Abstract
The phytochemical profiles of extracts from closed, semi-opened and opened leaf buds and the summer leaves of Ginkgo biloba were studied. The extraction and purification of bilobalide and ginkgolides, using andrographolide as an internal standard, were optimised. The terpene trilactone concentrations increased with bud development, from 1.07 mg/g dry wt in closed buds to a maximum of 3.75 mg/g dry wt in summer leaves. The major terpene trilactone was bilobalide at all developmental stages. The concentration of flavonol aglycones in hydrolysed extracts was also analysed. The flavonol glycoside concentration increased from the closed bud stage (0.21 ± 0.01% dry wt) to the summer leaf stage (1.15 ± 0.01% dry wt). A linear correlation was observed between the terpene trilactone and flavonoid content during gingko leaf development.
1. Introduction
Bud extracts are used as food supplements and nutraceuticals. Validation of the use of these products requires knowledge of their chemical composition. In most cases, the chemical composition of these young starting materials is unknown. However, scientific investigations of bud extracts have evolved rapidly over the past 10 years [1].
One of the most frequently used plants in herbal medicines and dietary supplements is Ginkgo biloba. Classified by Linnaeus in 1771, G. biloba is the only surviving species of the plant division Ginkgophyta [2]. Medicinally, G. biloba is used to treat and prevent a range of different diseases. Recently published reviews concerning G. biloba leaf extracts have provided substantial information regarding this plant’s specialized metabolites, including a description and evaluation of their chemical nature, their structural diversity and their biological activities [3]. Interest has also grown in G. biloba extracts in terms of their antioxidant and antimicrobial activities [4], as well as their effectiveness in treating diseases related to ageing [5,6] and cardiovascular disorders [7]. Ginkgo leaf extracts are recommended as treatments for cerebral insufficiency, vertigo of vascular origin, tinnitus and peripheral arterial disease [8]. Both ginkgo leaf and bud extracts are sold in the herbal medicine market, with a global value of USD 187.2 million in 2020 (estimated to reach USD 235.5 million by 2026) [9].
As one of the most popular medicinal plants, G. biloba accumulates pharmacologically active terpene trilactones and flavonoids [10,11]. The terpene trilactones are antagonists of the platelet-activating factor (PAF) receptor [12]. The major component in the family of terpene trilactones found in G. biloba is bilobalide, a bioactive molecule with neuroprotective activity [12,13]. This terpene trilactone also has known anti-ischemic, cardiovascular protective and anti-inflammatory activities [13]. The flavonoid components found in G. biloba are believed to act as protectants against capillary fragility, both as anti-inflammatory agents that reduce oedema caused by tissue injury and as free radical scavengers [6,14].
The recognised importance of G. biloba and its bioactive molecules points to the need for research to establish qualitative and quantitative methods for the detection and quantification of the major medicinal compounds of G. biloba bud and leaf extracts. Some articles in the literature have compared the phytochemical profiles of green and yellow leaves of G. biloba [15,16]. Many papers have described the quantification of G. biloba metabolites in leaf extracts and in pharmaceutical preparations available in drug stores [17], or in food supplements [18,19,20]. Many commercial products use ginkgo leaf buds as starting materials; however, phytochemical analysis of these young plant tissues is not yet well-developed.
In the present study, we provide the first report of a dynamic accumulation of bilobalide, ginkgolides and flavonoids in ginkgo buds during different physiological stages of development. Only a few reports have investigated the dynamics of flavonol glycoside [21] and terpene trilactone [22,23] accumulation in G. biloba, but those studies have focused on seasonal changes in fully developed leaves. Notably, only one publication [21] has taken into account the bud development stage. In the present paper, we describe significant improvements made using several methods, including extraction, purification, analysis and quantification by liquid chromatography-high resolution electrospray ionization mass spectrometry (LC-HR-ESI-MS), with buds and leaves as starting materials. We begin the paper by introducing a terpene trilactone study using a fortified mixture of commercial standards, consisting of bilobalide, ginkgolide A, ginkgolide B and ginkgolide C, with andrographolide as the internal standard. This mixture is used to determine the mass spectrometry ions and to obtain good peak resolution and conditions for the analysis. We then assess ultrasonic-assisted extraction and refluxing in oxidised water as two extraction methods for terpene trilactones, followed by comparison of two purification methods. After these optimisations, bilobalide and ginkgolide contents in buds and leaves of G. biloba are quantified. In the second part of the paper, three aglycones found in the G. biloba leaves (quercetin, kaempferol and isorhamnetin) are quantified after acidic hydrolysis of the extracts. Finally, we use Principal Component Analysis (PCA) to identify groups of similar individuals in the dataset.
2. Materials and Methods
2.1. Chemicals
Acetone (Me2CO, ACS reagent, 99.5%), chloridric acid (HCl, ACS reagent, 37%), phosphoric acid (99.99%), ethyl acetate (EtOAc, 99%), tetrahydrofuran (THF) (99.9%), distillate water (H2O), quercetin dehydrate (HPLC, ≥98%), kaempferol (HPLC, ≥97%), isorhamnetin (HPLC, ≥95%), Ginkgolides A (GA), Ginkgolides B (GB), Ginkgolides C (GC), bilobalide (BB) (all HPLC, ˃95%) and andrographolide (HPLC, ˃98%) were obtained from Sigma-Aldrich (Stennheim, Germany). Methanol (MeOH, Hypersolv Chromanorum for HPLC-Isocratic Grade) and 30% hydrogen peroxide (H2O2) were purchased from Prolabo VWR international.
2.2. Equipments
For the analysis of flavonoids, the equipment used was a reversed-phase HPLC Thermo Separation Product system (San Jose, CA, USA) fitted with an Interchim (Montluçon, France) Nucleosil C18 5 µm (250 mm × 4.6 mm I.D.) column. A UV/visible light detector (TSP, UV 2000) operating at 370 nm was also used. The HPLC software and data acquisition system we utilized was AZUR 5.0 (DATALYS, 38400 St Martin D’heres, France).
For terpene trilactone analysis and quantification, the equipment used was an LC system that consisted of a U3000-Dionex equipped with a 1 µL loop injector. Separations were carried out using an Acclaim Pepmap C18 1 mm I.D. column (150 mm × 3 µm × 100 µm) (Thermo Fischer, Illkirch, France).
All mass spectra were acquired with Electrospray Ionization—High Resolution Mass Spectrometry (ESI-HRMS) using a micrOTOFQTM (Bruker Daltonics, Bruker, Bremen, Germany) apparatus, equipped with an electrospray source in the negative mode at 10 eV.
2.3. Plant Materials
Three samples of each studied type of Ginkgo biloba material (three developmental stages of buds—closed buds (A), semi-opened buds (B), opened buds (C) and summer leaves (D)) were used. These were collected from twenty-five year old trees in a plantation in Champaubert, Marne region, France. Sample collection of the buds (stage A, B and C) took place in April–May 2018, and of the leaves in June 2018.
Closed buds were harvested on 15th April (stage A); semi-opened buds (stage B) were harvested 17 days later, on 2nd May; opened buds (stage C) were harvested 12 days later, on 14th May; and leaves (stage D) (500 g of fresh leaves) were harvested one month later, on 14th June.
Terminal and axillary buds (100 g of fresh buds of each development stage) from longer and lower branches of several G. biloba trees were harvested. All plant materials were dried at 40 °C in the oven for 48 h.
The plant material was authenticated by one of the authors, Pr. D. Laurain-Mattar, Professor in Pharmacognosy, University of Lorraine.
2.4. Procedure for Optimization LC-HR-ESI-MS Analysis and Terpene Trilactones Quantification
2.4.1. Standard Preparation
Five standards were used. All stock solutions of the reference standards (ginkgolides A, B, C, bilobalide) and the stock solution of the internal standard (andrographolide) were prepared in MeOH at a concentration of 1 mg/mL. All working solutions for the calibration curves were prepared by diluting the stock solutions using a mixture of methanol (MeOH) and distilled water (H2O) in a 1:1 volume ratio v/v). All stock solutions and working solutions were stored at 4 °C until further use.
2.4.2. Optimization of LC-HR-ESI-MS Analysis
For the LC analysis, mobile phase A consisted of an aqueous solution of 0.1% formic acid (HCOOH), and mobile phase B consisted of 50% aqueous MeOH. The LC method was optimized in order to obtain good peak resolutions. The LC column was eluted at a flow rate of 40 µL/min using a gradient ranging from 10% of mobile phase B to 70% of mobile phase B in a time span of 10 min, followed by a 15 min elution with A/B (30:70, v/v) prior to the next injection.
The conditions for MS analysis were the following: negative ion electrospray was used for ionization at a both a source temperature and a desolvation temperature of 190 °C, and nitrogen was used as a nebulizing and drying gas at a flow rate of 4 L/min.
A fortified mixture of reference standards (andrographolide; bilobalide; ginkgolide A, B and C) was prepared as detailed in the Section 2.4.1. The theoretical concentration of ginkgo terpene trilactones was 25 µg/mL. The theoretical concentration of andrographolide (internal standard) was 10 µg/mL.
2.4.3. Sample Extraction Techniques
For ultrasound-assisted extraction, 2 g of dried and pulverized ginkgo buds and leaves were extracted with 20 mL of 90% aqueous MeOH in an ultrasonic bath at 40 °C for 15 min. The extracts were filtered, dried, dissolved in 20 mL of distilled water and subjected to further purification using liquid–liquid extraction. The procedure was similar to that described by Kaur and co-workers [24], and we used the best conditions obtained by those authors.
For refluxing in oxidised water, 30 g of dried and powdered G. biloba leaves were boiled for 1 h in 250 mL of 5% aqueous hydrogen peroxide (H2O2), as proposed by Lichtblau and co-workers [25]. The macerate was then filtered through a Buchner funnel, and the filtered solution was used for liquid–liquid extraction. As our aim in this step was to optimise the extraction method, only the leaves were tested.
2.4.4. Liquid–Liquid Purification of Terpene Trilactones
An internal standard, andrographolide, was added to the extract prior to purification to quantify the losses of terpenes occurring during the purification steps. Andrographolide is a terpene trilactone from Andrographis paniculate, with a molecular weight and chemical and physical properties close to those of bilobalide and ginkgolides [26]. Recoveries were determined based on the recovery of the internal standard, as each crude extract contained a theoretical concentration of 10 µg/mL of andrographolide.
The aqueous extracts obtained at the end of both extraction methods were subjected to liquid–liquid extraction with a mixture of 4:1 (v/v) ethyl acetate (EtOAc): tetrahydrofuran (THF). The volume ratio of the aqueous phase to the organic phase was fixed at 1:2 (v/v). Two purification methods were compared.
- -
- Method A: the aqueous/organic mixture was placed in a conical flask and shaken for 2 h in a thermostated water bath set at 30 °C. This procedure was performed only once.
- -
- Method B: the aqueous phase was extracted three times with the organic mixture, and the resulting three layers were combined.
The organic layer containing excess peroxide (derived from the first extraction method) was washed successively with a saturated solution of sodium sulphite (Na2SO3) at pH 9.3, water and 80% aqueous sodium chloride (NaCl). The organic phases collected after each purification method were then dried over anhydrous sodium sulphate (Na2SO4). The solvent was removed by evaporation to dryness under reduced pressure.
2.4.5. Optimization of Purification Methods of Terpene Trilactones
The crude extracts obtained by the two extraction methods were spiked with 10 µg/mL of the andrographolide internal standard (Figure S1). The andrographolide was added to establish the efficiency of the purification steps and for quantification of terpene trilactones.
The crude extracts were extracted either once by heating for 2 h at 30 °C or three times with a mixture of EtOAc:THF (4:1, v/v) at room temperature to determine the best liquid–liquid extraction method for terpene trilactones. The solvent mixture was chosen based on the results presented by Lang and co-workers [27].
2.4.6. Quantification of Terpene Trilactones
The terpenes were quantitatively analysed using an internal standard calibration method and a nine-point calibration curve (R2 = 0.99), prepared using the standards of purchased ginkgolide and bilobalide. The concentration levels ranged from 5 to 100 µg/mL, and each reference solution contained 10 µg/mL of the andrographolide internal standard. The calibration curves were obtained with the quadratic mode between 2.3 and 50 µg/mL. The calibration curves for bilobalide (BB), for Ginkgolide A (GA), for Ginkgolide B (GB) and for Ginkgolide C (GC) quantification were presented in Figures S2–S5.
2.5. Procedures for Flavonoid Analysis and Quantification
2.5.1. Standard Preparation
Reference standards (quercetin, kaempferol and isorhamnetin) were dissolved in MeOH at a concentration of 0.5 mg/mL. A 1 mL sample of 37% hydrochloric acid (HCl) and 5 mL of distilled water were added to the solution. MeOH was added until the volume was completed, at 50 mL [28].
2.5.2. Hydrolyzed Sample Preparation
Approximately 2.5 g of dried and powdered ginkgo buds and leaves were placed into a 250 mL round-bottom flask with 50 mL of 60% v/v acetone (Me2CO) solution, then heated under reflux for 70 min according to the European Pharmacopoeia. The extracted liquid was filtered, and 40 mL of 60% v/v Me2CO solution was added to the remaining solid for a second extraction. A total of 50 mL of the final extract was evaporated until the steady flow of condensed solvent ceased. Then, 4.4 mL of 37% HCl was added to the obtained aqueous phase, and MeOH was added until the volume was complete at 50 mL. Next, 10 mL of the supernatant was transferred to a sealed glass vial and submerged in a boiling water bath for 25 min. An aliquot of each sample was filtered through a 0.4 µm solvent-resistant filter prior to injection [28].
2.5.3. HPLC Analytical Method
The mobile phase A consisted of an aqueous solution of phosphoric acid (0.3 g/L) adjusted to pH 2.2 and a mobile phase B of MeOH. The eluting mobile phase was A/B (60:40, v/v) for 1 min followed by a gradient of A/B (60:40, v/v) to A/B (45:55, v/v) over 19 min, then another gradient from A/B (45:55, v/v) to A/B (0:100, v/v) over 1 min. This was followed by a 4 min elution with A/B (0:100, v/v) prior to the next injection. The mobile phase was pumped at 1 mL/min, the column temperature was 35 °C, and the injection volume was 20 µL [28].
2.5.4. Calculation of Flavonol Content
Flavonol content (% dry wt) was calculated according to the European Pharmacopoeia [28] using the expression:
where F1 is the sum of the areas of all considered peaks in the chromatogram obtained with the sample solution, F2 is the area of the peak corresponding to quercetin in the chromatogram obtained with the standard solution, m1 is the mass (g) of quercetin used to prepare the standard solution, m2 is the mass (g) of the sample to be examined and used to prepare the sample solution and p is the percentage content of anhydrous quercetin in quercetin dehydrate standard solution.
2 × [(F1 × m1 × 2.514 × p)/(F2 × m2)]
2.6. Statistical Analysis
Each condition was repeated three to five times, depending on the experiment. For purification methods, the experiment were performed five times. The results were expressed as means with standard deviations (±SD).
For the quantification of the metabolites, the analyses were repeated three times, and the data were expressed as mean ± SD, p < 0.001.
For statistical analysis, Excel 2013 (Microsoft Office) and SPSS statistics 20 (IBM) were used. For PCA, SIMCA software version 16.0.2 (Umetrics, Umeå, Sweden) was used, and the PCA was realized using the Pareto scaling method.
3. Results and Discussion
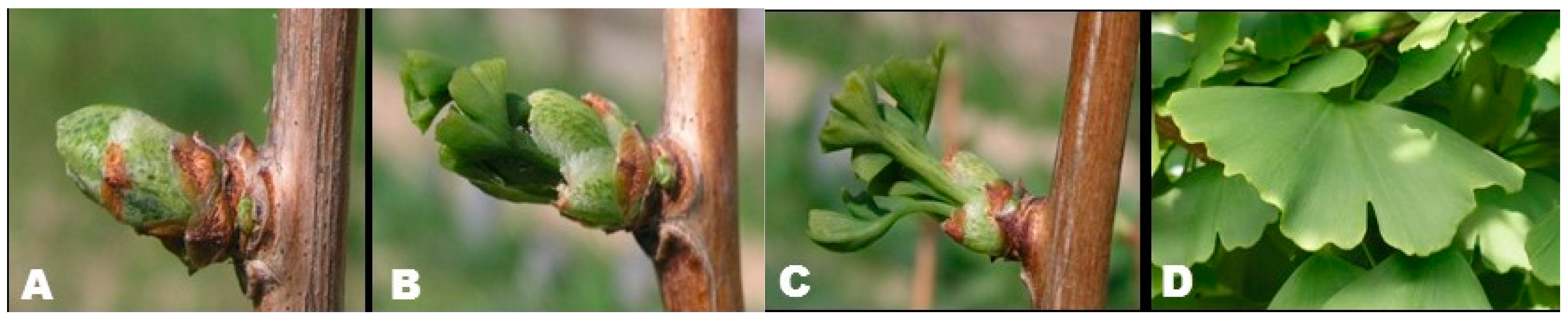
In this study, four different physiological development stages were identified for G. biloba leaf buds: closed buds covered with scales (Figure 1A), semi-opened buds (Figure 1B), opened buds with scales on the base (Figure 1C) and fully opened leaves (Figure 1D). The samples were collected from the lower branches of several G. biloba trees.

Figure 1.
G. biloba, from buds to leaves; (A) closed buds; (B) semi-opened buds; (C) opened buds; (D) summer leaves.
As the buds develop relatively rapidly in relation to the ambient temperature (all four developmental stages can occur within 48 h), successive harvests were made over a short period (from mid-April to mid-May for stages A, B and C). This was a tedious process, as we had to control bud development in the field and define several stages of physiological development.
3.1. Extraction and Purification of Terpene Trilactones
The bilobalide and ginkgolide contents in G. biloba buds and leaves were evaluated after ultrasonic-assisted extraction [24] and refluxing in oxidised water [25]. These two optimised extraction methods were chosen based on reports in the literature of high recovery of terpene trilactones from G. biloba leaves. For both methods, andrographolide was added to the crude extract before purification as an internal standard, and the recovery was evaluated by LC-HR-ESI-MS.
Two purification conditions were compared: a single liquid–liquid purification step with an EtOAc:THF mixture (method A) and heating for 2 h at 30 °C; and three room-temperature liquid–liquid purification steps using a mixture of EtOAc:THF (method B).
The first crude extract, obtained by ultrasonic-assisted extraction, showed an internal standard recovery of 92.47 ± 2.25% after purification using method A, and 87.28 ± 6.34% after purification using method B. Lower recoveries of andrographolide (between 68.43 ± 2.7% and 58.5 ± 4.2%) were obtained for the crude extract obtained by refluxing the plant tissues in oxidised water. A standard recovery of 68.43 ± 2.7% from the oxidised water extract was achieved using single purification (method A), and a recovery of 58.5 ± 4.2% was achieved using triplicate liquid–liquid purification (method B). The extraction yield was better with the EtOAc:THF mixture than with EtOAc alone. Table 1 shows all the techniques used (extraction and purification methods) and the recovery percentage of the andrographolide internal standard.

Table 1.
Recoveries of the internal standard andrographolide added to G. biloba leaf extracts obtained with two extraction and two purification methods.
Based on the recovery of the andrographolide, ultrasonic-assisted extraction, followed by single liquid–liquid purification for 2 h at 30 °C, was determined to have the best yield, and was also the most reproducible method.
3.2. Terpene Trilactone Content in G. biloba Buds and Leaves
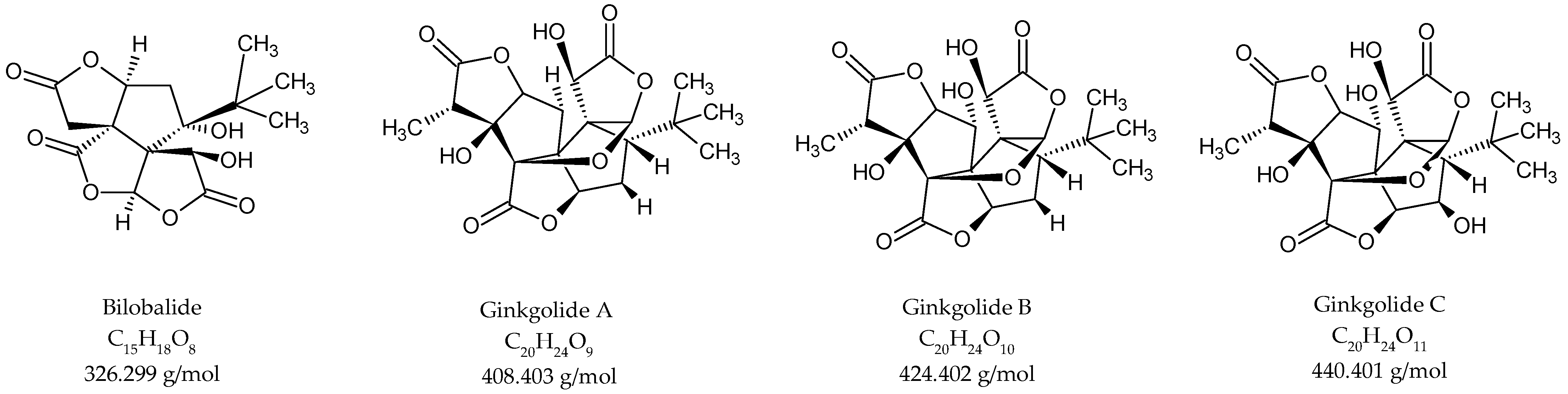
No standardised method has been developed for terpene trilactone quantification, although several studies have reported validated LC/MS protocols for the quantitative analysis of terpene trilactones [2,17,24,29]. The protocol presented in this work is closest to that previously reported by Sun and co-workers [26]. After optimisation of the previously described extraction and purification procedures, the terpene trilactone contents were evaluated, focusing on the concentrations of bilobalide, ginkgolide A, ginkgolide B and ginkgolide C in the plant tissue extracts. The chromatographic profiles of the different standards in the extracts are shown in Figure S6. The order of elution was bilobalide (12.7 min), ginkgolide C (13.6 min), ginkgolide A (15.4 min), ginkgolide B (15.7 min) and andrographolide (17.7 min). The chemical constituents were unambiguously identified by recording their mass spectra. The ESI-MS ionisation patterns of bilobalide and ginkgolides are shown in Figure S7. The ESI-HRMS profiles for bilobalide, ginkgolide C and ginkgolide B displayed [M − H]− at a m/z of 325.0879, 439.1197 and 423.1354 Da, respectively. Ginkgolide A and andrographolide displayed [M + HCOO]− at a m/z of 453.1347 and 395.2019 Da, respectively. The chemical structures of bilobalide and ginkgolides are presented in Figure 2.
Figure 2.
Structures of terpene trilactones quantified in G. biloba bud and leaf extracts.
The bilobalide and ginkgolide contents of G. biloba buds and leaves were quantified by analysing the extracts. The limit of quantification (LOQ) of terpene trilactones was 0.3 µg/mL. The limit of detection (LOD) was 0.06 µg/mL, as determined using ginkgolide C.
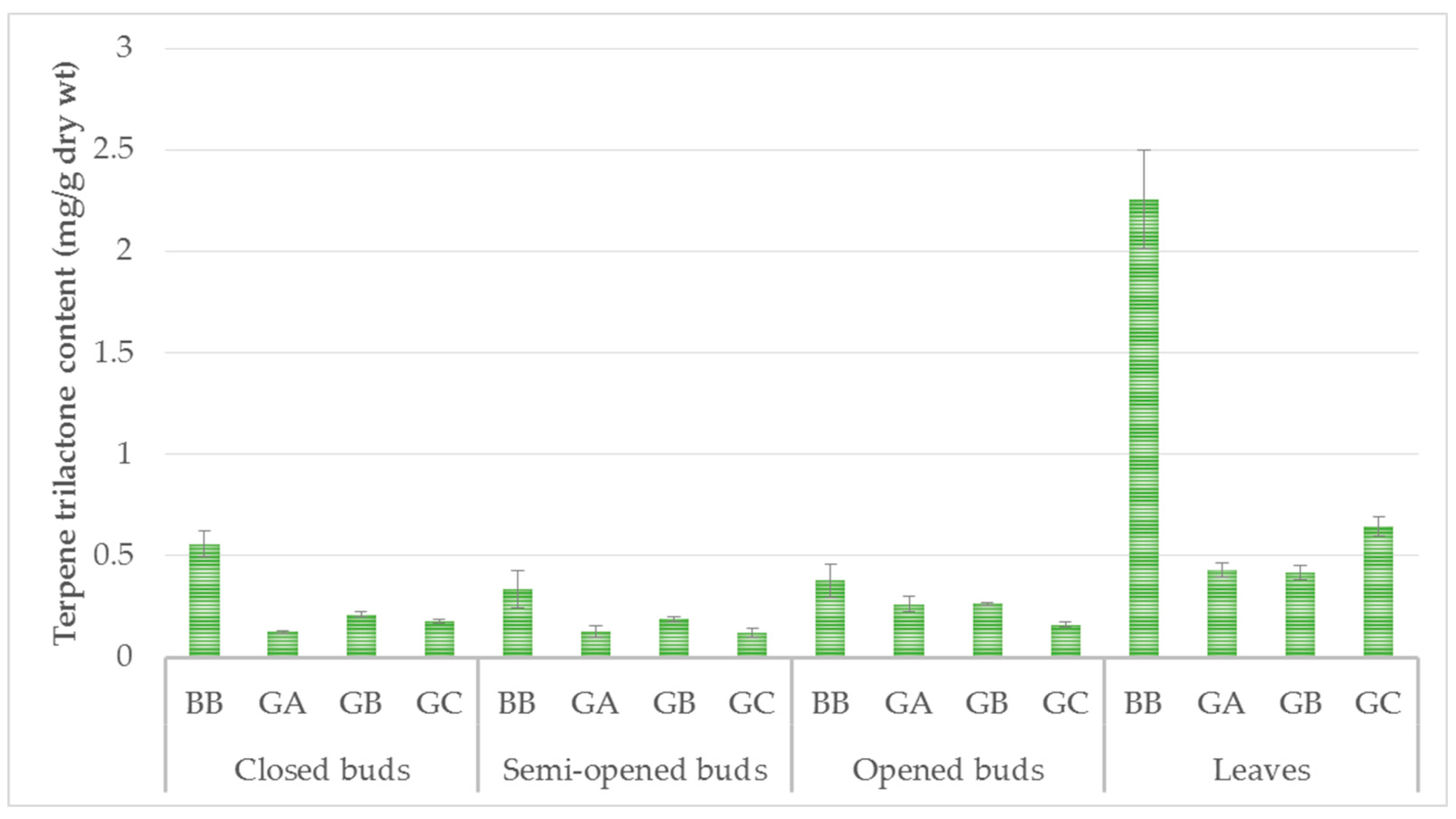
In the closed-bud stage, the content of the four terpene trilactones was 1.07 mg/g dry wt. In the semi-opened bud stage, the content was 0.77 mg/g dry wt, and increased to 1.06 mg/g dry wt in the opened-bud stage. The content of terpene trilactones increased from the closed-bud stage (A) (1.07 mg/g dry wt) to the summer leaf stage (D) (3.75 mg/g dry wt) (Figure 3). The content of ginkgolide A was stable in closed and semi-opened buds (0.12 ± 0.01 mg/g dry wt), but increased in opened buds (0,26 ± 0.06 mg/g dry wt) and leaves (0.43 ± 0.06 mg/g dry wt).
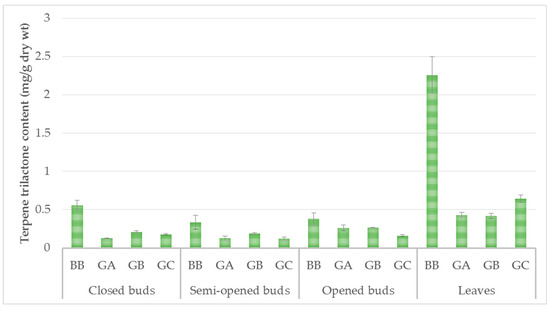
Figure 3.
Terpene trilactone content (mg/g dry wt) of three physiological states of ginkgo buds and summer leaves. GA = Ginkgolide A, GB = Ginkgolide B, GC = Ginkgolide C, BB = bilobalibe. Data are given as mean ± SD, replicates are n = 3 and p < 0.001.
According to the literature, the terpene trilactone content of ginkgo leaves varies between 1.45 and 2.85 mg/g dry wt [22]. Our results showed higher values (3.75 mg/g dry wt) than those previously reported. Bilobalide was the most important metabolite among the terpene trilactones at all four developmental stages. This compound was present at a concentration of 0.56 ± 0.06 mg/g dry wt in the closed buds, and reached 2.26 ± 0.24 mg/g dry wt in the summer leaves. This finding agreed with previous research published on ginkgo leaves. Two studies have previously investigated the seasonal dynamics of bilobalide and ginkgolide accumulation in ginkgo leaves [22,23], but they did not take into account the bud development stage.
3.3. Flavonoids Content in G. biloba Buds and Leaves
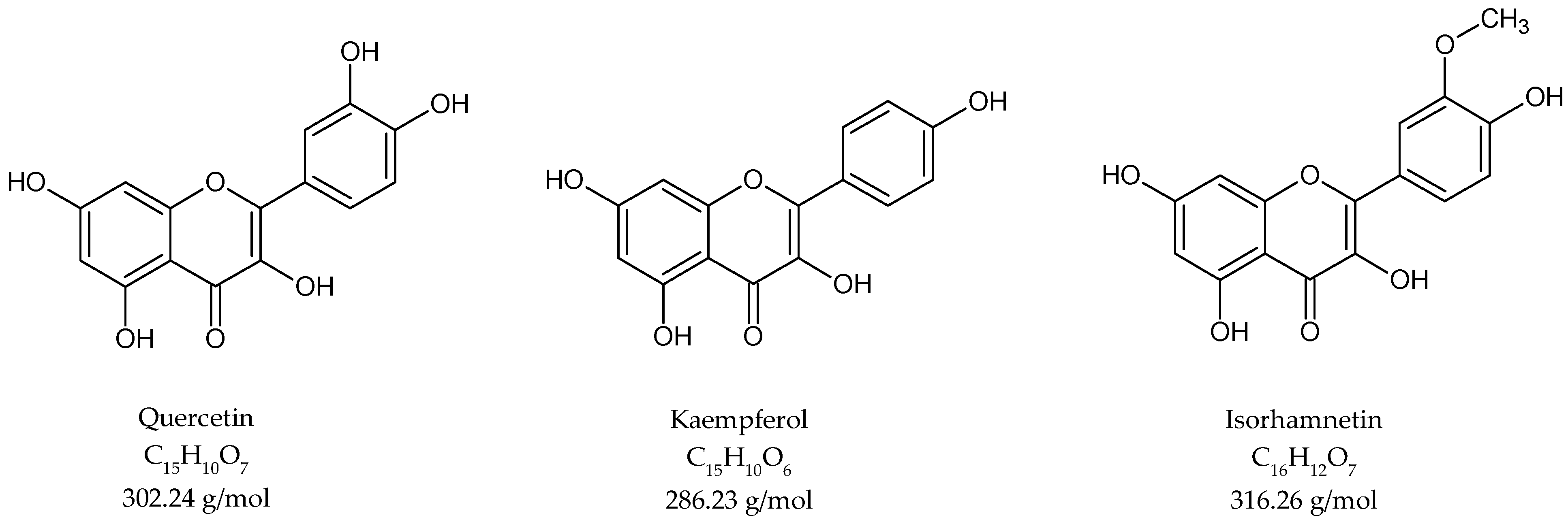
For the investigation of flavonoid content, an acetone extraction was performed, followed by acidic hydrolysis. The crude extract was analysed by high-performance liquid chromatography. The flavonoid fraction contained quercetin, kaempferol and isorhamnetin aglycones (Figure 4).
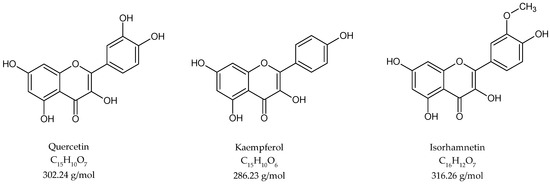
Figure 4.
Chemical structure of flavonoids in G. biloba acidic hydrolyzed extract.
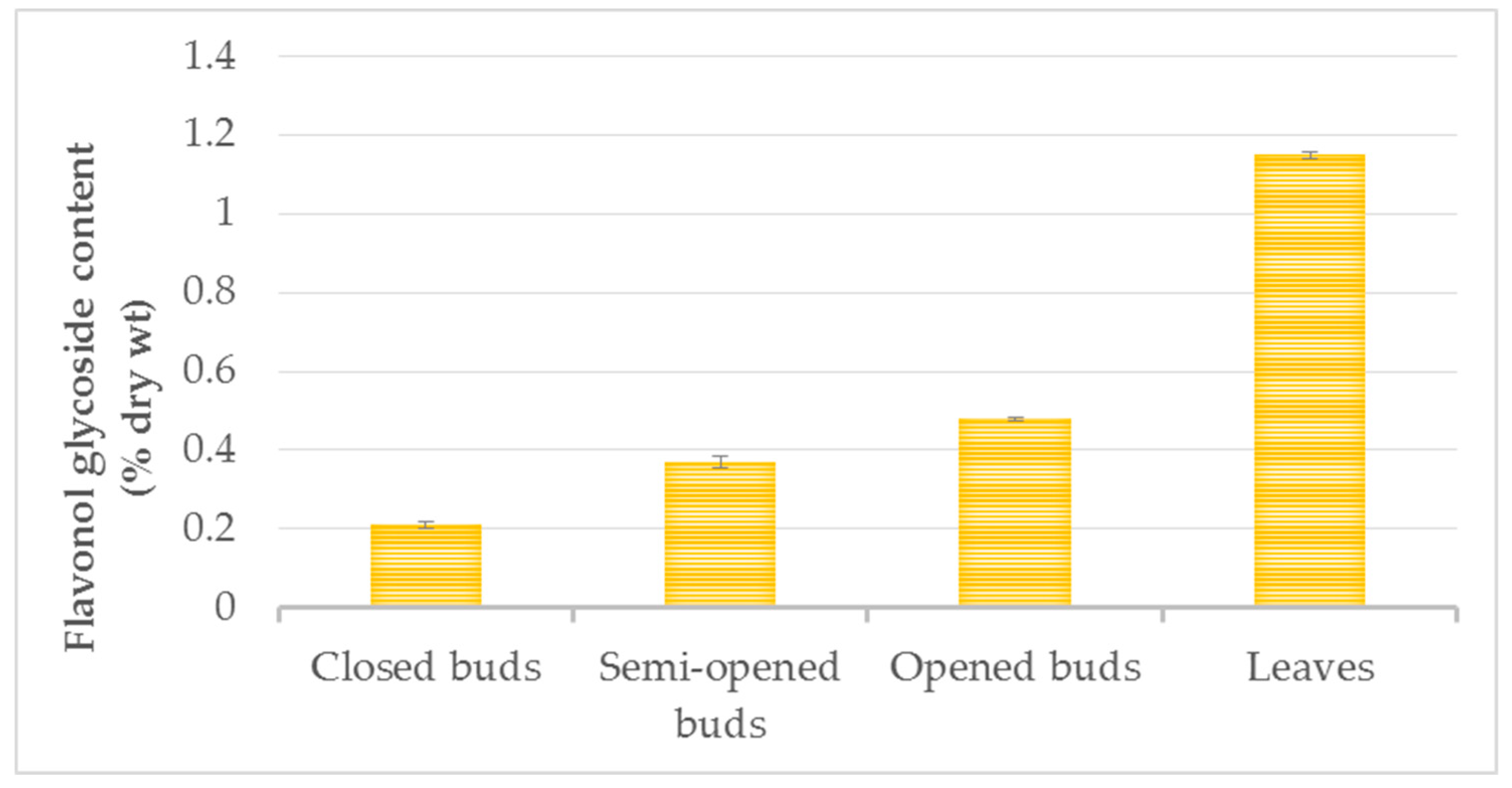
The amount of total flavonoids increased from the closed-bud stage (0.21 ± 0.01% dry wt) and the semi-open bud stage (0.37 ± 0.03% dry wt) to the opened-bud stage (0.48 ± 0.03% dry wt), with the greatest difference observed between the closed buds and the opened buds (Figure 5). The flavonol variations may be explained by the presence of scales that fully enclosed the closed buds, whereas the scales remained only at the base of the developed buds.
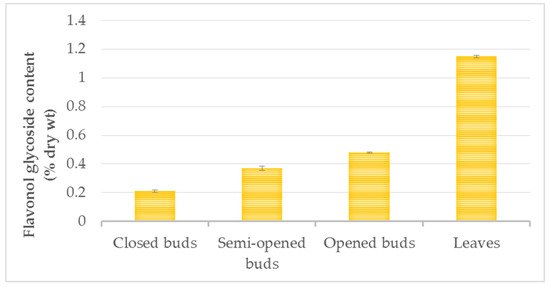
Figure 5.
Flavonol content (% dry wt) of three physiological states of ginkgo buds and summer leaves. Data are given as mean ± SD, replicates are n = 3 and p < 0.001.
The flavonol concentration continued to increase until the summer leaf stage, reaching levels of 1.15 ± 0.01% dry wt. The range of flavonol contents reported in the literature in control green leaves varied from 0.11 to 1.6% dry wt [11,30,31,32] in other plant materials and experimental conditions. Only one report in the literature has described the flavonol glycoside content in ginkgo buds [21]. The authors of that study examined the closed-bud stage and confirmed the presence of rutin, kaempferol and bilobetin. Acylflavonol glycosides have also been identified in buds [21].
3.4. Principal Components Analysis (PCA)
Comparison of the dynamics of the accumulation of both flavonol glycosides and terpene trilactones from the closed-bud stage to the summer leaf stage revealed similar profiles for both types of components. However, the accumulated quantities were greater for flavonoid glycosides than for terpene trilactones, regardless of the physiological stage of development.
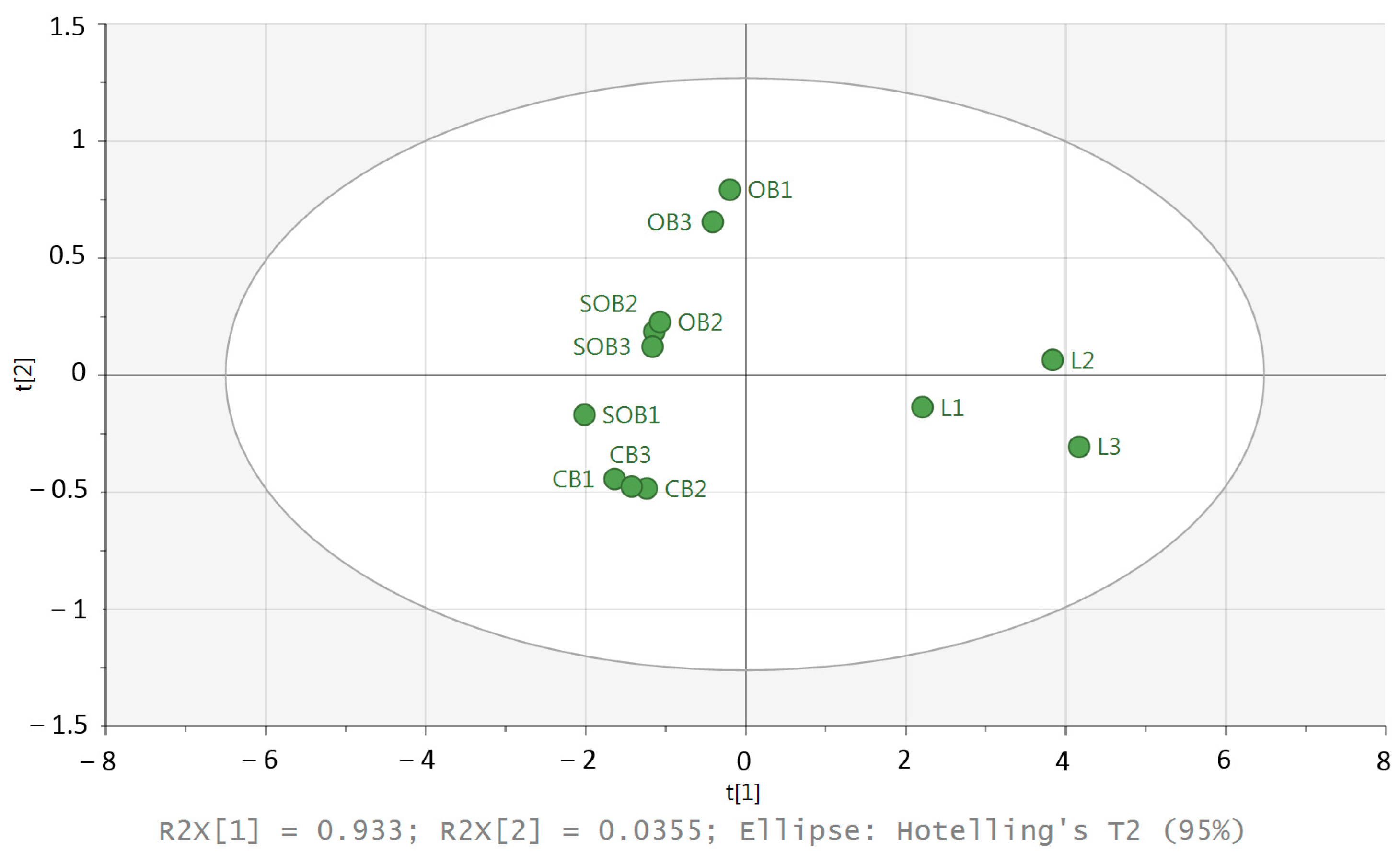
To identify groups of similar individuals in a dataset, principal components analysis (PCA) was chosen (Figure 6).
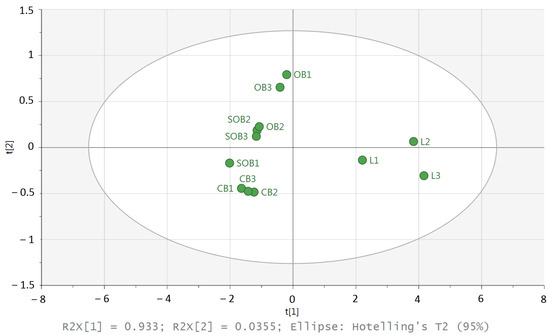
Figure 6.
PCA score plot of the G. biloba samples; L1–3 = leaves, OB1–3 = open buds, SOB1–3 = semi-open buds, CB1–3 = closed buds.
The PCA score plot revealed that the first and second principal axes accounted for 93.3% and 3.5% of the total variability, respectively. The score plot represents the proximity between the samples. The PCA ordination clearly discriminated buds and leaves. In addition, a weak variability between the three replicates of the leaves is visible. The leaves were located on the positive side of PC1, while the closed buds were located on the negative side of PC1, and a weak variability between the three replicates was visible. For the other samples (semi-open buds and open buds), the variability was higher than for the leaves and closed buds.
4. Conclusions
This study evaluated the terpene trilactone and flavonoid contents of three physiological development stages of G. biloba buds and leaves. The quantitative analysis of bilobalide and ginkgolides in the ginkgo buds and leaves was improved by using extracts obtained by ultrasonic-assisted extraction rather than by refluxing in oxidised water. The terpene trilactone concentrations varied with bud development, with contents of 1.07, 0.77 and 1.06 mg/g dry wt for the closed, semi-opened and opened buds, respectively. The contents reached a maximum of 3.75 mg/g dry wt in summer leaves.
The flavonol accumulation was calculated according to the European Pharmacopoeia requirements. The flavonol content increased from 0.21 ± 0.01% dry wt in the closed bud and reached its maximum in the summer leaves (1.15 ± 0.01% dry wt).
This paper provides the first significant phytochemical analysis of G. biloba bud extracts. Both flavonoids and terpene trilactones showed an ontogenetic dependence, as both phytochemical classes increased in quantity from the closed bud stage to the opened bud stage and reached their maximum at the summer leaf stage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nutraceuticals3010014/s1, Figure S1: Chemical structure of andrographolide. Figure S2: calibration curve for bilobalide (BB) quantification. Figure S3: calibration curve for Ginkgolide A (GA) quantification. Figure S4: calibration curve for Ginkgolide B (GB) quantification. Figure S5: calibration curve for Ginkgolide C (GC) quantification. Figure S6: LC-HR-ESI-MS chromatogram for standards reference in a fortified mixture. Figure S7: ESI-MS ionization patterns of terpene trilactones: bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and a reference standard, andrographolide.
Author Contributions
Conceptualization, D.L.-M., S.S. and J.M.; methodology, D.L.-M., S.S., A.K., R.S. and D.D.; formal analysis, D.L.-M., S.S., A.K., R.S. and D.D.; resources, J.M.; writing—original draft preparation, D.L.-M., S.S. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Lorraine.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to François Dupire in MassLor Plateform of L2CM in Lorraine University for LCMS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?otool=ifruhplib (accessed on 26 August 2022).
- van Beek, T.A.; Montoro, P. Chemical Analysis and Quality Control of Ginkgo Biloba Leaves, Extracts, and Phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef]
- Liu, X.-G.; Lu, X.; Gao, W.; Li, P.; Yang, H. Structure, Synthesis, Biosynthesis, and Activity of the Characteristic Compounds from Ginkgo biloba L. Nat. Prod. Rep. 2022, 39, 474–511. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; de Goulart, R.J.; Tofano, R.A.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo Biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Silva, H. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Savage, K.; Dowell, A.; Mouatt, P. Adulteration of Ginkgo Biloba Products and a Simple Method to Improve Its Detection. Phytomedicine 2014, 21, 912–918. [Google Scholar] [CrossRef]
- Global Ginkgo Biloba Extract Sales Market—Industry Reports. Available online: https://www.360researchreports.com/global-ginkgo-biloba-extract-sales-market-16698407 (accessed on 26 August 2022).
- Laurain, D.; Trémouillaux-Guiller, J.; Chénieux, J.-C.; van Beek, T.A. Production of Ginkgolide and Bilobalide in Transformed and Gametophyte Derived Cell Cultures of Ginkgo Biloba. Phytochemistry 1997, 46, 127–130. [Google Scholar] [CrossRef]
- Wang, J.; Cao, F.; Su, E.; Wu, C.; Zhao, L.; Ying, R. Improving Flavonoid Extraction from Ginkgo Biloba Leaves by Prefermentation Processing. J. Agric. Food Chem. 2013, 61, 5783–5791. [Google Scholar] [CrossRef]
- Strømgaard, K.; Nakanishi, K. Chemistry and Biology of Terpene Trilactones from Ginkgo Biloba. Angew. Chem. Int. Ed. 2004, 43, 1640–1658. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Liu, K.; Zhang, X.; Wang, X.; Dai, X.; Liang, Y.; Cao, Y.; Li, X. Bilobalide: A Review of Its Pharmacology, Pharmacokinetics, Toxicity, and Safety. Phytother. Res. 2021, 35, 6114–6130. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Goto, H.; Usuki, T. 1H-NMR Analysis of Terpene Trilactones (TTLs) in Ginkgo Biloba: Green Female Leaves Contain the Most TTLs. Phytochem. Anal. 2012, 23, 84–87. [Google Scholar] [CrossRef]
- Zheng, J.; Long, X.; Wang, X. Chemical Profiling and Anticoagulant Activity of Ginkgo Biloba Leaves under the Influence of Harvesting Time. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 55–62. [Google Scholar] [CrossRef]
- Sora, D.I.; Stefanescu, V.; David, V.; Medvedovici, A. Validation of an LC–MS/MS Assay of Terpene Trilactones in Ginkgo Biloba Extracts and Pharmaceutical Formulations through Standard Addition Method. J. Pharm. Biomed. Anal. 2009, 50, 459–468. [Google Scholar] [CrossRef]
- Czigle, S.; Tóth, J.; Jedlinszki, N.; Háznagy-Radnai, E.; Csupor, D.; Tekeľová, D. Ginkgo Biloba Food Supplements on the European Market—Adulteration Patterns Revealed by Quality Control of Selected Samples. Planta Med. 2018, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.J.; Kerns, S.P.; Aillon, K.; Mueller, G.; Rider, C.V.; DeRose, E.F.; London, R.E.; Harnly, J.M.; Waidyanatha, S. Comparison of Phytochemical Composition of Ginkgo Biloba Extracts Using a Combination of Non-Targeted and Targeted Analytical Approaches. Anal. Bioanal. Chem. 2020, 412, 6789–6809. [Google Scholar] [CrossRef]
- Walkowiak, A.; Wnuk, K.; Cyrankiewicz, M.; Kupcewicz, B. Discrimination of Adulterated Ginkgo Biloba Products Based on 2T2D Correlation Spectroscopy in UV-Vis Range. Molecules 2022, 27, 433. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, A.; Rietsch-Jako, L.; Haag-Berrurier, M.; Anton, R. Seasonal Variations of the Flavonoid Content from Ginkgo Biloba Leaves. Planta Med. 1991, 57, 430–433. [Google Scholar] [CrossRef]
- Ding, C.; Chen, E.; Lindsay, R.C. Natural Accumulation of Terpene Trilactones in Ginkgo Biloba Leaves: Variations by Gender, Age and Season. Eur. Food Res. Technol. 2007, 224, 615–621. [Google Scholar] [CrossRef]
- van Beek, T.A.; Lelyveld, G.P. Concentration of Ginkgolides and Bilobalide in Ginkgo Biloba Leaves in Relation to the Time of Year. Planta Med. 1992, 58, 413–416. [Google Scholar] [CrossRef]
- Kaur, P.; Chaudhary, A.; Singh, B. Gopichand Optimization of Extraction Technique and Validation of Developed RP-HPLC-ELSD Method for Determination of Terpene Trilactones in Ginkgo Biloba Leaves. J. Pharm. Biomed. Anal. 2009, 50, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Lichtblau, D.; Berger, J.M.; Nakanishi, K. Efficient Extraction of Ginkgolides and Bilobalide from Ginkgo Biloba Leaves. J. Nat. Prod. 2002, 65, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.; Fitzloff, J.F.; van Breemen, R.B. Liquid Chromatography-Electrospray Tandem Mass Spectrometry of Terpenoid Lactones in Ginkgo Biloba. J. Mass Spectrom. 2005, 40, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Wai, C.M.; Ang, C.Y.W.; Cui, Y.; Heinze, T.M.; Mattia, A.; Dinovi, M. Sample Preparation and Determination of Ginkgo Terpene Trilactones in Selected Beverage, Snack, and Dietary Supplement Products by Liquid Chromatography with Evaporative Light-Scattering Detection. J. AOAC Int. 2004, 87, 815–826. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.) 10th Edition. EDQM—European Directorate for the Quality of Medicines. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 10 February 2021).
- van Beek, T.A. Chemical Analysis of Ginkgo Biloba Leaves and Extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Gray, D.E.; Upton, R.; Chandra, A.; Porter, A.; Harris, R.K. Quantitative Analysis of Flavonol Glycosides in Ginkgo Biloba: A Comparison of Two Analytical Methods. Phytochem. Anal. 2006, 17, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; He, D.; Wang, T.; Han, J.; Li, Z.; Du, Y.; Zou, J.; Guo, M.; Tang, D. Separation and Characterization of Chemical Constituents in Ginkgo Biloba Extract by Off-Line Hydrophilic Interaction×reversed-Phase Two-Dimensional Liquid Chromatography Coupled with Quadrupole-Time of Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 146, 68–78. [Google Scholar] [CrossRef]
- Kobus, J.; Flaczyk, E.; Siger, A.; Nogala-Kałucka, M.; Korczak, J.; Pegg, R.B. Phenolic Compounds and Antioxidant Activity of Extracts of Ginkgo Leaves. Eur. J. Lipid Sci. Technol. 2009, 111, 1150–1160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).