Abstract
(1) The island of Mayotte, located in the Indian Ocean, possesses remarkable biodiversity. In a previous ethnobotanical study, we already highlighted 69 different plant species used in traditional medicine. Among those, 21 were traditionally employed for skin care by the local populations. The present study aimed to better understand the traditional use of those plants by investigating their in vitro biological activities and, more specifically, their anti-lipoxygenase, antioxidant and anti-tyrosinase properties. (2) These three activities were, respectively, determined by lipoxygenase inhibition, DPPH-reducing potency, and anti-tyrosinase activity assays. (3) Results revealed high biological activities for several plant species, with some of them displaying one strong single activity, while others had at the same time anti-lipoxygenase, antioxidant and anti-tyrosinase effects. (4) Those in vitro biological activities agreed with the traditional use of those plants by the local population. It also highlights the high potential of those species from Mayotte in the development of new cosmetic ingredients for the treatment of many skin affections, such as eczema.
1. Introduction
The island of Mayotte is part of the Comoros archipelago located in the Indian Ocean. One of the particularities of this island is its localization in a geographical area where the climate is appropriate for the development of exceptional biodiversity. Indeed, it has been reported that 25% of the earth’s biodiversity is present in the Indian Ocean area [1]. It was around 1820 that the interest in tropical islands from a botanical point of view began to grow, as De Candolle [2] notably suggested that “…‘Islands’ flora is worth studying due to the peculiar characteristics shown by their vegetation or to the fact that the study areas are clearly defined, thus the studies can be done thoroughly…”. Since then, this affirmation has been confirmed many times in numerous insular areas of the world. However, Mayotte was not one of them, as very little research has been done about this small island [2].
On the other hand, the history of the island of Mayotte and, notably, the successive settlement of people from African Bantu and Arab-Muslim origins [3] explains that the use of traditional medicine is still very common and deeply ingrained in the habits of the inhabitants [4,5,6]. Indeed, as these people have a strong bond to their cultural heritage, traditional cosmetic practices are still used nowadays. The conservation of this knowledge is based on an oral transmission that passes from one generation to another [7,8]. Mayotte has, therefore, much to offer when it comes to ethnobotanical studies [9].
During a previous ethnobotanical field study on the Island of Mayotte, we identified 69 plants used in traditional medicine for the treatment of many different conditions [10]. Among these plants, we noted a large number of uses related to skin care, whether to treat skin problems or for cosmetic purposes [10]. As the skin is the first protective layer of the human body, it withstands all types of stress, either endogenous, such as the effect of aging, or exogenous, such as sun exposition. These stresses are responsible for many skin imperfections and afflictions [11]. It did not take long for humanity to understand that these deleterious effects could be thwarted using what nature has to offer. Therefore, plants have been used to heal but also for aesthetic uses through makeup and perfume for as long as humanity can remember. These practices are deeply linked to man’s evolution [12,13].
In the present work, we focused more particularly on 21 plants traditionally used for skin care by the inhabitants of Mayotte based on our previous ethnobotanical study. Indeed, pharmaceutical industries are continuously searching for new bioactive molecules. The main purpose of the present study was, therefore, to investigate the potential of these plants for use as an ingredient in cosmetics through the study of three targeted biological activities: anti-lipoxygenase activity linked to anti-inflammatory properties, antioxidant activity, and anti-tyrosinase activity related to the treatment of pigmentation issues.
The inflammatory triggers can be divided into infections, tissue injuries, as well as tissue stress and malfunction. In the present work, we will focus on the second and third types of affection since the anti-inflammatory properties are meant to be paired with cosmetic practices. Among the different pathways linked to the appearance of inflammatory reactions, the research for lipoxygenase (LOX)-inhibitory compounds is crucial as LOX is responsible for the synthesis of leukotrienes, which are pro-inflammatory molecules [14]. Concerning antioxidant activity, we focused on the reaction involving DPPH (2,2-Diphenyl-1-picrylhydrazyl), which has previously been described as a good indicator of the antioxidative power [15]. This specific test assay indeed detects the scavenging potency of free radicals through the scavenging activity of the stable DPPH free radical. Finally, the tyrosinase inhibition capacity of plant extracts was used to evaluate their potential to treat skin pigmentation issues. Indeed, tyrosinase is the key enzyme in the biosynthesis of melanin that plays a major protective role against skin photocarcinogenesis, such as melanoma. Melanin is also responsible for phenomenon known as hyperpigmentation and hypopigmentation, often visible in the form of darker or lighter spots, zones or body parts [16]. The search for natural chemical agents capable of modulating pigmentation metabolism is, therefore, of great interest.
2. Materials and Methods
2.1. Reagents
Acetone (≥99%, technical), methanol (≥98.5%, technical), K2HPO4, KH2PO4, pure ethanol, NaOH, and H3BO3 were purchased from VWR chemicals (Leuven, Belgium). DPPH (2,2-Diphenyl-1-picrylhydrazyl), mushroom tyrosinase (EC 1.14.18.1), kojic acid, TROLOX ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), linoleic acid, Tween 80, L-DOPA (3,4-dihydroxiphenilalanine) and Glycine max lipoxygenase (EC 1.13.11.12) were purchased from Sigma-Aldrich (Darmstadt, Germany).
2.2. Plant Extracts Preparation
Based on the results of the previous ethnobotanical survey conducted by M. Saive in 2014 on the island of Mayotte [10], 21 different plant species were collected, and a specimen was stored for each sample at the CBNM herbarium in Mayotte [10]. All available organs at the time of the harvest were collected, leading to a total of 89 samples (see Table 1).

Table 1.
Recapitulative table presenting the acetone extracts obtained from the different plant species and organs with the biological activities investigated.
All samples were dried at 40 ± 1 °C in a drying oven for 48 h. When the vegetal material allowed it, the samples were powdered using an analytical grinder IKA A11 (Staufen, Germany). The global size of the ligneous parts of the vegetal material was reduced using shears. Once the size allowed it, the samples were then ground using a laboratory hammermill mounted with a 6 mm mesh. The ground samples were kept vacuum-packed at −22 ± 1 °C until extracted. The extraction process was carried out using a Soxhlet apparatus with acetone as a solvent. Acetone is indeed recognized as a solvent of choice for the extraction of bioactive compounds and, notably, antioxidants [17]. Two g of dried and ground samples were weighed and poured into the extraction chamber. Thirty mL of acetone was used [18], and the extraction lasted for 6 h. Once the extraction was completed, the crude extract was evaporated using a rotary evaporator (Heidolph, Laborota 4003, Schwabach, Germany). The dried crude extracts were kept at −22 ± 1 °C until used.
2.3. Lipoxygenase Inhibition Evaluation
The lipoxygenase inhibition capacity of plant extracts obtained by Soxhlet extraction with acetone was assessed by a spectrophotometric method described by Tanoh et al. (2019) with some minor modifications [19].
Lipoxygenase is sensitive to heat; therefore, all the following experimental steps were performed on an ice bath. A 0.1 mg/mL lipoxygenase solution (≥50,000 U/mg) was prepared using distilled water. The 1 mM linoleic acid substrate solution was prepared as follows: 140 mg of linoleic acid, 18 mg of Tween 80, and 100 µL of NaOH 5 mM were mixed and adjusted to 50 mL with distillate water. The enzyme solution, as well as the substrate, were divided into 1 mL aliquots and stored at −22 ± 1 °C until used, with only one thaw-out allowed.
As the expected product for the reaction was 13-HPOD, the optimal pH of the reaction matrix was obtained using a 0.1 M sodium borate buffer corrected to pH 9.5 using 5 M NaOH. To avoid false-positive results due to enzyme inactivation caused by acetone, pure ethyl alcohol was used for sample preparation. Five mL of pure ethanol was added to the dry extracts, which were then submitted to sonication and subsequently filtered using 0.45 µm PTFE syringe filters (Whatman, Puradisc 13, Maidstone, UK). As the color of the crude extracts was an issue, all samples were diluted in the borate buffer from factor 101 to factor 104. As oxygen is a co-substrate for the reaction, the borate solution was oxygenated for 30 min at room temperature prior to the reaction. The control was realized using 0.1 M borate buffer, leading to a non-inhibited reaction, which can be expressed as 0% of inhibition, and the blank was obtained by carrying out the experiment without the enzyme, which causes the absence of 13-HPOD synthesis and can be considered as 100% of inhibition.
The enzymatic reaction was performed by mixing 100 µL of plant sample with 35 µL of lipoxygenase and 800 µL of the oxygenated buffer. For the control, 900 µL of the oxygenated buffer was used. For the blank, 900 µL of oxygenated buffer was used, but no enzyme was added prior to the incubation. The mixtures were left to incubate for 15 min at room temperature and then added into quartz absorption cells along with 35 µL of the substrate solution. The synthesis of linoleic acid hydroperoxide was observed in real time as absorbance was measured every 20 s for 5 min at 234 nm. Each sample/control was done in triplicate. The relative activity was obtained by putting the slopes of the samples against the slopes of the control, according to Equation (1):
where the control slope is the slope of the control (0% inhibition) at the linear part of the reaction, and the sample slope is the slope of the samples at the linear part of the reaction, defined by an R2 higher than 0.9.
2.4. DPPH-Reducing Potency Evaluation
The evaluation of the DPPH-reducing potency of the plant samples was based on a protocol adapted from M. S. Blois (1958) [20]. A 2 × 10−4 M DPPH solution was prepared using technical-grade methanol. Once prepared, this solution was kept at 4 ± 1 °C in the dark and was used within the following 7 days.
The positive control for this test was a 2 × 10−3 M TROLOX solution prepared with methanol. The blank was likewise performed with methanol. For the sample analysis, 5 mL of methanol was added to the dry extracts, which were obtained by Soxhlet extraction with acetone. The crude extract was retrieved by sonication. The solution was then filtered using 0.45 µm PTFE syringe filter (Whatman, Puradisc 13, Maidstone, UK) and submitted to successive dilutions (10 to 10,000 times) in methanol. The analysis went as follows. A 1:1 DPPH:sample/blank/TROLOX mix was performed and left to incubate at room temperature for 10 min. Then the absorbance of the reaction mix was observed using an Ultrospec 9000 UV/Vis spectrophotometer from Biochrom (Cambridge, UK) at 517 nm. Each sample was measured in triplicate. The samples’ relative activity was established by comparing the final absorbance with the absorbance obtained with the blank and with the TROLOX, as seen in Equation (2):
where Abs TROLOX refers to the absorption at 517 nm of the positive control (100% of activity), and Abs BLANK refers to the absorption at 517 nm of the negative control (0% of activity). The corrected value is obtained by Equation (3):
where the final abs is equal to the absorbance (517 nm) of the reaction mix after 10 min, and the sample abs is equal to the absorbance (517 nm) of the sample at the studied concentration.
2.5. Anti-Tyrosinase Activity Evaluation
To evaluate the anti-tyrosinase activity of the different plant extracts obtained by Soxhlet extraction with acetone, the protocol used was based on and adapted from Rangkadilok et al. (2007) [21].
When working with tyrosinase, all reagents and solutions were kept on an ice bath to avoid any loss of activity due to temperature-linked enzyme degradation. A 625 U/mL tyrosinase solution was obtained by adding 4 mL of K3PO4 0.05 M pH 6.5 buffer directly into the commercial vial containing the enzyme (25.000 U) and by vortexing it for 10 s. One mL was retrieved and diluted in the same buffer to reach the required concentration. The total solution was divided into 1 mL aliquots stored at −22 ± 1 °C until used. The aliquots were only thawed once. A 1 mM L-DOPA solution was prepared using distilled water. For this analysis, the inhibition control was a 1 mM kojic acid solution in distilled water, and the blank was pure K3PO4 0.05 M pH 6.5 buffer. As for the lipoxygenase evaluation, pure ethyl alcohol was used for the sample preparation. Five mL of pure ethyl alcohol was added to the dry extracts and submitted to sonication. The solution was then filtered using a 0.45 µm PTFE syringe filter (Whatman, Puradisc 13, Maidstone, UK) and submitted to successive dilutions (from 10 to 10,000 times) in K3PO4 0.05 M pH 6.5 buffer. As oxygen is a co-substrate for the reaction, the buffer was oxygenated for 30 min at room temperature prior to the observation of the reaction.
The enzymatic reaction was performed by mixing 100 µL of sample/blank/control with 50 µL of tyrosinase and 250 µL of the oxygenated buffer. The mixture was left to incubate for 15 min at room temperature and then added into a quartz absorption cell along with 400 µL of the substrate solution. The synthesis of dopachrome was observed in real time, and absorbance was measured every 15 s for 5 min at 475 nm. Each sample/control/blank was carried out in triplicate. The relative activity was obtained according to Equation (4):
where the blank slope is the slope of the blank (0% inhibition) at the linear part of the reaction, and the sample slope is the slope of the samples at the linear part of the reaction, defined by an R2 higher than 0.9.
2.6. Statistical Analysis
For enzymatic inhibition assays, the relative activity was determined using the absorbance slope at its maximum intensity. For the DPPH-reducing potency evaluation assay, the relative activity was based on the absorbance variation after a 30-min reaction period at room temperature. In that case, the relative activity was obtained when comparing the positive control (TROLOX) with the sample results. Each assay was carried out in triplicate. For each activity and dilution, all samples were compared two by two using Tukey’s grouping test (Statistical software Minitab 19 Minitab, State College, PA, USA) with the following parameters: the null hypothesis was that all averages were equal, the significance limit was α = 0.05, the confidence interval was bilateral, and the error rate for the comparison was 5. This allowed the identification of samples or groups of samples showing significantly higher relative activities in comparison to the sample batch.
3. Results and Discussion
3.1. Anti-Lipoxygenase Activity Evaluation
A total of 77 out of the 89 collected plant samples were tested for their ability to inhibit lipoxygenase activity, which therefore indicates their anti-inflammatory potential. The remaining samples were not included in this assay as they showed a strong absorbance at 234 nm. Serial dilutions of plant extracts were performed, allowing us to determine which species presents the highest relative activity and, therefore, which species contains the highest amount of active compounds. Moreover, species showing results above the limit of quantification (LOQ) up to the 104 dilutions are expected to contain several active compounds in their crude extracts or one compound with a high degree of efficiency. The LOQ was established using the relative standard deviation (% RSD) of control replicates immediately after the reagents were thawed. Those %RSD values were not higher than 10%, which is in concordance with standard procedures [22,23,24].
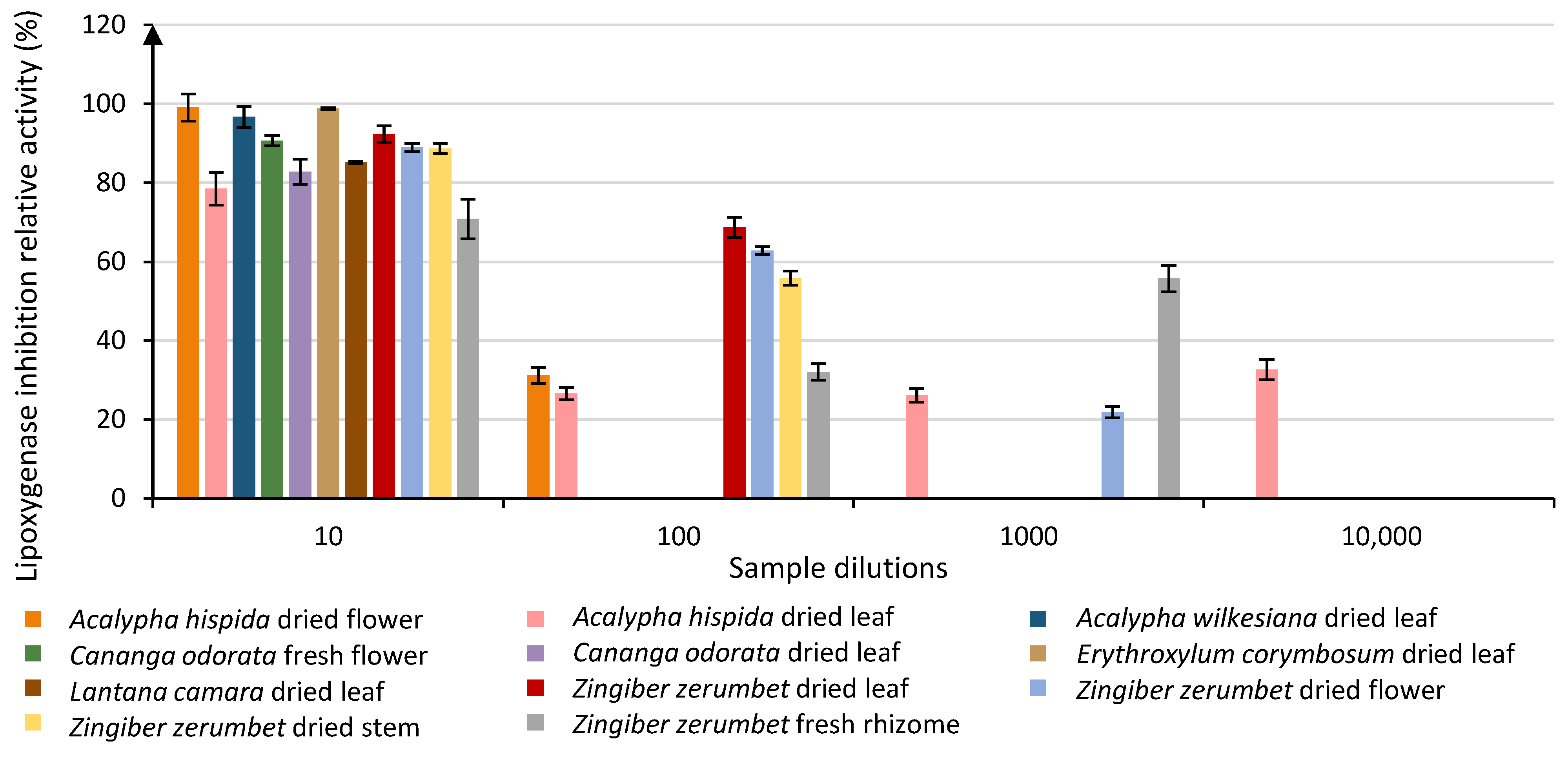
Out of the 77 samples, 17 absorbed too much at the first dilution but entered the evaluation process starting at the second dilution. Results showed that 27 plant extract samples were able to inhibit the lipoxygenase activity above the LOQ at a dilution of 101. Out of those 27 + 17 samples, 9 were still above the LOQ at a dilution of 102. Three of these were still above LOQ at a dilution of 103, and only 1 sample remained above the LOQ at a dilution of 104 (Table 2). Results (Figure 1 and Table 2) highlighted that the 27 plant extracts investigated for their anti-lipoxygenase activity at a dilution of 101 can be classified into 17 different groups. Some of these extracts displayed strong anti-lipoxygenase activities up to 99.05%, and 2 species particularly stand out as being only classified in the group with the highest relative activity (group A): Acalypha hispida dried flower (99.05% ± 4.22) and Erythroxylum corymbosum dried leaf (98.83% ± 2.91). Results (Table 2) also showed that samples with the highest relative activities, which are classified in group A even if there is some crossing over up to group D, ranged from 90.64% to 99.05% of relative activity. These samples can be considered the most effective ones. For the plant extracts diluted 102 times, there is a drop-in activity implying a dose-dependent response as only 9 samples were above the LOQ. Out of these 9 samples, 5 groups were observed. The first group (group A) contained two samples with the following activities: 68.73% ± 3.18 (Zingiber zerumbet dried leaf) and 62.84% ± 1.26 (Zingiber zerumbet dried flower). Interestingly enough, samples from groups A and B only came from one plant species, Zingiber zerumbet, but from different organs of that plant. When looking at the plant extracts diluted 103 times, only 3 samples remained, which were classified into 2 groups, A and B. At this level of dilution, the only sample that was classified in group A was zingiber zerumbet fresh rhizome with 55.73% ± 7.40 of relative activity. Among the samples from this dilution, one that was not considered in the most effective category in the two first dilutions still showed activity over the LOQ: Acalypha hispida dried leaf. Interestingly, only that extract remained above the LOQ with 32.62% ± 2.58 of relative activity at a dilution of 104.

Table 2.
Lipoxygenase inhibition activity results for the plant extracts diluted 10, 100 and 1000 times (n = 3). Results were statistically analyzed using Tukey’s grouping test (confidence level: 95%), and the observed group classifications (A, B, C……etc.) are shown in the present table.
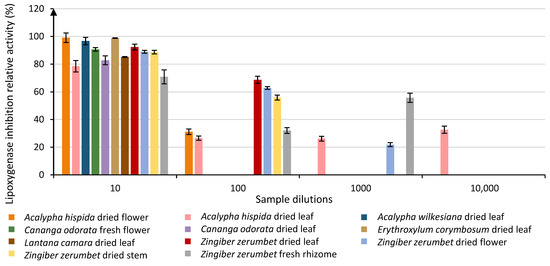
Figure 1.
Evolution of the relative lipoxygenase inhibition activity of the most efficient plant extract samples through 4 different dilutions (10, 100, 1000 and 10,000). Each column represents the mean ± SD (n = 3) per sample.
Through the different dilutions, a dose-response change can be observed for most plant extract samples (Figure 1) in their relative activities, except for Acalypha hispida dried leaves. This specific sample is indeed among the most active ones in the first dilution, though it then loses more than 50% of relative activity at the second dilution. It then remains around 30% of activity for the second, third and fourth dilutions as if the inhibition was caused by several compounds, some of these loosing activity as the dilution increases, while others are not impacted by the dilution. There is no simple explanation for these results, as the study of the enzyme inhibition potency of crude plant extracts containing many different molecules is not an easy process. Indeed, these molecules have many interaction options throughout the whole analysis process. They can react with one another but also with the substrate or with the enzyme. In the case of an enzyme interaction, several inhibition processes exist (competitive inhibition, noncompetitive inhibition, uncompetitive inhibition and mixed inhibition). Depending on the type of inhibition, the maximal speed of the reaction, the affinity between the enzyme and its substrate or both can be affected. As the inhibition evaluation was evaluated through kinetic observations, the inhibition phenomenon can also be affected by the incubation time. In addition, it can be due to other regulation modes and not be specifically linked to the enzyme inhibition process. All these phenomena render the study of inhibition arduous, and this is even more so when using an inhibition media as complex as a plant crude extract. This can lead to unexpected observations, such as the one observed for Acalypha hispida where the dilution does not seem to affect the results. Only an in-depth study of the different inhibition mechanisms occurring for this specific sample could help understand such a phenomenon. It is primordial to limit the uses of these results as a primary indication in a screening context.
3.2. Antioxidant Activity (DPPH Test)
The antioxidant properties of the collected plant samples were evaluated through the DPPH test. The average values were compared two by two and grouped using Tukey’s test. %RSD on replicates were not higher than 10%, which is in concordance with standard procedures [22,23,24].
For plant extracts diluted 101 times, 77 out of the 89 samples were above the LOQ. One could not be analyzed at this specific concentration as it impeded with the detector at the given wavelength (517 nm); the latter was re-integrated in the measurements for the following dilutions. These 77 samples were classified into 25 different groups (Table 3), and the group with the highest activity (Group A) showed activities ranging from 94.00% to 99.97%. Only one sample was only classified in that group: Erythroxylum corymbosum dried leaf (99.97% ± 0.06), as the other samples in group A presented some crossing over up to group L. At that dilution, a total of 26 samples were classified in Group A, which can be explained by the presence of compounds with known antioxidant properties, such as polyphenols, in many plants. The second and third dilutions will therefore allow a more precise classification of these samples.

Table 3.
Antioxidant activity results (DPPH assay) for the plant extracts diluted 10, 100 and 1000 times (n = 3). Results were statistically analyzed using Tukey’s grouping test (confidence level: 95%), and the observed group classifications (A, B, C……etc.) are shown in the present table.
Out of the initial samples and with the addition of the one removed from the plant extracts diluted 10 times, 56 plant extracts diluted 102 times remained above the LOQ. These samples were divided into 23 groups, and 19 samples were classified into group A, showing activities ranging from 92.58% to 98.76%. Only one sample was just in group A: Acalypha hispida fresh leaf (98.76% ± 1.08), as the others presented some crossing over up to group G (activities ranging from 86.63% up to 92.95%).
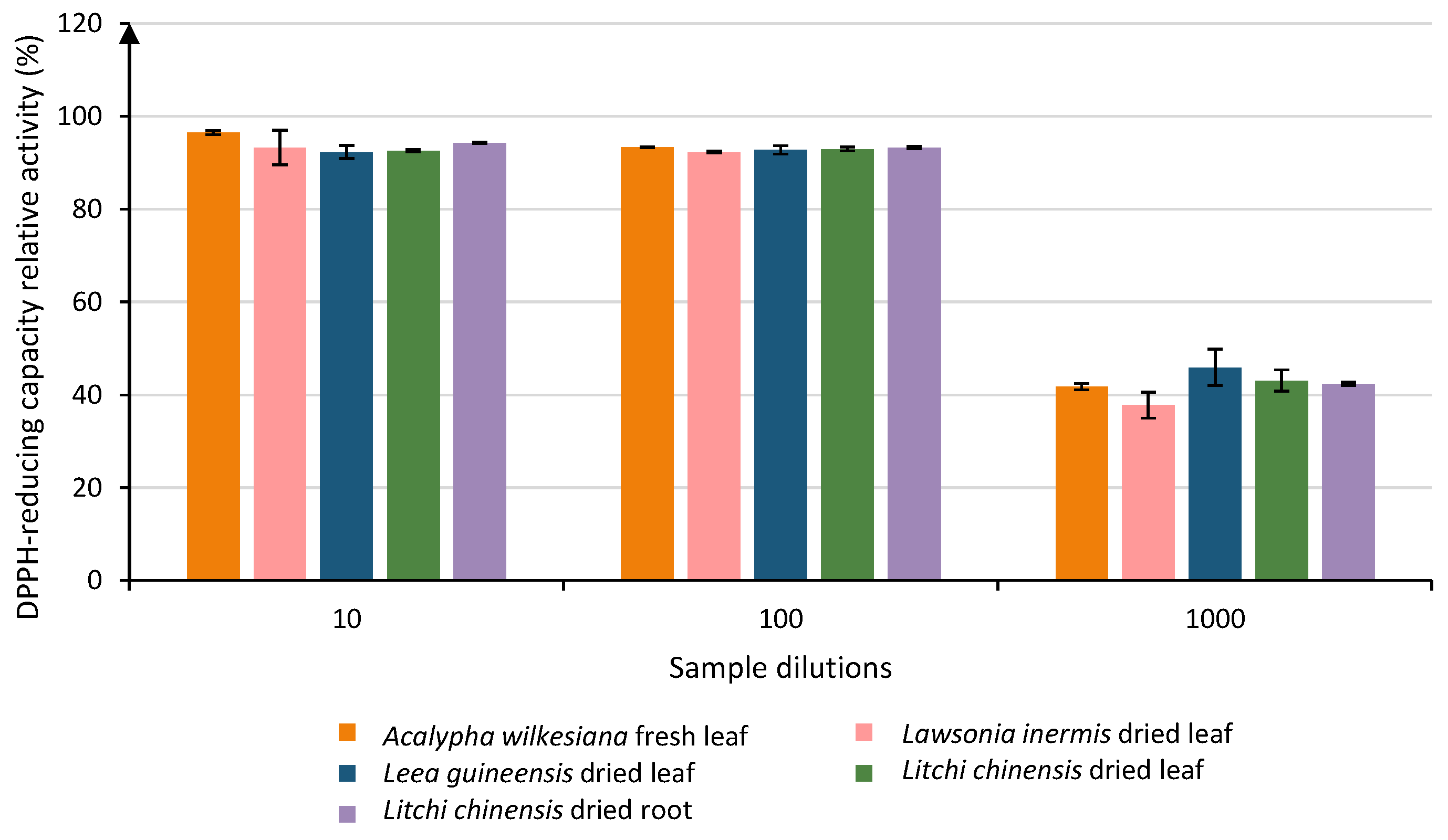
When looking at the plant extracts diluted 103 times, 21 samples remained above the LOQ and were classified into 9 groups. The group with the highest activities (Group A) contained 4 samples, of which 2 were only in group A: Leea guineensis dried leaf (45.91% ± 3.89) and Litchi chinensis dried leaf (43.10% ± 2.25). The other samples in group A presented some crossing over up to group B (activities ranging from 37.80% to 42.39%). A total of 5 samples were gathered among those high-relative-activity groups: Lawsonia inermis dried leaf, Acalypha wilkesiana fresh leaf, Litchi chinensis dried root, Litchi chinensis dried leaf, and Leea guineensis dried leaf (Figure 2).
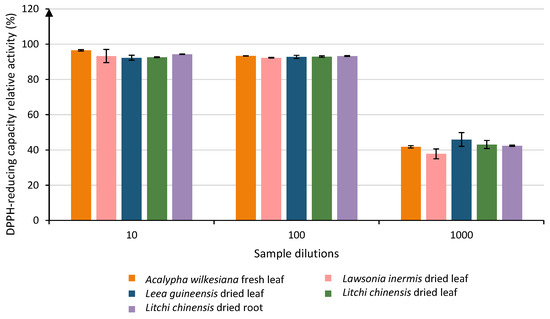
Figure 2.
Evolution of the relative DPPH-reducing capacity of the five most efficient samples at 3 different dilutions (101, 102 and 103). Each column represents the mean ± SD (n = 3) per sample.
Finally, no sample diluted 104 times showed results above the LOQ.
The present results, therefore, allowed the identification of plant species of interest for the isolation of antioxidant molecules. However, it should be noted that other tests could also be considered because of the complexity of oxidative stress and especially what might happen in the human organism.
3.3. Anti-Tyrosinase Activity
The anti-tyrosinase activity of the collected plant samples was evaluated through the observation of tyrosinase inhibition capacity. The activity averages of all samples were compared two by two and grouped using Tukey’s test. The observed limit %RSD to establish the validity of the results was based in concordance with standard procedures, this being a %RSD of 10 [22,23,24]. Out of the 89 samples tested at four different dilutions (samples were respectively diluted 101, 102, 103 and 104 times), 6 samples could not be tested using the anti-tyrosinase protocol, as even when strongly diluted, they strongly absorbed at 475 nm, impeding an accurate reading.
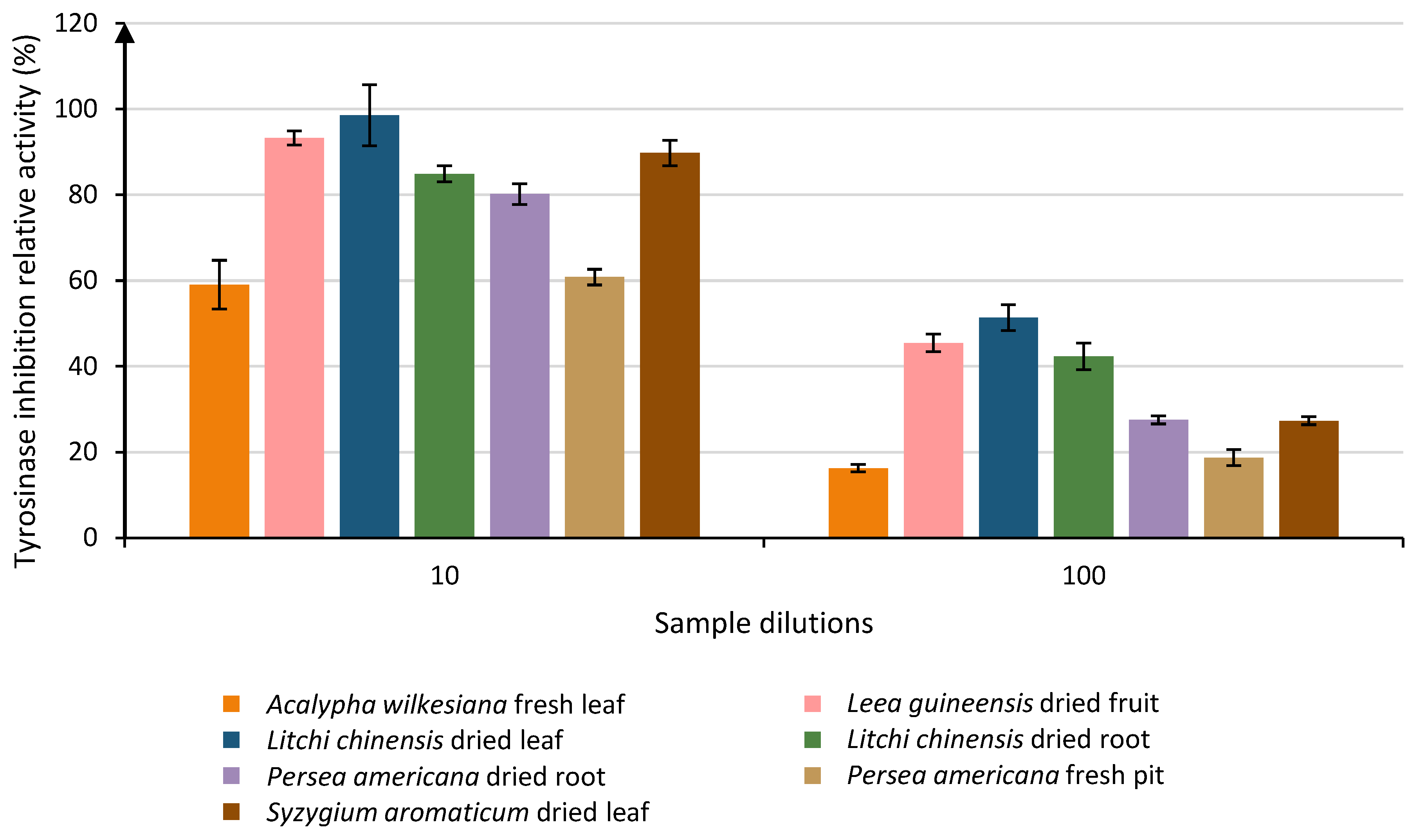
For the 52 tested sample extracts diluted 101 times, Tukey’s test highlighted 22 different groups above the LOQ (Table 4). Five samples were classified into the group with the best activity (Group A) and ranged from 89.74% to 98.57% of relative activity. Only one sample was only in this group: Litchi chinensis dried leaf (98.57% ± 7.09), as the others presented some crossing over up to group D (activities ranging from 80.07% to 89.82%). Together these groups counted for a total of 11 samples with interesting anti-tyrosinase potential.

Table 4.
Anti-tyrosinase activity results for the plant extracts diluted 10 and 100 times (n = 3). Results were statistically analyzed using Tukey’s grouping test (confidence level: 95%), and the observed group classifications (A, B, C……etc.) are shown in the present table.
When the samples were diluted 102 times, 7 samples remained above the LOQ. These samples were divided into 4 groups, and only one sample was classified into the group with the best activity (Group A): Litchi chinensis dried leaf (51.36% ± 3.00). Group B contained Leea guineensis dried fruit (45.45% ± 2.08) and Litchi chinensis dried root (42.29% ± 3.11). Groups C and D had activities ranging from 16.26% to 27.49%. The two other dilutions did not allow for any significant observations. The evolution of the activity of the most active sample is shown in Figure 3.
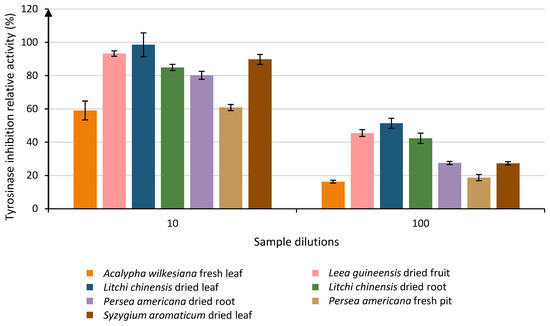
Figure 3.
Evolution of the relative tyrosinase inhibition activities of the most efficient samples through 2 different dilutions of 10 and 100 that were above the LOQ. Each column represents the mean ± SD (n = 3) per sample.
3.4. Comparison of the Different Activities
In the present work, different organs of different plant species collected in Mayotte were tested for their in vitro anti-lipoxygenase, DPPH-reducing and anti-tyrosinase activities. While the results presented above showed the high potential of certain plant extracts for a single specific activity, the results (Table 5) also highlighted some plant samples displaying more than one interesting activity. Such observations may help in the selection of species for further works. Based on the limit of the analytical methods, the samples showing the best potency for a specific activity were compared with the two other activities.
Therefore, the tested plants can be classified in two categories: the ones that have one strong biological activity, such as Lawsonia inermis and Zingiber zerumbet, and the others that show multiple biological properties. In this second category, different plants can be highlighted, such as Acalypha hispida and Litchi chinensis. The plant species presented here are of high interest for the isolation of novel natural molecules from which originates the determined in vitro activities and that may be incorporated into cosmetic products. Interestingly, Saive et al. (2020) already identified a proanthocyanidin that is responsible for the anti-tyrosinase and antioxidant properties of Litchi chinensis root. In the same way, different compounds, notably belonging to the procyanidin and catechin families, were previously highlighted as interesting antioxidant molecules in Persea americana pit [25]. In addition, zerumbone, a sesquiterpenoid, has already been identified in the fresh rhizome of Zingiber zerumbet and is recognized for its anti-inflammatory properties [26]. By continuing in this perspective, new molecules could be identified and valorized for health purposes.

Table 5.
Recapitulative table presenting a comparison of the extracts obtained from different plant species and organs for their investigated biological activities. The comparison is based on the plant organ extracts diluted 100 times for the 3 activities. + the plant organ extract has between 10% and 30% activity, ++ the plant organ extract has an activity superior to 30%, +++ the plant organ extract has an activity that is among the 3 best at the highest dilution, n/a means not applicable.
Table 5.
Recapitulative table presenting a comparison of the extracts obtained from different plant species and organs for their investigated biological activities. The comparison is based on the plant organ extracts diluted 100 times for the 3 activities. + the plant organ extract has between 10% and 30% activity, ++ the plant organ extract has an activity superior to 30%, +++ the plant organ extract has an activity that is among the 3 best at the highest dilution, n/a means not applicable.
| Sample | Status of the Plant Species in Mayotte | Anti-Lipoxygenase Activity | DPPH-Reducing Agent | Anti-Tyrosinase Activity |
|---|---|---|---|---|
| Acalypha hispida dried flower | Cultivated [27] | ++ | ++ | n/a |
| Acalypha hispida dried leaf | +++ | ++ | n/a | |
| Acalypha wilkesiana dried leaf | Cultivated [27] | n/a | ++ | n/a |
| Acalypha wilkesiana fresh leaf | n/a | ++ | + | |
| Cananga odorata dried leaf | Cultivated [10] | n/a | ++ | n/a |
| Cananga odorata fresh flower | n/a | ++ | n/a | |
| Erythroxylum corymbosum dried leaf | Indigenous [28] | n/a | ++ | n/a |
| Lantana camara dried leaf | Exotic [10] | n/a | ++ | n/a |
| Lawsonia inermis dried leaf | Cultivated [10] | n/a | ++ | n/a |
| Leea guineensis dried fruit | Indigenous [10] | n/a | ++ | +++ |
| Leea guineensis dried leaf | n/a | +++ | n/a | |
| Litchi chinensis dried leaf | Cultivated [10] | + | +++ | +++ |
| Litchi chinensis dried root | n/a | +++ | +++ | |
| Persea americana dried root | Cultivated [10] | n/a | ++ | + |
| Persea americana fresh pit | n/a | ++ | + | |
| Persea americana dried pit | + | ++ | n/a | |
| Zingiber zerumbet dried flower | Exotic [10] | +++ | + | n/a |
| Zingiber zerumbet dried leaf | ++ | n/a | n/a | |
| Zingiber zerumbet dried stem | ++ | n/a | n/a | |
| Zingiber zerumbet fresh rhizome | +++ | n/a | n/a |
4. Conclusions
On the basis of previous ethnobotanical research in Mayotte, 21 different plants were selected for their potential as skin care agents. Different organs of these were collected and tested in vitro for their capacity to reduce the stable free radical molecule DPPH to inhibit the activity of tyrosinase and/or lipoxygenase in order to better understand why these plants are used in traditional medicine and to discover new biological sources of natural skin care agents.
When looking at the perspectives for future research, two interesting facts stood out. Firstly, 10 promising species were identified based on their in vitro biological activity for skin care. Among these species, some showed yet-unknown biological activities. These specific species could enter future research targeting activities such as topical anti-inflammatory activities. Such plants would be of interest in the development of treatments against dermatitis/eczema and could possibly help to replace the common use of corticosteroids. Secondly, some other species did not show an outstanding specific activity but an average potency for several activities, leading to more generalist applications such as anti-ageing and pigmentation issues or for a relieving balm. When looking at these two points of view, both are of interest: a strong, unique activity or a mild intensity but for multiple activities.
In conclusion, this study highlighted the interesting potential of many plants from Mayotte for their incorporation in cosmetic formulations. Among the 10 plant species of high interest highlighted in the present work, 6 are cultivated in Mayotte, 2 are indigenous to this area, and 2 are exotic. Further studies are now needed, including in vivo tests, in order to confirm the activities shown here in vitro. Additionally, toxicological tests also should be conducted to ensure their innocuity. More studies must also be initiated to identify other molecules of interest. Moreover, if some of these plants begin to be used extensively for their properties, it would also be necessary to set up conservation measures in order to avoid their disappearance. In that case, the target collected organ of the plant will be very important as the collection of fruits, as an example, will not lead to species disappearance, while more attention should be paid when roots or leaves are collected.
Author Contributions
Conceptualization, M.S. and M.-L.F.; Data curation, M.G., L.L., M.S. and C.M.; Formal analysis, M.S. and C.M.; Funding acquisition, M.-L.F.; Investigation, M.S. and C.M.; Methodology, M.S. and M.-L.F.; Project administration, M.-L.F.; Resources, M.-L.F.; Supervision, M.-L.F.; Validation, M.-L.F.; Visualization, M.G., L.L. and M.S.; Writing—original draft, M.G., L.L. and M.S.; Writing—review and editing, M.G., L.L. and M.-L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Danny Trisman and Thomas Bertrand for their efficient technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gurib-Fakim, A. Traditional roles and future prospects for medicinal plants in health care. Asian Biotechnol. Dev. Rev. 2011, 13, 77–83. [Google Scholar]
- Pascal, O. Plantes et forêts de Mayotte. In Collection Patrimoines Naturels; French National Museum: Paris, France, 2002. [Google Scholar]
- Liszkowski, H.D. Mayotte et les Comores: Escales sur la Route des Indes aux XVe et XVIIIe Siècles. In Collection Mémoires; Editions du Baobab: Mamoudzou, France, 2000. [Google Scholar]
- Saive, M.; Genva, M.; Istasse, T.; Frederich, M.; Maes, C.; Fauconnier, M.-L. Identification of a Proanthocyanidin from Litchi Chinensis Sonn. Root with Anti-Tyrosinase and Antioxidant Activity. Biomolecules 2020, 10, 1347. [Google Scholar] [CrossRef]
- Conco, W.Z. The African Bantu traditional practice of medicine: Some preliminary observations. Soc. Sci. Med. 1972, 6, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A. How Islam changed medicine. Br. Med. J. 2005, 331, 1486–1487. [Google Scholar] [CrossRef]
- Soidrou, S.H.; Mohamedb, N.A.; Abdellah Faraha, S.O.; Said Hassaneb, D.B. Ethnopharmacoligical investigation of five plants used in comorian folkloric medicine. Int. J. Phytopharm. 2013, 4, 230–236. [Google Scholar]
- Kaou, A.M.; Mahiou-Leddet, V.; Hutter, S.; Aïnouddine, S.; Hassani, S.; Yahaya, I.; Azas, N.; Ollivier, E. Antimalarial activity of crude extracts from nine African medicinal plants. J. Ethnopharmacol. 2008, 116, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Boullet, V. Index de la flore Vasculaire de Mayotte (Trachéophytes): Statuts, Menaces et Protections. Version 2016.1. Conservatoire Botanique National de Mascarin, Antenne de Mayotte, Coconi. Available online: http://floremaore.cbnm.org (accessed on 9 September 2022).
- Saive, M.; Frederich, M.; Fauconnier, M.-L. Plants used in traditional medicine and cosmetics in Mayotte Island (France): An ethnobotanical study. Indian J. Tradit. Knowl. 2018, 17, 645–653. [Google Scholar]
- Rogers, J.; Harding, C.; Mayo, A.; Banks, J.; Rawlings, A. Stratum corneum lipids: The effect of ageing and the seasons. Arch. Dermatol. Res. 1996, 288, 765–770. [Google Scholar] [CrossRef]
- Edwards, S.; Nebel, S.; Heinrich, M. Questionnaire surveys: Methodological and epistemological problems for field-based ethnopharmacologists. J. Ethnopharmacol. 2005, 100, 30–36. [Google Scholar] [CrossRef]
- Weller, S.C. Cultural consensus theory: Applications and frequently asked questions. Field Methods 2007, 19, 339–368. [Google Scholar] [CrossRef]
- Charles, P.; Elliott, M.J.; Davis, D.; Potter, A.; Kalden, J.R.; Antoni, C.; Ferdinand, C.; Smolen, J.S.; Eberl, G.; Feldmann, M.; et al. Regulation of Cytokines, Cytokine Inhibitors, and Acute-Phase Proteins Following Anti-TNF-α Therapy in Rheumatoid Arthritis. J. Immunol. 1999, 163, 1521–1528. [Google Scholar] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Tanoh, E.A.; Nea, F.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, F.Z.; Fauconnier, M. Antioxidant and Lipoxygenase Inhibitory Activities and Zanthoxylum psammophilum Ake Assi. Molecules 2019, 24, 2445. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- National Association of Testing Authorities, Australia (NATA). Guidelines for the Validation and Verification of Quantitative and Qualitative Test Methods; National Association of Testing Authorities: Sydney, Australia, 2012. [Google Scholar]
- National Association of Testing Authorities, Australia (NATA). Technical Note 17 Guidelines for the Validation and Verification of Quantitative and Qualitative Test Methods; National Association of Testing Authorities: Sydney, Australia, 2013. [Google Scholar]
- Sengul, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2015, 24, 56–62. [Google Scholar] [CrossRef]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.Y.; Huang, S.K.H.; Lee, C.J.; Tsai, P.W.; Wang, C.C. Antinociceptive and anti-inflammatory effects of zerumbone against mono-iodoacetate-induced arthritis. Int. J. Mol. Sci. 2016, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.M.; Cardiel, J.M.; Levin, G.A. Nomenclatural review of Acalypha (Euphorbiaceae) of the Western Indian Ocean Region (Madagascar, the Comoros Archipelago, the Mascarene Islands and the Seychelles Archipelago). PhytoKeys 2018, 116, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Pascal, O.; Labat, J.-N.; Pignal, M.; Soumille, O. Systematics and Geography of Plants. In Plant Systematics and Phytogeography for the Understanding of African Biodiversity; Botanic Garden Meise: Meise, Belgium, 2001; Volume 71, p. 1101. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).