Abstract
Several alternative proteins have emerged that may improve the environmental footprint of our food system. Evaluations into the impact of these protein sources on gastrointestinal health is limited. A study was performed to determine whether aqueous extracts from dietary protein sources, both traditional and alternative, had a differential impact on a leaky gut cell culture model. Aqueous extracts of soybean meal, fish meal, Cyberlindnera jadinii, Saccharomyces sp., Bio-Mos, Chlorella pyrenoidosa, Methylobacterium extorquens, Escherichia coli, and Hermetia illucens were administered onto a Caco-2/THP-1 co-culture and the transepithelial electrical resistance (TEER) and IL-1β, IL-6, IL-8, IL-10, TNF-α, CXCL10, and MCP-1 concentrations, and NF-κB activity were determined. Principal components analysis and K means clustering were performed. Three clusters were identified: one for soybean meal, one for bacterial meals, and one for the remaining sources. The bacterial meal cluster exhibited pro-inflammatory properties, i.e., correlated with TNF-α, IL-1β, IL-8, and NF-κB. The soybean meal cluster exhibited both pro- and anti-inflammatory properties, whereas the third cluster containing the remaining proteins exhibited anti-inflammatory properties (correlated with TEER and IL-10). These results suggest that aqueous extracts from yeast proteins contribute more positively, and bacterial proteins contribute the least positively, towards intestinal health in a leaky gut model.
1. Introduction
A range of alternative protein sources have emerged in recent years to reduce the environmental footprint of food animal production. Many of these proteins have also seen applications within human foods as well. Alternative protein products include single cell proteins and insect meals. However, dietary ingredients can play a critical role in gastrointestinal health. In the case of animal livestock production, this may be particularly crucial as livestock production transitions towards the removal of antibiotics. Often, detrimental substances that are present in protein sources may be removed by processing, and, in other instances, pathogenesis is age- and species-dependent. However, bioactive substances have also been found originating from protein products that may be beneficial towards gastrointestinal health. Animal-derived proteins such as fish meal or spray-dried plasma protein are generally regarded to be of high quality and may in some instances confer functional benefits. Nevertheless, plant proteins, and soy in particular, are often seen as the gold standard from a price–performance standpoint and are common in protein-enriched food products. Some evidence of bioactive properties in soy extracts has also been reported, e.g., isoflavones [1]. Nonetheless, there are scenarios wherein even soy inclusion is limited. Soy products may contain the presence of anti-nutritional factors in the form of lectins, non-starch polysaccharides and oligosaccharides. Weanling pigs exhibit a transient hypersensitivity and fish such as Atlantic salmon (Salmo salar), zebrafish (Danio rerio), and Rainbow trout (Oncorhynchus mykiss) are susceptible to soybean meal-induced enteritis [2,3,4,5,6]. Soy is also considered one of the major food allergens among humans [1].
Alternative protein sources are viewed as potentially environmentally-friendly protein sources. In particular, single cell proteins (yeast, bacteria, and algae) and insect meals are gaining commercial traction, e.g., Calysta (Menlo Park, CA, USA) or Innovafeed (Nesle, France). Bacterial proteins may be propagated, i.e., grown, on methane or be a by-product from amino acid/enzyme production [7,8]. Insect meals such as black soldier fly larvae and crickets, as well as microalgae and yeast, are also environmentally-friendly alternatives [9]. New protein sources are regularly evaluated for nutritional quality, and some have been evaluated for functional properties [9]. However, these evaluations are often limited in scope and rarely compare similar endpoints across different sources.
Common to many gastrointestinal disorders is the dysregulation of the intestinal epithelial barrier [10]. When the intestinal barrier function is disrupted, the trafficking of molecules is no longer under control, so that luminal contents may enter the lamina propria and activate the immune system, thereby leading to uncontrolled immune responses (a process known as ‘leaky gut’) [10]. The intestinal epithelial barrier is formed by intercellular tight junctions, a complex protein–protein network that mechanically links adjacent cells and seals the intercellular space. Therefore, the intestinal epithelial barrier controls the equilibrium between immune tolerance and immune activation, and so it has a prominent role in ‘leaky gut’ pathogenesis. The improper functioning or regulation of these tight junctions seems to be responsible for larger intercellular spaces allowing luminal element passage through the barrier, with a consecutive local and systemic inflammation [10].
Various in vitro models of intestinal permeability exist. The human colonic Caco-2 cell line is a common method of evaluating the impact of various products and molecules on intestinal barrier function [10]. Species-specific cell lines are also used, e.g., intestinal porcine enterocyte cell line (IPEC). Different techniques are then deployed to induce intestinal permeability such as dextran sodium sulfate (DSS) to mimic colitis. However, DSS often induces rapid and severe development of colitis and loss of barrier function that does not resemble inflammatory bowel disease (IBD) [11]. Another technique is through coculture of Caco-2 cell line with the human acute monocytic leukemia THP-1 cell line, which has been differentiated to activated macrophages [12,13]. Indeed, this model has been shown to disrupt barrier function and elicit a cytokine and chemokine profile similar to that associated with IBD [12,13,14]. Therefore, this coculture approach has been suggested as an effective model for screening drugs or food substances against irritable bowel syndrome or leaky gut [12]. The particular benefit of the coculture approach is that it can capture abnormal activation of immune cells and resulting epithelial cell damage as would be observed in inflammatory bowel diseases.
The aim of this study was to perform an initial in vitro investigation on the potential bioactive effects of soluble extracts from nine protein products on intestinal health in terms of modulation of a ‘leaky gut’ under inflammatory conditions, and to allow for a comparative ranking of protein sources.
2. Materials and Methods
2.1. Products and Aqueous Extracts
Nine traditional and alternative protein products were sourced and samples (between 2 and 100 g) were received in August of 2020 (Table 1). They were maintained at ambient temperature and humidity until testing. Soybean meal (48% crude protein) and fish meal (62% crude protein) can be considered the incumbent traditional proteins representing plant and animal protein, respectively. Cyberlindnera jadinii (yeast), Saccharomyces sp. (yeast), Chlorella pyrenoidosa (microalgae), Methylobacterium extorquens (bacteria), Escherichia coli (bacteria), and Hermetia illucens larvae (black soldier fly) were included as presentations of single cell and insect proteins, respectively. The feed additive Bio-Mos was included, although it is not considered a protein source per se, but it is marketed as a product to maintain gastrointestinal health and integrity in production animals. All products except for Black Soldier Fly were powders and were tested without further processing or refinement. The non-powder product, whole Black Soldier Fly larvae, was ground down to a powder using a mortar and pestle. Products were weighed out and dissolved in dH2O at a concentration of 32 mg/mL and mixed for 1 h at room temperature. Samples were centrifuged for 5 min at 2655× g and the supernatant was collected. Finally, the supernatant was diluted to 60% in Caco-2 complete medium, obtaining a final concentration of 19.2 mg/mL of initial product. This concentration was selected based off of previous experimentation with torula yeast, showing no cytotoxicity at this concentration (unpublished data). Proximate composition of products was determined at Eurofins Labs (Madison, WI, USA) (Table 1). Protein contents of the extracts were determined by Pierce BCA assay following the manufacturer’s recommendations (ThermoFisher, Waltham, MA, USA). Total solids were determined by weighing the solid content of 1 mL of extract after lyophilization for 24 h.

Table 1.
Proximate composition of traditional and alternative protein products and aqueous extracts.
2.2. Caco-2/THP1-Blue™ Co-Culture
The co-culture experiment was performed as previously described by Daguet et al. [13]. Briefly, Caco-2 cells (HTB-37; American Type Culture Collection) and THP1-Blue™ cells (InvivoGen Europe, Toulouse, France) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing glucose and glutamine and supplemented with HEPES and 20% (v/v) heat-inactivated (HI) fetal bovine serum (FBS) and Roswell Park Memorial Institute (RPMI)1640 medium containing glucose and glutamine, supplemented with HEPES, sodium pyruvate, and 10% (v/v) HI-FBS, resp, at 37 °C in a humidified atmosphere of air/CO2 (95:5, v/v). For the co-culture experiment, Caco-2 cells were seeded in 24-well semi-permeable inserts (Millicell Hanging cell culture insert, 0.4 µm, Merck KGaA, Darmstadt, Germany) and cultured for 14 days, with three medium changes/week, until a functional cell monolayer with a transepithelial electrical resistance (TEER) of more than 300 Ω.cm2 was obtained by using an Epithelial Volt-Ohm meter Millicell ERS-2 (Merck KGaA, Darmstadt, Germany). In addition, THP1-Blue™ cells were seeded in 24-well plates and treated with 100 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, Merck KgaA, Darmstadt, Germany) for 48 h prior to the co-culture. Before the start of the co-culture, TEER of the Caco-2 monolayers was measured (=0 h time point). Then, Caco-2-bearing inserts were placed on top of the PMA-differentiated THP1-Blue™ cells and treated apically for 24 h with the products. After 24 h, TEER was measured again (=24 h time point) and cells were restimulated at the basolateral side with 500 ng/mL of ultrapure LPS (Escherichia coli K12, InvivoGen Europe, Toulouse, France). Cells were also treated apically with 12 mM Sodium butyrate (NaB) as a positive control. Sodium butyrate has been reported to have a protective effect on barrier function and has been observed to increase TEER [15]. Complete medium-only, with no protein product addition, was considered the negative control. After 6 h of LPS stimulation, basolateral supernatants were collected for cytokine measurement (human IL-1β, IL-6, IL-8, IL-10, TNF-α, CXCL10 and MCP-1 by ProcartaPlex Immunoassays (Thermo Fisher Scientific, Life Technologies Europe BV, Merelbeke, Belgium) using a MAGPIX® system (xMAP instrument, Luminex Corporation) according to the manufacturers’ instructions. Basolateral supernatants were also measured for NF-κB activity; they were measured as secreted embryonic alkaline phosphatase activity in the stably transfected THP1-Blue™ cells using the QUANTI-Blue reagent (InvivoGen Europe, Toulouse, France). Cells were also stimulated with LPS in combination with 1 μM hydrocortisone (HC), as a known immunosuppressant, and medium without LPS (LPS-) as additional quality controls. All treatments were performed in triplicate.
2.3. Statistics
All products were taken as technical replicates (n = 3) in the cell assay. The CM/LPS+ control was included in the statistical comparison. Differences between treatments were compared, using a one-way ANOVA with Tukey’s multiple comparisons test. Significance was declared at p < 0.05. A principal components analysis and K means clustering were performed. The two components with the highest eigenvalues were retained. Three clusters were selected after visual inspection. All statistics were performed using JMP 15.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. TEER
Quality control assessments were confirmed as TEER was reduced with only complete medium and TEER increased with the administration of NaB (Table 2). Nine different products were applied at the apical side to the Caco-2 cells for 24 h, while in the basolateral compartment, activated THP1 cells were added. All products numerically increased the TEER compared to the CM control, and were able to maintain the TEER at its initial levels, although soybean meal and E. coli were not significantly different from the CM control (Table 3). Moreover, torula yeast, dried yeast, Chlorella, fish meal, black soldier fly, Methylobacterium, and Bio-Mos significantly increased the TEER, compared to the initial value.

Table 2.
Quality control measurements for the leaky gut cell culture model (Caco-2/THP-1). LPS positive control was stimulated with LPS, but no protein was administered.

Table 3.
Effects of various protein sources on transepithelial electrical resistance (TEER) and IL-6, IL-10, IL-1β, TNF-α, IL-8, CXCL10, MCP-1, and NF-κB production in a leaky gut cell culture model (Caco-2/THP-1) after LPS stimulation. LPS positive control was stimulated with LPS, but no protein was administered. p values of one-way ANOVA described as p > F; values within a column that do not share a letter are significantly different (p < 0.05). SEM is the standard error of the mean.
3.2. Immune Markers
Quality control assessments were confirmed as LPS stimulated an immune response, but this response was attenuated with the inclusion of HC (Table 2). Significant differences across treatments were observed for all cytokines/chemokines (p < 0.05). Amongst the traditional protein products, fish meal administration induced significantly lower levels of IL-6, IL-10, CXCL10, and MCP-1 production (p < 0.05). Whereas, soybean meal only induced significantly higher levels of IL-10 compared with the LPS control (p < 0.05). No other cytokines were significantly different from the control.
Amongst the bacterial meals, E. coli induced significantly higher levels of IL-1β and significantly lower levels of IL-6, CXCL10, and MCP-1 compared with the LPS control (p < 0.05). Whereas, Methylobacterium induced significantly higher levels of IL-1β and IL-8 and significantly lower levels of CXCL10 and MCP-1 compared with the LPS control (p < 0.05). No other cytokines/chemokines were significantly different from the control.
Torula yeast and dried yeast both significantly lowered NF-κB, IL-6, IL-10, CXCL10, and MCP-1 production compared with the LPS control (p < 0.05). Bio-Mos had a similar cytokine profile to the other yeasts; however, it did not have a significantly different NF-κB production from the LPS control and IL-1β was significantly higher (p < 0.05). Chlorella significantly lowered IL-6, CXCL10, and MCP-1 production, whereas black soldier fly lowered NF-κB, IL-6, CXCL10, and MCP-1 production (p < 0.05).
3.3. Principal Components Analysis and K Means Clustering
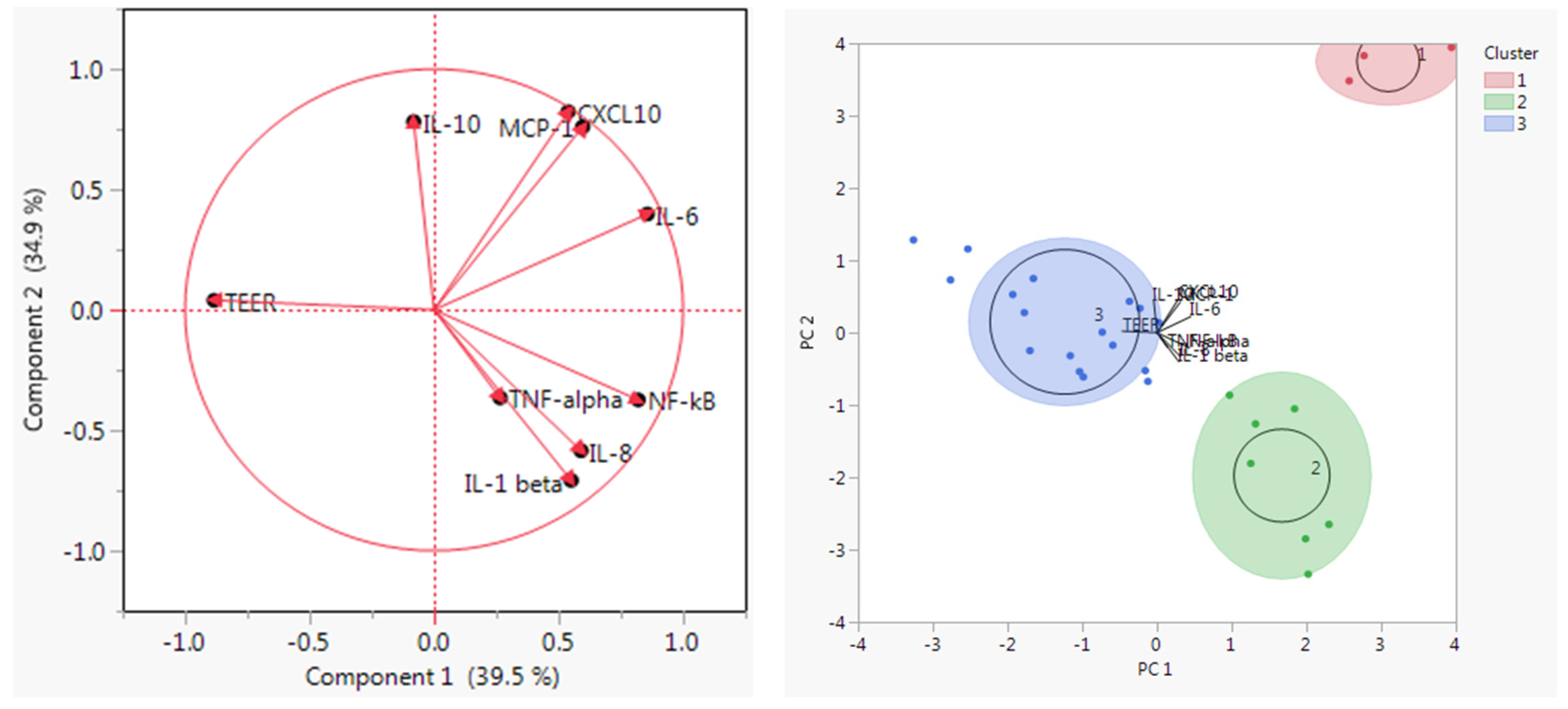
A principal cluster analysis and K means clustering revealed three distinct clusters (Figure 1): one for soybean meal, one for bacterial meals, and one for the remaining protein sources. The eigenvalue for component 1 was 3.56 and for component 2 was 3.14. The bacterial meal cluster was associated with higher levels of pro-inflammatory cytokines and the lowest TEER values. Soybean meal exhibited both pro- and anti-inflammatory features. The remaining products within cluster 3 had generally neutral or anti-inflammatory properties and higher TEER values, and yeasts were more prominently anti-inflammatory.
Figure 1.
Principal Components Analysis and K means clustering. The principal components were selected based on the two highest eigenvalues and explained 74.4% of the variability. Three distinct clusters were detected after visual inspection: the red cluster (1) contained soybean meal, the green cluster (2) contained the bacterial meals, and the blue cluster (3) contained the yeast, algae, fish meal, and insect meal.
4. Discussion
Dietary ingredients may play a critical role in gastrointestinal health and, in some instances, may contribute towards the development or resolution of gastrointestinal disorders. Three clusters were identified: one for soybean meal, one for bacterial meals, and one for the remaining products (Figure 1). Soybean meal was unique in its significant production of CXCL-10 and MCP-1, and low protective effects on intestinal permeability as measured by TEER. However, the highest IL-10 values were observed with soybean meal. The soy extracts used herein had the highest total solids content of the tested products. Although the measured protein content of the extracts was high, it is clear that there is a significant amount of non-protein constituents present, including soluble non-starch polysaccharides. Weanling pigs exhibit a transient hypersensitivity to soy due to the presence of β-conglycinin, glycinin, and agglutinins, wherein growth is impaired, diarrhea incidences increase, and intestinal permeability increases [2,3,6,16,17]. Several fish species exhibit a soybean-meal induced enteritis and/or intestinal permeability that may be used as a leaky gut model [5,18,19]. The supplementation of certain soy components, e.g., isoflavones, has been observed to improve animal health and performance [20,21]. However, evaluation of soybean meal on a mouse small intestinal organoid monolayer did not reveal differentially expressed genes associated with gastrointestinal health [22]. Whereas, spray-dried porcine plasma revealed differentially expressed genes related to wound healing within the same organoid monolayer [22]. However, soy protein concentrate showed a protective effect on H2O2-induced cell death in Caco-2 culture [23].
Bacterial meals are promising protein sources due to their high crude protein content and lower environmental impact. Bacterial meals are typically the spent products from synthetic amino acid or enzyme production, or purpose-grown meals from natural gas [7,8]. The bacterial cluster was pro-inflammatory, i.e., it correlated well with TNF-α, IL-1β, IL-8, and NF-κB. It is likely that the soluble cell wall components, e.g., LPS, play a role in the response. The soluble protein content in both bacterial products was low relative to the total solids (~5%). There is little research on the impact of inactivated bacterial biomass on gastrointestinal health in mammals. In swine, for example, it is reported that after weaning, only up to 10% of the bacteria in the intestines are Gram negative [24,25]. Similarly, dietary LPS altered inflammation and permeability in mice [26,27], and in Caco-2 cells [26,28].
Bacterial products may also have a beneficial impact on gut function. Romarheim et al. [7] suggest that the inclusion of a methanotroph may alleviate soybean meal-induced enteritis in Atlantic salmon. However, benefits were most pronounced once soluble cell wall fractions were removed. This may suggest that bacterial cell wall fractions may attenuate positive elements, e.g., antimicrobial peptides, found within certain bacterial meals. In the current study, both the Methylobacterium and E. coli exhibited significant pro-inflammatory effects. After treatment with Methylobacterium, IL 1β and IL-8 were induced, while IL-10 was strongly reduced. Moreover, E. coli increased IL-1β, TNF-α, and IL-8. As the soluble fractions of the bacterial meals were tested herein, it is likely that detrimental components reside within the soluble fraction, in support of the results by Romarheim et al. [7]. It may also be likely that digestive processes could sufficiently degrade these cell wall components.
The third major cluster contained the yeast, microalgae, fish meal, and black soldier fly products. All test products protected from inflammation-induced intestinal epithelial barrier disruption. There was no observed correlation between proximate composition or extract composition on inflammatory response. The highest total solids content of the extracts was observed with fish meal and resulted in a neutral inflammatory response, while the yeast products spanned the spectrum of protein concentrations. Nonetheless, the most beneficial effects were observed after treatment with yeast, wherein the strongest correlations with TEER and IL-10 were observed with yeast. Yeasts are also believed to improve gastrointestinal health through a variety of mechanisms including pathogen binding, immune modulation, and microbiome modulation (via prebiotic effects) [29]. The lack of a microbiota within this model precludes pathogen binding and microbiome modulation as a mode of action, but does not preclude immune modulation. Yeasts exerted anti-inflammatory properties through cytokine IL-10, and by reducing the secretion of the pro-inflammatory cytokines and chemokines. The resulting improved TEER may be a consequence of the resolution of this inflammatory process. Yeast cell wall fractions are considered potent immunomodulators and pathogen binders [30]. Herein, we observed a relatively high contribution of soluble protein as a proportion to total solids relative to the other protein products, but it is unclear whether this is a driver of the observed effect. They have regularly been shown to increase cytokine release, including IL-1, IL-2, IL-6, and TNF-α, and to enhance the immune response. So, in this regard, the present results run contrary to what is the reported consensus for yeast. Lee et al. [31], however, reported that the inclusion of the yeast cell wall reduced inflammation, as evidenced by lower serum TNF-α and IL-1β in weaner pigs, as well as increased expression of tight junction proteins. Torula yeast has also been reported to reduce the severity of soybean meal-induced enteritis in Atlantic salmon [32,33]. The model used herein is intended to represent a disease state, and the observed anti-inflammatory response may reflect the resolution of the inflammatory response.
Microalgae, fish meal, and insect meal all exhibited similar effects to the yeast products, though less pronounced. Microalgae (e.g., Spirulina, Chlorella, and Chlamydomonas) have been shown to exert positive effects in various IBS and GI distress models through antioxidative and/or anti-inflammatory properties [17,26,34,35,36]. Furbeyre et al. [35] indicated that Spirulina, but not Chlorella, reduced the incidence of diarrhea in weaning pigs, though no changes to local inflammation were observed. There have been reports of anti-oxidative and anti-inflammatory properties in mealworm, cricket, and locust protein, as well, due to bioactive peptides [37]. Kar et al. [22] reported that the inclusion of black soldier fly larvae may have beneficial effects on swine intestinal microbiota and gastrointestinal health. It is likely that the presence of lauric acid (C12:0) may contribute extensively towards the positive gut health impacts of insect meals [38]. To that end, the extent of oil removal during insect processing may dictate the efficacy of intestinal health benefits of insect proteins. As such, within the present study, a fully fatted black soldier fly product was used.
5. Conclusions
In conclusion, the results reported herein suggest that extracts from yeasts contribute most positively towards improved outcomes in a challenge model and bacteria the least. However, critical elements remain to be elucidated. The present study evaluated aqueous extracts and did not include insoluble components, nor the impacts of gastrointestinal digestion, as well their effects when administered with whole or complex meals. Further characterization of the protein sources is needed to determine which components exert a bioactive function in those proteins eliciting a strong positive or negative effect. Lastly, the cell culture model used was a human leaky gut model and more refined assessments, e.g., in vivo assessments, will be needed to evaluate species-specific effects. Nonetheless, these data provide an initial ranking of proteins for their potential bioactive impacts on gastrointestinal health.
Author Contributions
M.M., P.V.d.A., L.V. and R.D.E. designed the research; L.V. conducted the research; L.V. and J.D.M. analyzed data; and M.M., L.V. and R.D.E. wrote the paper. R.D.E. had primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Arbiom Inc., Durham, North Carolina, USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
R.D.E. is an employee of Arbiom Inc., a company that develops and markets microbial proteins. R.D.E. was involved in the planning, analysis, and writing of this work. All other authors declare no conflicts of interest.
References
- Hassan, S.M. Soybean, Nutrition and Health. In Soybean—Bio-Active Compounds; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Li, D.F.; Nelssen, J.L.; Reddy, P.G.; Blecha, F.; Hancock, J.D.; Allee, G.L.; Goodband, R.D.; Klemm, R.D. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 1990, 68, 1790–1799. [Google Scholar] [CrossRef]
- Engle, M.J. The role of soybean meal hypersensitivity in postweaning lag and diarrhea in piglets. J. Swine Health Prod. 1994, 2, 7–10. [Google Scholar]
- Merrifield, D.L.; Dimitroglou, A.; Bradley, G.; Baker, R.T.; Davies, S.J. Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2009, 32, 755–766. [Google Scholar] [CrossRef]
- Hedrera, M.I.; Galdames, J.A.; Jimenez-Reyes, M.F.; Reyes, A.E.; Avendaño-Herrera, R.; Romero, J.; Feijóo, C.G. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS ONE 2013, 8, e69983. [Google Scholar] [CrossRef]
- Zheng, L.; Duarte, M.E.; Loftus, A.S.; Kim, S.W. Intestinal Health of Pigs Upon Weaning: Challenges and Nutritional Intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Øverland, M.; Mydland, L.T.; Skrede, A.; Landsverk, T. Bacteria Grown on Natural Gas Prevent Soybean Meal-Induced Enteritis in Atlantic Salmon. J. Nutr. 2011, 141, 124–130. [Google Scholar] [CrossRef]
- Almeida, F.N.; Sulabo, R.C.; Stein, H.H. Amino acid digestibility and concentration of digestible and metabolizable energy in a threonine biomass product fed to weanling pigs. J. Anim. Sci. 2014, 92, 4540–4546. [Google Scholar] [CrossRef]
- Ornan, E.M.; Reifen, R. Revisiting Protein Quality Assessment to Include Alternative Proteins. Foods 2022, 11, 3740. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2003, 28, 619. [Google Scholar] [CrossRef]
- Cochran, K.E.; Lamson, N.G.; Whitehead, K.A. Expanding the utility of the dextran sulfate sdium (DSS) mouse model to induce a clinically relevant loss of intestinal barrier function. Peer J. 2020, 8, e8681. [Google Scholar] [CrossRef]
- Satsu, H.; Ishimoto, Y.; Nakano, T.; Mochizuki, T.; Iwanaga, T.; Shimizu, M. Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp. Cell Res. 2006, 312, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosachharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Possemiers, S.; Pinheiro, I.; Verhelst, A.; Abbeele, P.V.D.; Maignien, L.; Laukens, D.; Reeves, S.G.; Robinson, L.E.; Raas, T.; Schneider, Y.-J.; et al. A Dried Yeast Fermentate Selectively Modulates both the Luminal and Mucosal Gut Microbiota and Protects against Inflammation, As Studied in an Integrated In Vitro Approach. J. Agric. Food Chem. 2013, 61, 9380–9392. [Google Scholar]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Kumar, V.; Hossain, M.S.; Ragaza, J.A.; Rubio Benito, M. The Potential Impacts of Soy Protein on Fish Gut Health, Soybean for Human Consumption and Animal Feed. In Soybean for Human Consumption and Animal Feed; Sudarić, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Sealey, W.M.; Barrows, F.T.; Smith, C.E.; Overturf, K.; LaPatra, S.E. Soybean meal level and probiotics in first feeding fry diets alter the ability of rainbow trout Oncorhynchus mykiss to utilize high levels of soybean meal during grow-out. Aquaculture 2009, 293, 195–203. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, H.; Zhang, B.; Xing, K.; Guo, Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018, 8, 5161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef]
- Kar, S.K.; van der Hee, B.; Loonen, L.M.P.; Taverne, N.; Taverne-Thiele, J.J.; Schokker, D.; Smits, M.A.; Jansman, A.J.M.; Wells, J.M. Effects of undigested protein-rich ingredients on polarised small intestinal organoid monolayers. J. Anim. Sci. Biotechnol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Wopperer, A.L.; Chrisfield, B.J.; Tao, L.; Cooper, T.K.; Vanamala, J.; Elias, R.J.; Hayes, J.E.; Lambert, J.D. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J. Nutr. Biochem. 2017, 40, 201–208. [Google Scholar] [CrossRef]
- Leser, T.D.; Amenuvor, J.Z.; Jensen, T.K.; Lindecrona, R.H.; Boye, M.; Møller, K. Culture-Independent Analysis of Gut Bacteria: The Pig Gastrointestinal Tract Microbiota Revisited. Appl. Environ. Microbiol. 2002, 68, 673–690. [Google Scholar] [CrossRef]
- Knecht, D.; Cholewińska, P.; Jankowska-Mąkosa, A.; Katarzyna, C. Development of Swine’s Digestive Tract Microbiota and Its Relation to Production Indices—A Review. Animals 2020, 10, 527. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, S.; Feng, G.; Wu, L.; Feng, Y.; Guo, T.; Yang, Y.; Wu, H.; Zeng, M. Microalgae aqueous extracts exert intestinal protective effects in Caco-2 cells and dextran sodium sulphate-induced mouse colitis. Food Funct. 2020, 11, 1098–1109. [Google Scholar] [CrossRef]
- Lindenberg, F.C.; Ellekilde, M.; Thörn, A.C.; Kihl, P.; Larsen, C.S.; Hansen, C.H.; Metzdorff, S.B.; Aalbæk, B.; Hansen, A.K. Dietary LPS traces influences disease expression of the diet-induced obese mouse. Res. Vet. Sci. 2019, 123, 195–203. [Google Scholar] [CrossRef]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Shurson, G. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed. Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Kogan, G.; Kocher, A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest. Sci. 2007, 109, 161–165. [Google Scholar] [CrossRef]
- Lee, J.; Kyoung, H.; Cho, J.; Choe, J.; Kim, Y.; Liu, Y.; Kang, J.; Lee, H.; Kim, H.; Song, M. Dietary Yeast Cell Wall Improves Growth Performance and Prevents of Diarrhea of Weaned Pigs by Enhancing Gut Health and Anti-Inflammatory Immune Responses. Animals 2021, 11, 2269. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida utilis and Chlorella vulgaris counteract intestinal inflammation in Atlantic salmon (Salmo salar L.). PLoS ONE 2013, 8, e83213. [Google Scholar] [CrossRef]
- Reveco-Urzua, F.E.; Hofossæter, M.; Rao Kovi, M.; Mydland, L.T.; Ånestad, R.; Sørby, R.; Press, C.M.; Lagos, L.; Øverland, M. Candida utilis yeast as a functional protein source for Atlantic salmon (Salmo salar L.): Local intestinal tissue and plasma proteome responses. PLoS ONE 2019, 14, e0218360. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).