reactive Oxygen Species (ROS) Assay in MNT-1 Cells
2.8. Tyrosinase and MITF Protein Levels in MNT-1 Cells
2.9. Statistical Analysis
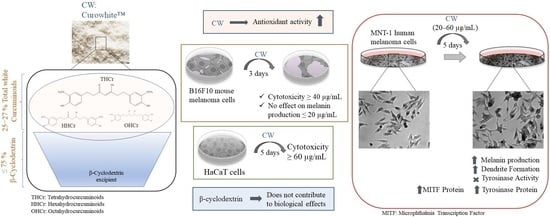
3. Results
3.1. CW Exhibits Antioxidant Activity
3.2. CW Did Not Affect B16F10 Mouse Melanoma Cells Melanogenesis at Nontoxic Concentrations
3.3. CW Stimulated Melanogenesis in MNT-1 Cells at Nontoxic Concentrations
3.4. CW Effects on Tyrosinase Activity and ROS Production in MNT-1 Cells
3.5. Effects of CW on Cytotoxicity in Human Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raposo, G.; Marks, M.S. The dark side of lysosome-related organelles: Specialization of the endocytic pathway for melanosome biogenesis. Traffic 2002, 3, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J. Hum. Evol. 2013, 65, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef] [Green Version]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef]
- Madu, P.N.; Syder, N.; Elbuluk, N. Postinflammatory hypopigmentation: A comprehensive review of treatments. J. Dermatol. Treat. 2022, 33, 704–708. [Google Scholar] [CrossRef]
- Patel, R.; Pandya, A.G.; Sikirica, V.; Gandhi, K.; Daniel, S.R.; Anastassopoulos, K.P.; Yamaguchi, Y.; Napatalung, L.; Baik, R.; Ezzedine, K. Prevalence of Vitiligo among Children and Adolescents in the United States. Dermatology 2023, 239, 227–234. [Google Scholar] [CrossRef]
- Mahama, A.N.; Haller, C.N.; Ahmed, A.M. Psychosocial considerations in the management of vitiligo. Clin. Dermatol. 2023, 41, 82–88. [Google Scholar] [CrossRef]
- Tobin, D.J. Biology of hair follicle pigmentation. In Hair Growth and Disorders; Springer: Berlin/Heidelberg, Germany, 2008; pp. 51–74. [Google Scholar]
- Sánchez-Ferrer, Á.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Bae-Harboe, Y.-S.C.; Park, H.-Y. Tyrosinase: A central regulatory protein for cutaneous pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachmin, I.; Ostrowski, S.M.; Weng, Q.Y.; Fisher, D.E. Topical treatment strategies to manipulate human skin pigmentation. Adv. Drug Deliv. Rev. 2020, 153, 65–71. [Google Scholar] [CrossRef] [PubMed]
- A Ali, S.; Naaz, I.; Uddin Zaidi, K.; S Ali, A. Recent updates in melanocyte function: The use of promising bioactive compounds for the treatment of hypopigmentary disorders. Mini Rev. Med. Chem. 2017, 17, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Sreeraj, G.; Jacob, J.; George, R.; Sreeraj, T. A unique formulation of hydrogenated curcuminoids with higher bio availability and the application in food matrices. J. Nutr. Food Sci. 2016, 6, 1. [Google Scholar]
- Gopi, S.; Jacob, J.; Mathur, K.Y. Acute and subchronic oral toxicity studies of hydrogenated curcuminoid formulation ‘CuroWhite’ in rats. Toxicol. Rep. 2016, 3, 817–825. [Google Scholar] [CrossRef]
- Ravikumar, A.N.; Jacob, J.; Gopi, S.; Jagannath, T.S. A Toxicological Evaluation of a Standardized Hydrogenated Extract of Curcumin (CuroWhite). J. Toxicol. 2018, 2018, 5243617. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Amalraj, A.; Raj, K.K.J.; Divya, C.; Kunnumakkara, A.B.; Gopi, S. A novel bioavailable hydrogenated curcuminoids formulation (CuroWhite) improves symptoms and diagnostic indicators in rheumatoid arthritis patients—A randomized, double blind and placebo controlled study. J. Tradit. Complement. Med. 2019, 9, 346–352. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Pezzella, A.; Guerra, F.; Maione, F.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Promelanogenic Effects by an Annurca Apple-Based Natural Formulation in Human Primary Melanocytes. Clin. Cosmet. Investig. Dermatol. 2021, 14, 291. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Die Pharm. 2002, 57, 820–824. [Google Scholar]
- Tomren, M.; Másson, M.; Loftsson, T.; Tønnesen, H.H. Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007, 338, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Loron, A.; Gardrat, C.; Tabary, N.; Martel, B.; Coma, V. Tetrahydrocurcumin encapsulation in cyclodextrins for water solubility improvement: Synthesis, characterization and antifungal activity as a new biofungicide. Carbohydr. Polym. Technol. Appl. 2021, 2, 100113. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.S.; Palkar, M.B.; Diwan, P.V. Stability studies of pure and mixture form of curcuminoids by reverse phase-HPLC method under various experimental stress conditions. Food Sci. Biotechnol. 2017, 26, 591–602. [Google Scholar] [CrossRef]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akao, N.; Kondo, K.; Tsuda, Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef] [Green Version]
- Natural Actives Product List, Sabinsa Cosmetics. Available online: https://sabinsacosmetics.com/product-list (accessed on 1 July 2023).
- Asawanonda, P.; Klahan, S.-O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: A preliminary randomized controlled study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J. Cannabidiol and cannabigerol exert antimicrobial activity without compromising skin microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Solid and liquid state characterization of tetrahydrocurcumin using XRPD, FT-IR, DSC, TGA, LC-MS, GC-MS, and NMR and its biological activities. J. Pharm. Anal. 2020, 10, 334–345. [Google Scholar] [CrossRef]
- Majeed, M.; Badmaev, V. Use of Tetrahydrocurcuminoids to Regulate Physiological and Pathological Events in the Skin and Mucosa. AU Patent 2,006,235,807, 11 September 2008. [Google Scholar]
- Majeed, M.; Badmaev, V. Cross-Regulin Composition of Tumeric-Derived Tetrahydrocurcuminoids for Skin Lightening and Protection against UVB Rays. U.S. Patent 6,653,327, 25 November 2003. [Google Scholar]
- Goenka, S. Novel Hydrogenated Derivatives of Chemically Modified Curcumin CMC2. 24 Are Potent Inhibitors of Melanogenesis in an In Vitro Model: Influence of Degree of Hydrogenation. Life 2023, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative Study of Curcumin and Its Hydrogenated Metabolites, Tetrahydrocurcumin, Hexahydrocurcumin, and Octahydrocurcumin, on Melanogenesis in B16F10 and MNT-1 Cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Kuswandi, B.; Hidayat, M.A. A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Comparative Study of Δ9-Tetrahydrocannabinol and Cannabidiol on Melanogenesis in Human Epidermal Melanocytes from Different Pigmentation Phototypes: A Pilot Study. J. Xenobiot. 2022, 12, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Organogold drug Auranofin exhibits anti-melanogenic activity in B16F10 and MNT-1 melanoma cells. Arch. Dermatol. Res. 2020, 312, 213–221. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef] [Green Version]
- Villareal, M.O.; Kume, S.; Neffati, M.; Isoda, H. Upregulation of Mitf by phenolic compounds-rich cymbopogon schoenanthus treatment promotes melanogenesis in b16 melanoma cells and human epidermal melanocytes. BioMed Res. Int. 2017, 2017, 8303671. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, K.B.; Palú, É.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Morita, M.; Ichikawa, C.; Takahashi, H.; Toriyama, M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci. Biotechnol. Biochem. 2008, 72, 2589–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roméro, C.; Aberdam, E.; Larnier, C.; Ortonne, J.-P. Retinoic acid as modulator of UVB-induced melanocyte differentiation. Involvement of the melanogenic enzymes expression. J. Cell Sci. 1994, 107, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a multifunctional topical hypopigmenting agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape extract promoted α-msh-induced melanogenesis in b16f10 melanoma cells, which was inverse to resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef]
- Hu, M.; Chen, C.; Liu, J.; Cai, L.; Shao, J.; Chen, Z.; Lin, L.; Zheng, T.; Ding, X.; Li, Z. The melanogenic effects and underlying mechanism of paeoniflorin in human melanocytes and vitiligo mice. Fitoterapia 2020, 140, 104416. [Google Scholar] [CrossRef]
- Wen, S.Y.; Wu, Y.S.; Liu, H.; Ng, S.C.; Padma, V.V.; Huang, C.Y.; Kuo, W.W. Paeoniflorin found in Paeonia lactiflora root extract inhibits melanogenesis by regulating melanin-related signal transduction in B16F10 cells. J. Cosmet. Dermatol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Ku, B.; Kim, D.; Choi, E.-M. Tetrahydrocurcumin Inhibits α-MSH-induced Melanogenesis via GSK3β Activation in B16F10 Melanoma Cells. Toxicol. Environ. Health Sci. 2019, 11, 210–218. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Pineda, R.T.V.; Chan, G.; Gabriel, T.; Dayrit, J.; Pelayo, C.A.; Prakash, L. A randomized, double-blind, placebo-controlled, comparative study, The safety and efficacy of 0.25% tetrahydrocurcumin (tumeric) cream as depigment agent against 4% hydroquinone cream. HPC Today 2010, 3, 44–46. [Google Scholar]
- Benediktsdottir, B.E.; Baldursson, O.; Gudjonsson, T.; Tønnesen, H.H.; Masson, M. Curcumin, bisdemethoxycurcumin and dimethoxycurcumin complexed with cyclodextrins have structure specific effect on the paracellular integrity of lung epithelia in vitro. Biochem. Biophys. Rep. 2015, 4, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, L.; Wu, L.; Wang, H.; Chen, K.; Wu, H.; Li, Y. Kaempferol, the melanogenic component of Sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J. Pharmacol. Sci. 2021, 147, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, I.S.; Dong, Y.; Lee, I.-S.; Kim, J.S.; Kim, J.-S.; Woo, J.-T.; Cha, B.-Y. Melanogenesis-inducing effect of cirsimaritin through increases in microphthalmia-associated transcription factor and tyrosinase expression. Int. J. Mol. Sci. 2015, 16, 8772–8788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Yokoyama, K.; Yasumoto, K.-i.; Suzuki, H.; Shibahara, S. Cloning of the human DOPAchrome tautomerase/tyrosinase-related protein 2 gene and identification of two regulatory regions required for its pigment cell-specific expression. J. Biol. Chem. 1994, 269, 27080–27087. [Google Scholar] [CrossRef]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.; Hearing, V. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Aoki, H.; Moro, O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci. 2002, 71, 2171–2179. [Google Scholar] [CrossRef]
- Han, H.; Hyun, C.-G. Syringetin Promotes Melanogenesis in B16F10 Cells. Int. J. Mol. Sci. 2023, 24, 9960. [Google Scholar] [CrossRef]
- Moon, E.; Kim, A.-J.; Kim, S.Y. Sarsasapogenin increases melanin synthesis via induction of tyrosinase and microphthalmia-associated transcription factor expression in melan-a cells. Biomol. Ther. 2012, 20, 340. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Kim, J.H.; Kim, M.O.; Jang, S.; Kang, M.; Oh, S.W.; Nho, Y.H.; Kang, S.H.; Kim, M.H.; Park, S.-H. Afzelin positively regulates melanogenesis through the p38 MAPK pathway. Chem.-Biol. Interact. 2016, 254, 167–172. [Google Scholar] [CrossRef]
- Zhou, J.; Song, J.; Ping, F.; Shang, J. Enhancement of the p38 MAPK and PKA signaling pathways is associated with the pro-melanogenic activity of Interleukin 33 in primary melanocytes. J. Dermatol. Sci. 2014, 73, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Leopardi, S.; Printup, S.; Madden, B.C. Filopodia are conduits for melanosome transfer to keratinocytes. J. Cell Sci. 2002, 115, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, Y.; Kawakishi, S.; Osawa, T. Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996, 52, 519–525. [Google Scholar] [CrossRef]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Wu, S.; He, Y.; Nian, Q.; Lei, J.; Yao, Y.; Guo, J.; Zeng, J. Plant-derived compounds as promising therapeutics for vitiligo. Front. Pharmacol. 2021, 12, 685116. [Google Scholar] [CrossRef]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Tang, L.; Wang, G.; Lin, J.; Liao, W.; Pan, W.; Xu, J. Molecular hydrogen protects human melanocytes from oxidative stress by activating Nrf2 signaling. J. Investig. Dermatol. 2020, 140, 2230–2241.e9. [Google Scholar] [CrossRef]
- Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/β-catenin signaling. Lab. Investig. 2018, 98, 1527–1537. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine 2009, 16, 1137–1143. [Google Scholar] [CrossRef]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2021, 35, 1830–1835. [Google Scholar] [CrossRef] [PubMed]







| CW (µg/mL) | Tyrosinase Protein Levels (%) | MITF Protein Levels (%) |
|---|---|---|
| 0 | 99.97 ± 5.32 | 100.20 ± 4.17 |
| 20 | 106.44 ± 2.93 | 115.32 ± 12.52 |
| 40 | 123.04 ± 3.40 a | 116.62 ± 4.85 c |
| 60 | 152.24 ± 10.52 b | 117.56 ± 16.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Effects of a Standardized Hydrogenated Extract of Curcumin (Curowhite™) on Melanogenesis: A Pilot Study. Nutraceuticals 2023, 3, 421-437. https://doi.org/10.3390/nutraceuticals3030031
Goenka S. Effects of a Standardized Hydrogenated Extract of Curcumin (Curowhite™) on Melanogenesis: A Pilot Study. Nutraceuticals. 2023; 3(3):421-437. https://doi.org/10.3390/nutraceuticals3030031
Chicago/Turabian StyleGoenka, Shilpi. 2023. "Effects of a Standardized Hydrogenated Extract of Curcumin (Curowhite™) on Melanogenesis: A Pilot Study" Nutraceuticals 3, no. 3: 421-437. https://doi.org/10.3390/nutraceuticals3030031
APA StyleGoenka, S. (2023). Effects of a Standardized Hydrogenated Extract of Curcumin (Curowhite™) on Melanogenesis: A Pilot Study. Nutraceuticals, 3(3), 421-437. https://doi.org/10.3390/nutraceuticals3030031