Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies
Abstract
1. Introduction
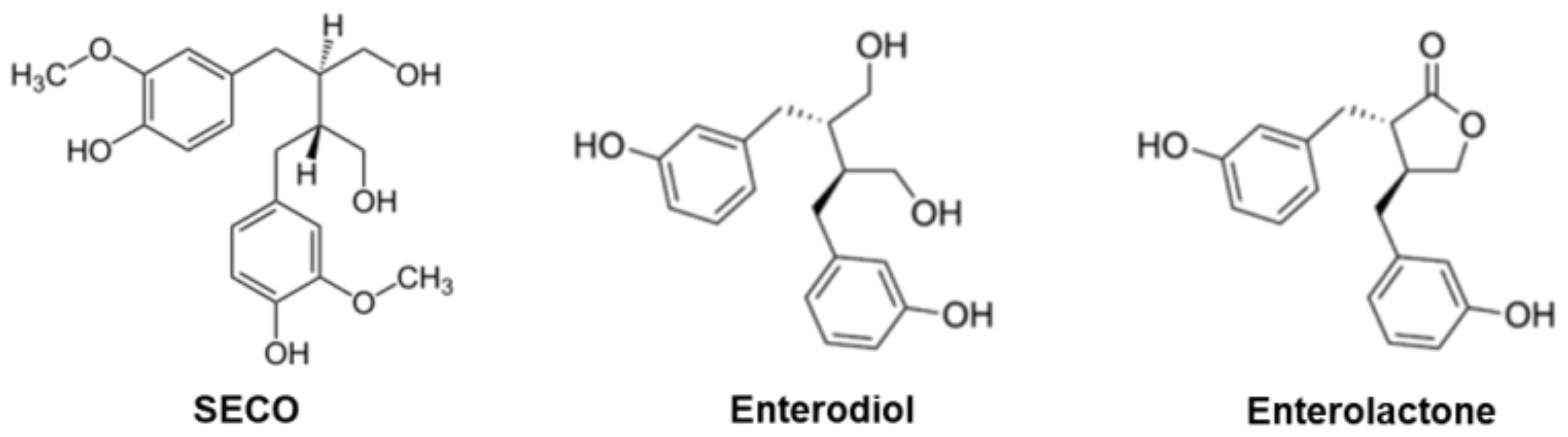
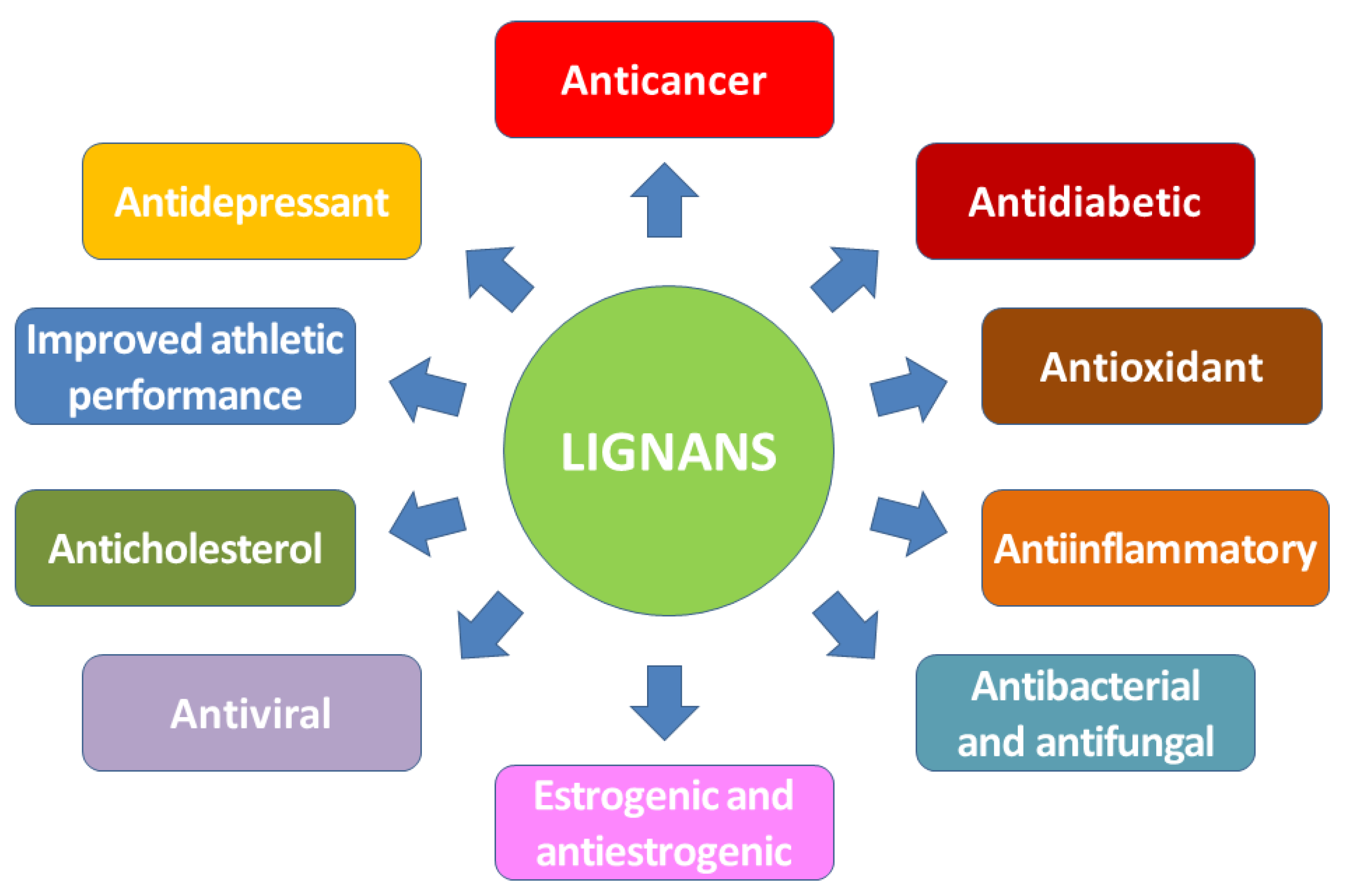
2. Biological Properties of Lignans
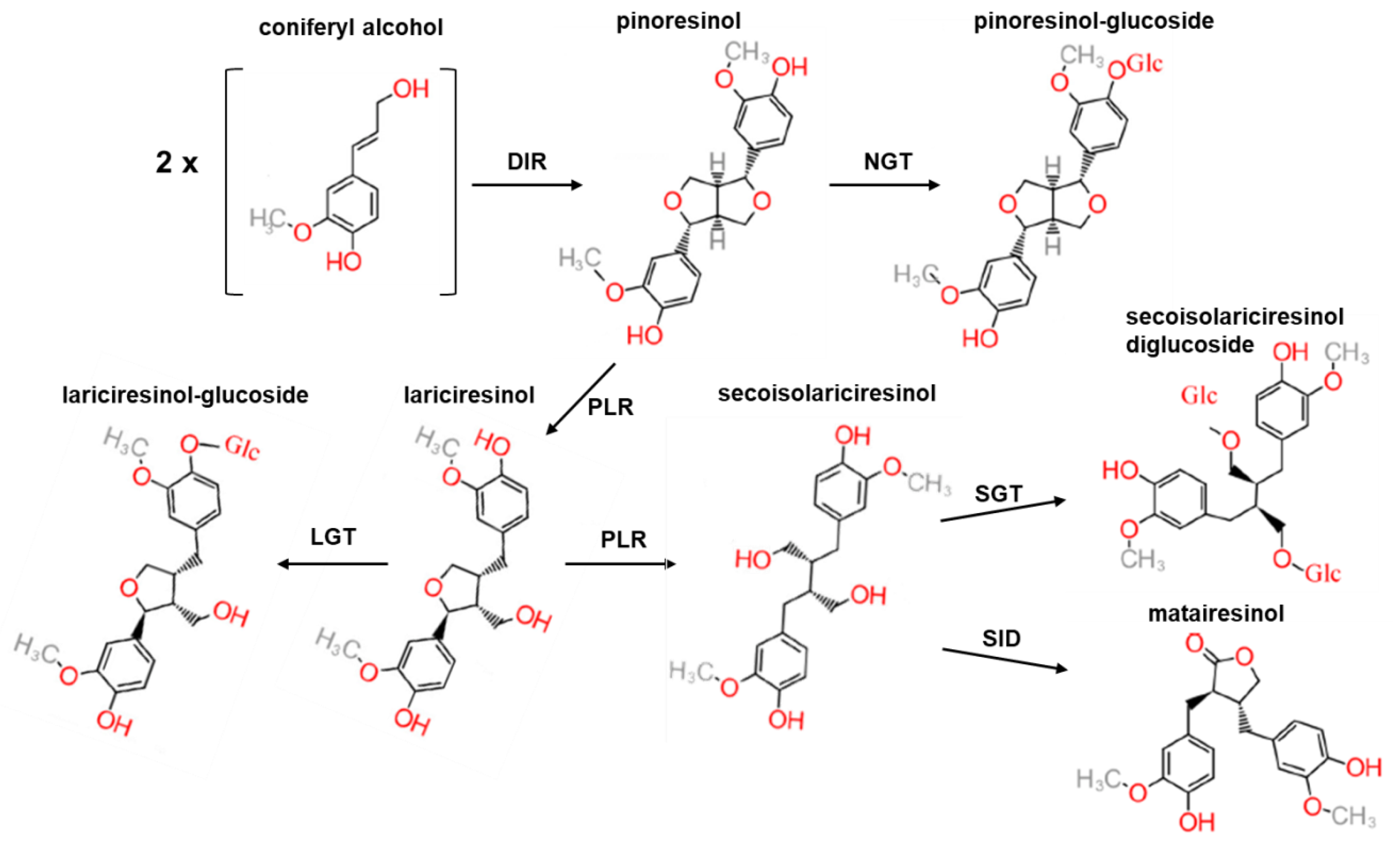
3. Flaxseed: A Source of Lignans
4. Recovery Technologies of Lignans from Flaxseed
4.1. Recovery Technologies from the Scientific Literature
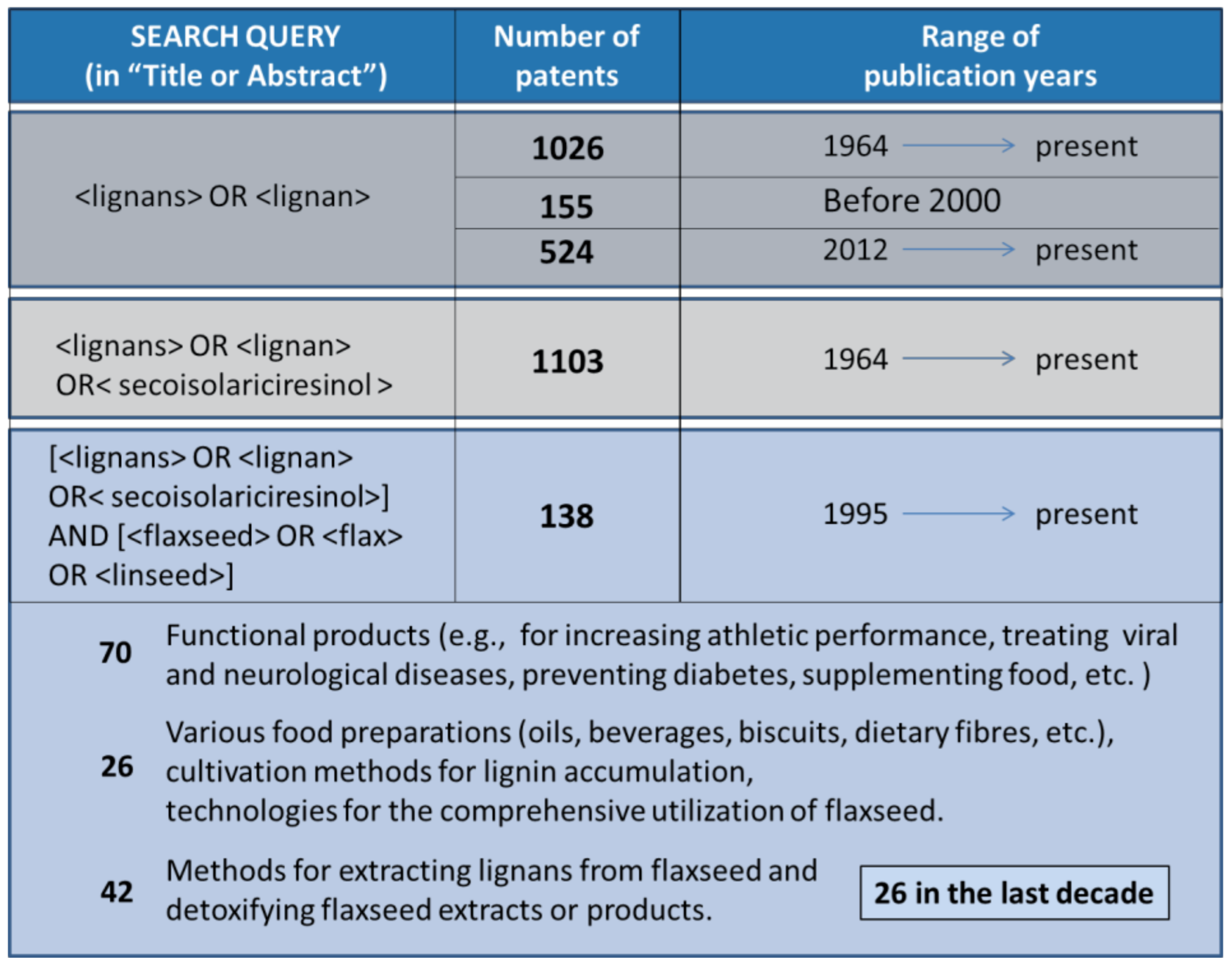
4.2. Patented Recovery Technologies
4.3. Comparison between the Literature Results and Patents of the Last Decade
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHS | anhydrosecoisolariciresinol |
| DIR | dirigent proteins |
| Glc | Glucoside |
| HMGA | 3-hydroxy-3-methylglugaric acid |
| HVED | High voltage Electric Discharge |
| LGT | lariciresinol glycosyltransferase |
| MAE | Microwave-assisted extraction |
| NGT | pinoresinol glucosyltransferase) |
| PEF | Pulsed electric fields |
| PLR | pinoresinol/lariciresinol reductase |
| SDG | secoisolariciresinol diglucoside |
| SECO | secoisolariciresinol |
| SG | secoisolariciresinol glucoside |
| SGT | secoisolariciresinol glycosyltransferase |
| SID | matairesinol O-methyltransferase |
| UAE | Ultrasound Assisted Extraction |
References
- Shekhara Naik, R.; Anurag, A.P.; Prakruthi, M.; Mahesh, M.S. Flax Seeds (Linum usitatissimmum): Nutritional composition and health benefits. IP J. Nutr. Metab. Health Sci. 2021, 3, 35–40. [Google Scholar] [CrossRef]
- Food Data Central, FlaxSeeds. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1100610/nutrients (accessed on 5 November 2022).
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; De Lacour, J.L.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (Linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Dhirhi, N.; Shukla, R.; Patel, N.B.; Sahu, E.; Gendley, T.; Mehta, N. “Lignan”-Antioxidant of Linseed. Plant Arch. 2016, 16, 12–17. [Google Scholar]
- Cichonska, P.; Pudło, E.; Wojtczak, A.; Ziarno, M. Effect of the Addition of Whole and Milled Flaxseed on the Quality Characteristics of Yogurt. Foods 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Waghmare, R.; Kumar, V.; Rasane, P.; Kaur, S.; Gat, Y. Recent advances in utilization of flaxseed as potential source for value addition. OCL 2018, 25, A304. [Google Scholar] [CrossRef]
- Shadyro, O. Effect of biologically active substances on oxidative stability of flaxseed oil. J. Food Sci. Technol. 2020, 57, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, R.; Punia, S. Characterization of mucilages extracted from different flaxseed (Linum usitatissiumum L.) cultivars: A heteropolysaccharide with desirable functional and rheological properties. Int. J. Biol. Macromol. 2018, 117, 919–927. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Grasl-kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Evaluation of the health risks related to the presence of cyanogenic glycosides in foods other than raw apricot kernels. EFSA J. 2019, 17, e05662. [Google Scholar] [CrossRef]
- Sainvitu, P.; Nott, K.; Richard, G.; Blecker, C.; Jérôme, C.; Wathelet, J.P.; Paquot, M.; Deleu, M. Structure, properties and obtention routes of flaxseed lignan secoisolariciresinol: A review. Biotechnol. Agron. Soc. Environ. 2012, 16, 115–124. [Google Scholar]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary phytoestrogens and their metabolites as epigenetic modulators with impact on human health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Á.; Bjelková, M. Biological functions of lignans in plants. Agriculture 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Chhillar, H.; Chopra, P.; Ashfaq, M.A. Lignans from linseed (Linum usitatissimum L.) and its allied species: Retrospect, introspect and prospect. Crit. Rev. Food Sci. Nutr. 2021, 61, 2719–2741. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, Bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Di, Y.; Jones, J.; Mansell, K.; Whiting, S.; Fowler, S.; Thorpe, L.; Billinsky, J.; Viveky, N.; Cheng, P.C.; Almousa, A.; et al. Influence of Flaxseed Lignan Supplementation to Older Adults on Biochemical and Functional Outcome Measures of Inflammation. J. Am. Coll. Nutr. 2017, 36, 646–653. [Google Scholar] [CrossRef]
- Draganescu, D.; Ibanescu, C.; Tamba, B.I.; Andritoiu, C.V.; Dodi, G.; Popa, M.I. Flaxseed lignan wound healing formulation: Characterization and in vivo therapeutic evaluation. Int. J. Biol. Macromol. 2015, 72, 614–623. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.F.; Alcorn, J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment. Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, biosynthesis and therapeutic potential of lignans. 3 Biotech 2022, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Global Market Size. Available online: https://www.globenewswire.com/news-release/2020/03/25/2006051/0/en/Lignans-Market-revenue-to-hit-90-million-by-2026-Says-Global-Market-Insights-Inc.html (accessed on 7 November 2022).
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E.; Karakas, S.E. Lignan content of the flaxseed influences its biological effects in healthy men and women. J. Am. Coll. Nutr. 2013, 32, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef]
- Lemay, A.; Dodin, S.; Kadri, N.; Jacques, H.; Forest, J.C. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstet. Gynecol. 2002, 100, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and their derivatives from plants as antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Mukhija, M.; Joshi, B.C.; Bairy, P.S.; Bhargava, A.; Sah, A.N. Lignans: A versatile source of anticancer drugs. Beni-Suef Univ. J. Basic Appl. Sci 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The what and who of dietary lignans in human health. Special focus on prooxidant and antioxidant effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Mridula, D.; Singh, K.K.; Barnwal, P. Development of omega-3 rich energy bar with flaxseed. J. Food Sci. Technol. 2013, 50, 950–957. [Google Scholar] [CrossRef]
- Prasad, K.; Dhar, A. Flaxseed and Diabetes. Curr. Pharm Des. 2016, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Martini, M.C.; Olson, B.A.; Thomas, W.; Slavin, J.L. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr. Cancer 2001, 39, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Ma, X.; Wang, R.; Zhao, X.; Zhang, C.; Sun, J.; Li, J.; Zhang, L.; Shao, T.; Ruan, L.; Chen, L.; et al. Antidepressant-like effect of flaxseed secoisolariciresinol diglycoside in ovariectomized mice subjected to unpredictable chronic stress. Metab. Brain Dis. 2013, 28, 77–84. [Google Scholar] [CrossRef]
- Han, Y.; Deng, X.; Zhang, Y.; Wang, X.; Zhu, X.; Mei, S.; Chen, A. Antidepressant-like effect of flaxseed in rats exposed to chronic unpredictable stress. Brain Behav. 2020, 10, e016. [Google Scholar] [CrossRef]
- Narender, B.R.; Tejaswini, S.; Sarika, M.; Karuna, N.; Shirisha, R.; Priyanka, S. Antibacterial and Antifungal activities of Linum Usitatissimum Antibacterial and Antifungal activities of Linum Usitatissimum (Flax seeds). Int. J. Pharm. Educ. Res. 2016, 3, 4–8. [Google Scholar]
- Zhang, L.; Wang, X.; Sun, D.; Liu, X.; Hu, X.; Kong, F. Regulation of zinc transporters by dietary flaxseed lignan in human breast cancer xenografts. Mol. Biol. Rep. 2008, 35, 595–600. [Google Scholar] [CrossRef]
- Calado, A.; Neves, P.M.; Santos, T.; Ravasco, P. The Effect of Flaxseed in Breast Cancer: A Literature Review. Front. Nutr. 2018, 5, 4. [Google Scholar] [CrossRef]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol diglucoside of flaxseed and its metabolites: Biosynthesis and potential for nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef]
- Spence, J.D.; Thornton, T.; Muir, A.D.; Westcott, N.D. The effect of flax seed cultivars with differing content of alpha-linolenic acid and lignans on responses to mental stress. J. Am. Coll. Nutr. 2003, 22, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Patyra, A.; Kołtun-Jasion, M.; Jakubiak, O.; Kiss, A.K. Extraction Techniques and Analytical Methods for Isolation and Characterization of Lignans. Plants 2022, 11, 2323. [Google Scholar] [CrossRef] [PubMed]
- Phenol-Explorer Database. Available online: http://phenol-explorer.eu/ (accessed on 3 November 2022).
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of Extraction Method on the Phenolic and Cyanogenic Glucoside Profile of Flaxseed Extracts and their Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Al-jumaily, E.F.; Al-azawi, A.H. Isolation and Purification of Secoisolariciresinoldiglucoside oligomers (Lignan) from Flax seed and its evaluation of antioxidant activity. IOSR J. Appl. Chem. 2013, 5, 17–24. [Google Scholar] [CrossRef]
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en#thematicareas (accessed on 7 November 2022).
- Sharma, M.; Dadhwal, K.; Gat, Y.; Kumar, V.; Panghal, A.; Prasad, R.; Kaur, S.; Gat, P. A review on newer techniques in extraction of oleaginous flaxseed constituents. OCL 2019, 26, 14. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lamblin, F.; Lainé, F. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Charlet, S.; Bensaddek, L.; Raynaud, S.; Gillet, F.; Mesnard, F.; Fliniaux, M.A. An HPLC procedure for the quantification of anhydrosecoisolariciresinol. Application to the evaluation of flax lignan content. Plant Physiol. Biochem. 2002, 40, 225–229. [Google Scholar] [CrossRef]
- Eliasson, C.; Kamal-Eldin, A.; Andersson, R.; Aman, P. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J. Chromatogr. A 2003, 1012, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.C.; Liu, C.R.; Shan, C.B.; Liu, Z.; Liu, L.; Ma, C.M. High-yield production of secoisolariciresinol diglucoside from flaxseed hull by extraction with alcoholic ammonium hydroxide and chromatography on microporous resin. Food Prod. Process Nutr. 2021, 3, 35. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; González-Muñoz, A.; Muñoz, O. Optimization of secoisolariciresinol diglucoside extraction from flaxseed (Linum usitatissimum L.) and isolation by a simple HPLC-UV method. CyTA J. Food 2015, 13, 273–281. [Google Scholar] [CrossRef]
- Beejmohun, V.; Fliniaux, O.; Grand, E.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.A.; Mesnard, F. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem. Anal. 2007, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, S. Microwave-assisted extraction of secoisolariciresinol diglucoside from flaxseed hull. J. Sci. Food Agric. 2007, 87, 1455–1462. [Google Scholar] [CrossRef]
- Nemes, S.M.; Orsat, V. Microwave-Assisted Extraction of Secoisolariciresinol Diglucoside-Method Development. Food Bioprocess Technol. 2011, 4, 1219–1227. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Zhao, M.; Bergaentzlé, M.; Flieller, A.; Marchioni, E. Development and validation of an ultra-high performance liquid chromatography-high resolution mass spectrometry method for simultaneous quantification of cyanogenic glycosides and secoisolariciresinol diglucoside in flaxseed (Linum usitatissimum L.). J. Chromatogr. A 2019, 1601, 214–223. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.A. Supercritical CO2 extraction of flax lignans. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 707–715. [Google Scholar] [CrossRef]
- Ho, C.H.; Cacace, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT Food Sci Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE). Eur. Food Res. Technol. 2014, 238, 85–91. [Google Scholar] [CrossRef]
- Boussetta, N.; Turk, M.; De Taeye, C.; Larondelle, Y.; Lanoisellé, J.L.; Vorobiev, E. Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind. Crop. Prod. 2013, 49, 690–696. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.-Y.; Xiao, D.; Li, Z.-M.; Wang, J.-W. Negative-pressure cavitation extraction of secoisolariciresinol diglycoside from flaxseed cakes. Molecules 2015, 20, 11076–11089. [Google Scholar] [CrossRef] [PubMed]
- Espacenet-Patent search. Available online: https://worldwide.espacenet.com/ (accessed on 3 November 2022).
- Zhang, W.; Jiang, J.; Yang, R.; Xu, S.; Wang, Z. Method for Extracting Secoisolariciresinol Diglucoside from Flax Seeds or Husks, Extract Obtained and Use Thereof. CN Patent 101570556B, 4 January 2012. [Google Scholar]
- Li, F.; Ye, T.; Tian, Y. Production Process of Secoisolariciresinol Diglucoside. CN Patent 102558252A, 11 July 2012. [Google Scholar]
- Zhao, X.; Liu, G.; Hu, W.; Qian, X. Method for Extracting, Separating and Purifying Flax Lignans from Flax Cakes. CN Patent 102796148A, 28 November 2012. [Google Scholar]
- Zhao, X.; Liu, G.; Hu, W.; Qian, X. Method for Extracting Secoisolariciresinol Diglucoside. CN Patent 102816190A, 12 December 2012. [Google Scholar]
- Ma, C.; Wang, Y.; Ma, J.; Liu, Z.; Liu, Q. Detoxification Method of Flaxseed Product. CN Patent 102823782A, 19 December 2012. [Google Scholar]
- Wang, W.; Zhang, H.; Wang, W. Extraction of Lignans from Flaxseeds through Ultrasonic Enzymolysis. CN Patent 102125261B, 2 January 2013. [Google Scholar]
- Biskupski, M.; Ksiazkiewicz, M.; Gromek, M.; Stelmaszczyk, M. Method for Isolation of Flaxseed Lignans. PL Patent 397907A1, 5 August 2013. [Google Scholar]
- Shuai, T. Method for Directly Extracting Flax Lignans from Flaxseed Meal and detoxifying High-Protein Flaxseed Meal. CN Patent 104478954A, 1 April 2015. [Google Scholar]
- Hu, X.; Li, Q.; Xu, G.; Gao, Z.; Qiu, X.; Gu, X. Method for Continuously Extracting Flaxseed Gum and Lignan from Flaxseed Husks. CN Patent 102850414B, 8 April 2015. [Google Scholar]
- Jiang, Q. Method for Extracting Lignan from Linseed Oil Residue. CN Patent 102766174B, 20 May 2015. [Google Scholar]
- Qiu, Y.; Lin, D.; Zhang, F. Method for Preparing Fruit Antioxidant by Efficiently Extracting Flax Lignans. CN Patent 105145806A, 16 December 2015. [Google Scholar]
- Zhao, Y.; Jia, H.; Liao, X. Separation and Purification Method for Secoisolariciresinol Diglucoside. CN Patent 103396461B, 2 March 2016. [Google Scholar]
- Lu, H.; Lu, C. Method for Preparing Flax Lignans from Flax Cake. CN Patent 105585599A, 18 May 2016. [Google Scholar]
- Sun, J.; Liu, T. Method for Extracting Secoisolariciresinol Diglucoside, Obtained Extract and Use of Extract. CN Patent 103860649B, 4 January 2017. [Google Scholar]
- Zhang, H.; Chen, J.; Xi, H.; Zhang, Z.; Lin, N. Method for Obtaining High Biological Activity Lignan and Mushroom by Using Edible Mushroom to Degrade Flax Seed Shell. CN Patent 105000934B, 6 April 2018. [Google Scholar]
- Liao, J.; Li, J. Extraction Method for Flax Lignan from Flax Seed Husks. CN Patent 108129526A, 8 June 2018. [Google Scholar]
- Deng, F.; Wang, F.; Zhang, Z.; Liang, S.; Ge, J. Method for Continuously Extracting Flaxseed Gum and Decoisolariciresinol Diglucoside from Flaxseed Meal. CN Patent 108409813A, 17 August 2018. [Google Scholar]
- Zhang, Z.; Deng, F.; Wang, Y.; Ge, J.; Liang, S. Method for Extracting Flax Lignans from Flaxseed Husks by Microwaves. CN Patent 108659064A, 16 October 2018. [Google Scholar]
- Liu, J. Extraction Method of Flax Gum and Lignan in Flax Cake. CN Patent 109535276A, 29 March 2019. [Google Scholar]
- Su, L. Lignan Extraction Technology. CN Patent 111116687A, 8 May 2020. [Google Scholar]
- Tang, S.; Dou, W.; Cai, Y. High-Performance Liquid Phase Method for Simulta-Neously Preparing and Separating four Lignan Compo-Nents. CN 111233944A, 5 June 2020. [Google Scholar]
- Wang, Z.; Li, Q.; Xu, G.; Gao, Z.; Yin, L.; Chen, X.; Zhao, X.; Mao, K. Extraction Method of Flaxseed Extract. CN Patent 111543644A, 18 August 2020. [Google Scholar]
- Huang, D.; Li, C.; Zhao, K.; Qian, H.; Chen, W.; Li, B.; Long, Y. Method for Extracting Secoisolariciresinol Diglucoside from Flaxseeds by Using Subcritical Water. CN Patent 112480192A, 12 March 2021. [Google Scholar]
- Li, S. Method for Simultaneously Producing Five Components in Flaxseeds. CN Patent 113527385A, 22 October 2021. [Google Scholar]
- Yang, L. Method for Removing Benzopyrene in Flax Extract. CN Patent 113980066A, 28 January 2022. [Google Scholar]
- Li, Y.; Huang, J.; Wang, Y. Subcritical Composite Solvent Extraction Separation Method for Multiple Components in Flaxseed Meal. CN Patent 113786641B, 12 August 2022. [Google Scholar]
- Hosseinian, F.S.; Beta, T. Patented Techniques for the Extraction and Isolation of Secoisolari- ciresinol Diglucoside from Flaxseed. Recent Pat. Food Nutr. Agric. 2009, 1, 25–31. [Google Scholar] [CrossRef] [PubMed]
| Name | Amount (%) |
|---|---|
| Water | 6.96 |
| Protein | 18.29 |
| Total fat | 42.16 |
| Saturated Fatty Acids | 3.66 |
| Monounsaturated Fatty Acids | 7.52 |
| Polyunsaturated Fatty Acids | 28.73 |
| Linolenic acid | 22.81 |
| Linoleic acid | 5.90 |
| Carbohydrate | 28.88 |
| Fiber, total dietary | 27.30 |
| Sugars, Total | 1.55 |
| Others (minerals, vitamins, carotenoids, lignans, etc.) | 3.71 |
| Lignan | Amount (mg) per 100 g of Flaxseed |
|---|---|
| Secoisolariciresinol (SECO) | 257.60 |
| Lariciresinol | 11.46 |
| Pinoresinol | 8.64 |
| Matairesinol | 6.68 |
| Extraction Techniques | Raw Materials | Extracted Lignans | Operating Conditions | Results | Ref. |
|---|---|---|---|---|---|
| Cellulase-assisted extraction | Whole flaxseed, Flax hull | SDG | Sequential methanol/ethanol extraction followed by alkaline hydrolysis and subsequently enzyme-assisted extraction of SECO | SDG: 40.75 mg/g in the hull; 15.20 mg/g in whole flaxseed. Cellulase is more efficient than β-glucosidase | [54] |
| Direct alkaline hydrolysis | Milled defatted flaxseed flour | SDG | Direct hydrolysis with 5 mL of 2 M NaOH for 1 h at 20 °C. | SDG yields are higher than those obtained by hydrolysis after alcoholic extraction | [56] |
| One-pot hydrolysis and extraction | Flaxseed hull | SDG | Alcoholic ammonium hydroxide (pH = 12.9) for 4.9 h at 75.3 °C allows the direct hydrolysis and extraction of SDG. | Very simple method with very high yields of SDG. | [57] |
| Microwave-assistedExtraction (MAE) | Flaxseed | SDG | 70% ethanol added with 0.1 M NaOH. 50–150 W power for 1–15 min | MAE has higher yields of SDG (16.1 mg/g) than that of traditional methods. | [59] |
| Flaxseed hull | SDG | 0–100% ethanol, liquid to solid ratio from 5:1 to 40:1 mL/g, 50–390 W microwave energy for 10–330 s | SDG recovery with MAE (11.7 mg/g) is higher than that of Soxhlet extraction (7.6 mg/g) | [60] | |
| Defatted flaxseed meal | SDG | 1 g sample extracted with 50 mL 0.5 M NaOH, with the microwave power (135 W) applied intermittently for 3 min | 97% SDG recovery. Fast and efficient method. | [61] | |
| Ultrasound Assisted Extraction (UAE) | Flaxseeds | SDG | Water with 0.2 N NaOH to get free SDG from its HMG complex. Extraction: 60 min at 25 °C and 30 kHz ultrasound frequency | Higher yield for SDG, particularly compared to MAE and conventional alkaline extraction | [62] |
| Ground defatted flaxseeds | SDG |
| No purification step is necessary before analysis.Efficient recovery of SDG. | [63] | |
| Supercritical CO2 Extraction | Ground (hydrolyzed) flaxseed, ground hulls | SDG | Extraction of SDG by using SC-CO2 modified with 7.8 mol% ethanol at 60 °C and 45 MPa | Very low yields compared to the original SDG content. SC-CO2 extraction can be an advantageous pretreatment. | [64] |
| Subcritical water extraction | Defatted flaxseed meal | SDG | Extraction using subcritical water at 170 °C, at pH 9 and 5.2 MPa, and solvent to solid ratio of 100 mL/g | Simultaneous extraction of SDG (21 mg/g), proteins, and carbohydrates. | [65] |
| Flaxseed meal sticks | SDG | High SDG yields at 170–180 °C for 15 min, 1.500 psi and 40% fresh water | SDG: 72.57% (at 180 °C), 70.67% (at 170 °C) | [66] | |
| High voltage Electric Discharge (HVED) | Flaxseed cake | SDG | HVED treatment at 20–40 kV and 20–60 °C, 0–25% ethanol for subsequent extraction | Lower levels of SDG extracted compared to the literature data | [67] |
| Pulsed electric fields (PEF) | Flaxseed hull | Polyphenols (lignans, flavonoids, ferulic, and p-coumaric acids) | PEF treatment (at 20 kV/cm for 10 ms) after hull rehydration using water, ethanol (20%), and 0.3 mol/L NaOH for 40 min at 20 °C. | PEF extracts uo to 80% of polyphenols (including lignans) | [68] |
| Negative-pressure cavitation extraction | Flaxseed cakes | SDG | Extraction at −0.04 MPa, at 35 °C, for 35 min with ethanol 65% (v/v), ventilation volume 90 L/h, NaOH 1.39%, and the liquid/solid rate (mL/g) 13.16:1. | SDG yields (16.25 mg/g) and SDG extraction purity (3.86%) are comparable to UAE results. | [69] |
| Title | Flaxseed Materials | Extracted Lignans | Number and Ref. |
|---|---|---|---|
| Method for extracting secoisolariciresinol diglucoside from flax seeds or husks, extract obtained and use thereof | Flax seeds or husks | SDG | CN101570556B [71] |
| Production process of secoisolariciresinol diglucoside | Flaxseed oil residue | SDG | CN102558252A [72] |
| Method for extracting, separating, and purifying flax lignans from flax cakes | Flaxseed cake | Not specified: SECO or SDG | CN102796148A [73] |
| Method for extracting secoisolariciresinol diglucoside | Flaxseed cake | SDG | CN102816190A [74] |
| Detoxification method of flaxseed product | (Defatted) flaxseed powder | Not specified | CN102823782A [75] |
| Extraction of lignans from flaxseeds through ultrasonic enzymolysis | Defatted flaxseeds | Not specified | CN102125261B [76] |
| Method for isolation of flaxseed lignans | Flaxseed cake | Not specified | PL397907A1 [77] |
| Method for directly extracting flax lignans from flaxseed meal and detoxifying high-protein flaxseed meal | Flaxseed meal | SDG | CN104478954A [78] |
| Method for continuously extracting flaxseed gum and lignan from flaxseed husks | Flaxseed husks | SDG | CN102850414B [79] |
| Method for extracting lignan from linseed oil residue | Flaxseed oil residue | Not specified | CN102766174B [80] |
| Method for preparing fruit antioxidant by efficiently extracting flax lignans | Flaxseeds | Not specified | CN105145806A [81] |
| Separation and purification method for secoisolariciresinol diglucoside | Flax seeds or flax shells | SDG | CN103396461B [82] |
| Method for preparing flax lignans from flax cake | Flax cake | Not specified | CN105585599A [83] |
| Method for extracting secoisolariciresinol diglucoside, obtained extract and use of extract | Flaxseed oil residue | SDG | CN103860649B [84] |
| Method for obtaining high biological activity lignan and mushroom by using edible mushroom to degrade flax seed shell | Flax seed shell | SDG | CN105000934B [85] |
| Extraction method for flax lignan from flax seed husks | Flaxseed husks | Not specified | CN108129526A [86] |
| Method for continuously extracting flaxseed gum and secoisolariciresinol diglucoside from flaxseed meal | Flaxseed meal | SDG | CN108409813A [87] |
| Method for extracting flax lignans from flaxseed husks by microwaves | Flaxseed husks | SDG | CN108659064A [88] |
| Extraction method of flax gum and lignan in flax cake | Flax cake | Not specified | CN109535276A [89] |
| Lignan extraction technology | Flaxseeds | Not specified | CN111116687A [90] |
| High-performance liquid phase method for simultaneously preparing and separating four lignan components | Flaxseed hulls or meals | SDG, SG, SECO, AHS | CN111233944A [91] |
| Extraction method of flaxseed extract | Flaxseeds | Not specified | CN111543644A [92] |
| Method for extracting secoisolariciresinol diglucoside from flaxseeds by using subcritical water | Flaxseeds powder | SDG | CN112480192A [93] |
| Method for simultaneously producing five components in flaxseeds | Flaxseeds | SDG | CN113527385A [94] |
| Method for removing benzopyrene in flax extract | Flaxseed meal | SDG | CN113980066A [95] |
| Subcritical composite solvent extraction separation method for multiple components in flaxseed meal | Flaxseed meal | Not specified | CN113786641B [96] |
| Extraction Technologies | Results in the Literature of the Last Decade (Total Number 9) | Patents of the Last Decade (Total Number 26) |
|---|---|---|
| Conventional solvent extraction | 4 | 12 |
| Microwave-assisted Extraction (MAE) | 0 | 4 |
| Ultrasound Assisted Extraction (UAE) | 2 | 7 |
| Supercritical CO2 Extraction | 0 | 1 |
| Subcritical solvent extraction | 1 | 2 |
| Electrical methods (PEF and HVED) | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangiorgio, P.; Errico, S.; Verardi, A.; Moliterni, S.; Tamasi, G.; Rossi, C.; Balducchi, R. Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies. Nutraceuticals 2023, 3, 58-74. https://doi.org/10.3390/nutraceuticals3010005
Sangiorgio P, Errico S, Verardi A, Moliterni S, Tamasi G, Rossi C, Balducchi R. Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies. Nutraceuticals. 2023; 3(1):58-74. https://doi.org/10.3390/nutraceuticals3010005
Chicago/Turabian StyleSangiorgio, Paola, Simona Errico, Alessandra Verardi, Stefania Moliterni, Gabriella Tamasi, Claudio Rossi, and Roberto Balducchi. 2023. "Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies" Nutraceuticals 3, no. 1: 58-74. https://doi.org/10.3390/nutraceuticals3010005
APA StyleSangiorgio, P., Errico, S., Verardi, A., Moliterni, S., Tamasi, G., Rossi, C., & Balducchi, R. (2023). Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies. Nutraceuticals, 3(1), 58-74. https://doi.org/10.3390/nutraceuticals3010005







