Abstract
Solanum lycopersicum and Moringa oleifera are two essential nutraceutical-containing plants from two different families, and are sources of abundant metabolites. They have a variety of applications in medicines, functional food additives and even water purification. This review aims to complement earlier reviews by comparing the metabolite profiles and modern-day pharmacological relevance of both plants. The metabolome of Moringa oleifera was compared to that of Solanum lycopersicum, to evaluate the common metabolites found within the two plants and how these compounds can be used for same pharmacological and nutritional benefits. While these plants contain similar metabolites, they also contain different compounds of the same class that differ in terms of their biological functions. In such instances, Moringa oleifera and Solanum lycopersicum may have similar applications, but remain distinguishable from each other in terms of pharmacological potential.
1. Introduction
Moringa oleifera and Solanum lycopersicum are vegetable crops that are in high demand in the agricultural sector for various economic, social, and cultural purposes. Furthermore, Moringa oleifera and Solanum lycopersicum are abundant sources of bioactive compounds and are therefore essential nutraceuticals [1,2]. Their edible nature makes them popular; for instance, Moringa pods are commonly used in Thailand for sour soup (also known as Keang-Som) while in other countries the leaves, flowers, and seeds of Moringa oleifera are boiled and served as side dishes for chili pastes [3]. Additionally, Moringa leaves, flowers, and seeds are made into dry powder and packed in capsules or tea bags for infusion drinks [3]. Similarly, Solanum lycopersicum is a common staple in many countries, with a global production of over 42.3 million tons [4]. A large portion of its produce is served as vegetable drinks, sauces, salads, or stews in restaurants and supermarkets. Hence, Moringa oleifera and Solanum lycopersicum both serve as pivotal nutritional supplements for the well-being of mankind [5,6].
The nutritional qualities of Moringa oleifera and Solanum lycopersicum are derived from their phytochemical composition. The metabolic profile of Moringa oleifera includes proteins, vitamins, beta-carotene, amino acids, and various phenolics [7]. Similarly, metabolites such as systemin, phenolic acids such as ferulic acids, flavonoids, organic acids, and glycoalkaloids have been reported in Solanum lycopersicum [1,8]. A common feature of Moringa oleifera and Solanum lycopersicum is the abundance of flavonoids and phenolic acid metabolites in both. Flavonoids and phenolic acids have been observed to exhibit antioxidant properties [9]. For instance, Ojiako et al. [10] reported that extracts of Moringa oleifera contained abundant phenolic-based antioxidants such as vitamins A, C, and E, which inhibited bacterial infections, reduced inflammation, and eliminated toxins associated with venomous bites and gout.
Previously, reviews by Tomás-Barberán et al. [11], Fernández-Moriano et al. [12], Chutulo et al. [13], and Kazmi et al. [14] have reported on the phytoconstituents and bioactivities of plants including Arthothelium awastii, Parmotreme tinctorum, Azadirachta indica, Nigella sativa, and Prunus avium. However, to the best of our knowledge, there no review has placed emphasis on differences and similarities in flavone, phenolic acid, or alkaloid metabolic profiles of plants obtained from different families, particularly Moringaceae and Solanaceae. Hence, this review aims to complement earlier reviews by providing collective information, comparing and contrasting the metabolic profiles and metabolic relevance in the modern era of compounds obtained from Moringa oleifera and Solanum lycopersicum. The reader is presented with an update on the health-promoting applications of phenolic and alkaloid-based metabolites obtained from Moringa oleifera and Solanum lycopersicum. Thereafter, challenges and recommendations are discussed to educate the reader on how better to handle and obtain the most nutritional value from these versatile nutraceuticals, Moringa oleifera and Solanum lycopersicum [11,12,13,14].
2. Comparison of Polyphenolic Profiles of Solanum lycopersicum and Moringa oleifera and Thier Resultant Antioxidant Activities
Structural Variation in Phenolic Acids and Flavones in Solanum lycopersicum and Moringa oleifera
Solanum lycopersicum and Moringa oleifera have been reported to contain polyphenolic phytochemicals. These include quinic and hydroxylated or methoxylated cinnamic acid derivatives [15,16,17,18]. Common polyphenols in Solanum lycopersicum and Moringa oleifera include cinnamic acids (phenolic acids), some of which include gallic, ferulic, caffeoyl, and p-coumaric acids, and cyclic polyphenols such as quinic acids. Solanum lycopersicum and Moringa oleifera were both reported to contain the esterification product of cinnamic acid and quinic acid. One example of an ester is caffeoyl quinic acid (CQA), which is derived from the reaction of quinic acid and caffeic acid, and has been reported in Solanum lycopersicum [19,20]. Examples include 5-CQA [21], CQA and tricaffeoylquinic acid [22], 3-CQA [23], 3-CQA, 5-CQA, and 4-CQA [16], diCQA, and p-coumaroylquinic acid [24].
Hamany-Djande et al. [25] reported identification of three chlorogenic acids in Moringa oleifera; caffeoyl quinic acid (CQA), coumaroyl quinic acid (CoQA), and feruloyl quinic acid (FQA). Rodriguez-Perez et al. [26] characterized eleven phenolic acids and derivatives from Moringa oleifera. Seven of the identified phenolic acids were identified for the first time in Moringa oleifera leaves and in the Moringaceae family. They reported four isomers of CQA and two isomers of FQA, characterized for the first time in Moringa oleifera leaves. One fragment corresponded to [M-CH3-CO2-H]- from ferulic acid and another fragment corresponded to the loss of ferulic acid and five isomers of CoQA, with one fragment being identified as 4-p-CoQA, also identified for the first time in Moringa oleifera and the Moringaceae family [26]. A study by Bennett et al. [27] identified 3- and 5- CQA in Moringa oleifera leaves. Ziani et al. [28] also reported the presence of 3-CQA, 4-CQA and 3-CoQA in Moringa oleifera leaves.
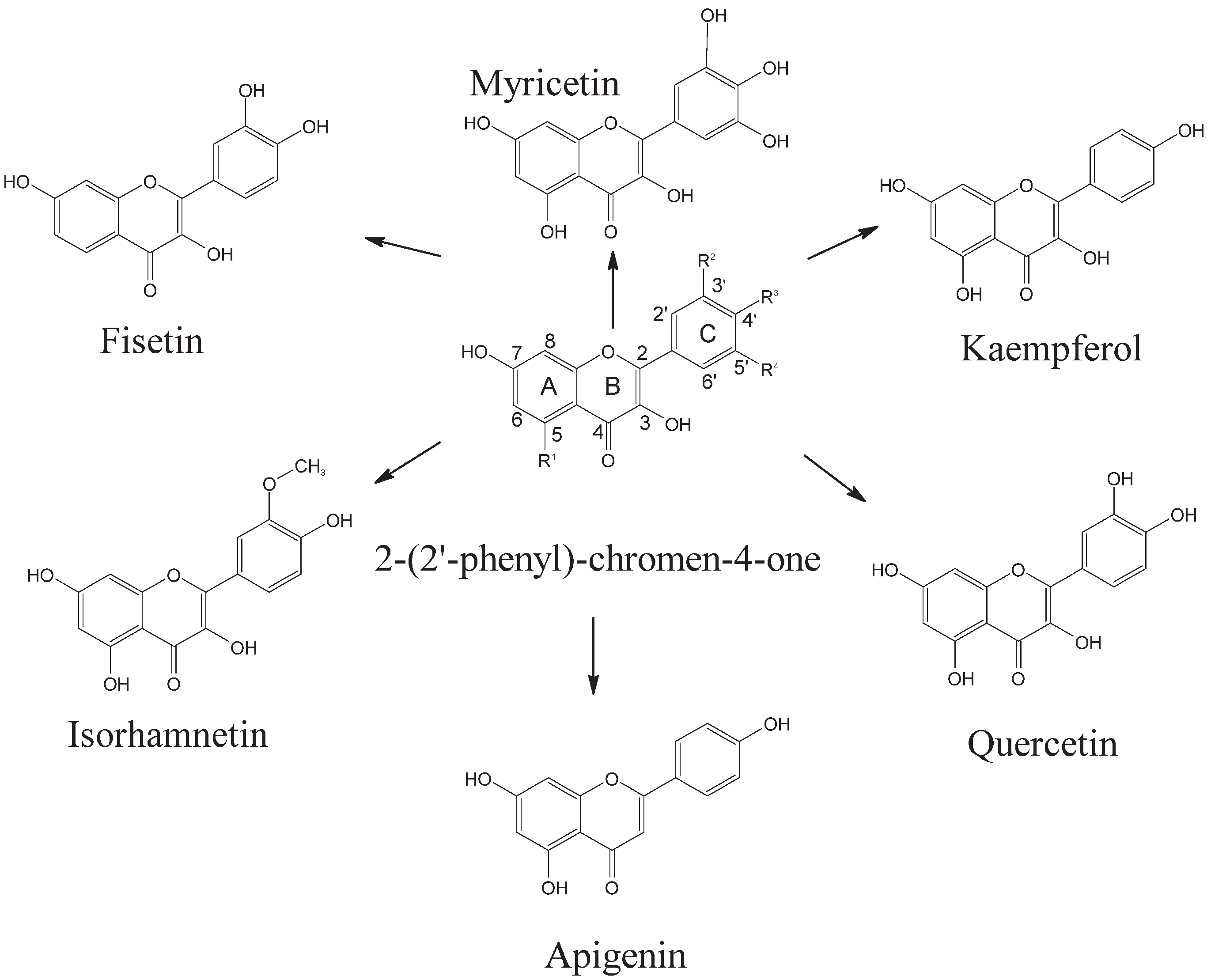
Flavonoids are a wide-ranging group of metabolites with estimates of over 10,000 compounds reported [29,30], some of which have been observed in Moringa oleifera and Solanum lycopersicum. From this cluster, 6500 of these flavonoids are made up of a 15-carbon skeleton [31]. The flavonoids highlighted therein can be further broken down into flavonols, flavones, flavanones, isoflavones, anthocyanidins, chalcones, aurones, and flavanols [32]. The basic skeletal design of flavonoid molecules is based on the (2-(2′-phenyl)-chromen-4-one) which consists of two benzene rings denoted as A and C, connected by a three-carbon chain that forms a closed pyran ring (B ring), which fuses with ring A as shown in Figure 1 [33]. Additionally, the skeleton (2-(2′-phenyl)-chromen-4-one) structure of flavonoids consists of a C2–C3 double bond and 3-hydoxy group with 4-Oxo group in ring B. The basic skeletal structure (2-(2′-phenyl)-chromen-4-one) is so versatile that it permits other combinations of multiple hydroxyls, methoxyl, and glycoside substituents to attach to R’ (Figure 1), some of which include solabiose (galactose + glucose+ rhamnose), chacotriose (glucose + rhamnose + rhamnose), and rutinoside (rhamnose + glucose) [33]. Examples of flavones include isorhamnetin, quercetin, kaempferol, and myricetin; Isorhamnetin-3-O-rutinoside was reported in Moringa oleifera [34] while the carbohydrate side chain linked to isorhamnetin at carbon 3, sophoroside (glucose + glucose), was detected in Solanum lycopersicum [35]. This indicates that though the aglycone unit of the flavones may be the same, the arrangement of glycosides attached there can differ, giving rise to unique biological properties [33].

Figure 1.
Flavones reported in Moringa oleifera and Solanum lycopersicum. Mechanistic antioxidant activity of phenolic acids and flavones obtained from Solanum lycopersicum and Moringa oleifera.
Polyphenolic compounds in both Solanum lycopersicum and Moringa oleifera are renowned for their anticancer and anti-inflammatory activities [36,37,38]. Table 1 lists metabolites derived from Moringa oleifera, such as kaempferol, isorhamnetin, and quercetin, which can provide antioxidant activity, reduce human leukeamia cells, and promote anti-inflammatory and anti-alzheimer’s activity, respectively [39,40]. For instance, flavones such as quercetin, morin, fisetin, and rutin from Solanum lycopersicum [39,40] and kaempferol, isorhamnetin, myrecytin, and apigenin from Moringa oleifera leaves were observed to form hydrogen bonds with bovine serum albumin [41]. This indicated that the higher free-radical-scavenging and antioxidant abilities of flavones were probably due to the presence of the 4′-OH group on ring C, as well as the 3-OH group on ring B, in addition to other hydroxyl groups on ring C (Figure 1). Kitagawa et al. [42] studied the inhibitory effect of flavonoids on P-gp-mediated transport in KB-C2 cells and found that the inhibitory effect on P-gp decreased in the order kaempferol > quercetin > myricetin > fisetin. Results revealed that the double bonds between C2–C3, 3-OH, 5-OH, 7-OH, and 4′-OH groups (Figure 1) were responsible for the higher activity of flavones, whereas the presence of other hydroxyl group at ring C with the exception of 4′-OH resulted in decreased activity. Flavones in Moringa oleifera including kaempferol, isorhamnetin, and quercetin were reported to prevent DNA damage [40], reduce human leukaemia cells [43,44,45]. Solanum lycopersicum, through querctin glucoside and kaempferol rutinoside, has been reported to provide inhibition of sodic-alkaline stress [46]. This arises due to the pro-oxidant action and electrophilic conjugation reaction of polyphenols. For instance, electrophilic conjugation reaction includes the oxidation of flavonol into electrophilic quinones. The quinones then function as effective electron-pair acceptors, to form nucleophiles such as thiols, amino-containing proteins, and glutathiones yielding biologically active flavonol adducts. The chemical sites responsible for anti-inflammatory activity are the C2–C3 double bond, 5-OH and 7-OH groups at ring A, and the 4′-OH group at ring B (Figure 1). Furthermore, as indicated in Table 1, both Solanum lycopersicum and Moringa oleifera have been observed to produce cinnamic acids such as chlorogenic acid (caffeoyl quinic acid), gallic acid, and caffeic acid, which have been reported to inhibit galectin-3, trichorderma harzianum, and diabetes.
Solanum lycopersicum and Moringa oleifera were observed to contain common polyphenolic metabolites including gallic, ferulic, caffeoyl, and p-coumaric acids, quercetin, and kaempferol derivatives, the majority of which have been shown to be efficient antioxidants. Additionally, mono/polysaccharides attached to the (2-(2′-phenyl)-chromen-4-one) were reported with various bioactivities (Table 1). There were also differences in polyphenolic composition between Moringa oleifera and Solanum lycopersicum, with isorhamnetin-3-O-rutinoside and isorhamnetin sophoroside observed in Moringa oleifera and Solanum lycopersicum, respectively, as a result of differences in the arrangement of the carbohydrate sugar side chains glycosylated to (2-(2′-phenyl)-chromen-4-one) (Table 1).


Table 1.
Bioactivities exhibited by flavones and phenolic acids in Solanum lycopersicum and Moringa oleifera.
Table 1.
Bioactivities exhibited by flavones and phenolic acids in Solanum lycopersicum and Moringa oleifera.
| Plant Species | Flavones | Phenolic acids | Bioactivity | Reference |
|---|---|---|---|---|
| Moringa oleifera | Apigenin | - | Anti-inflammatory, anti-Alzheimer’s activity | [7] |
| - | Caffeic acids; gallic acid | Anti-diabetic and anti-obese properties; inhibits gluCose-6-phosphate translocase in rat liver | [39] | |
| Isorhamnetin | - | Reduces human leukaemia cells | [40] | |
| Kaempferol | - | Prevents DNA damage, antioxidant | [40,43] | |
| Solanum lycopersicum | Quercetin glycoside | - | Inhibition of sodic-alkaline stress | [46] |
| Kaempferol rutinoside | - | Inhibition of sodic-alkaline stress | [46] | |
| - | Chlorogenic acids | Inhibition of galectin-3 | [47] | |
| - | Ferulic acids | Inhibition of galectin-3 | [47] | |
| - | Gallic acid | Inhibition of trichoderma harzianum | [48] | |
| - | Salicylic acid | Inhibition of trichoderma harzianum | [48] | |
| - | Caffeic acid | Nematode (Meloidogyne incognita) resistance | [49] | |
| - | Phenylalanine | Resistance to drought stress | [50] | |
| - | Tyrosine | Resistance to drought stress | [50] |
3. Similarities and Differences Due to Cytotoxic Potency against Cancerous Cells of Alkaloids Contained in Moringa oleifera and Solanum lycopersicum
3.1. Alkaloids in Moringa oleifera and Solanum lycopersicum
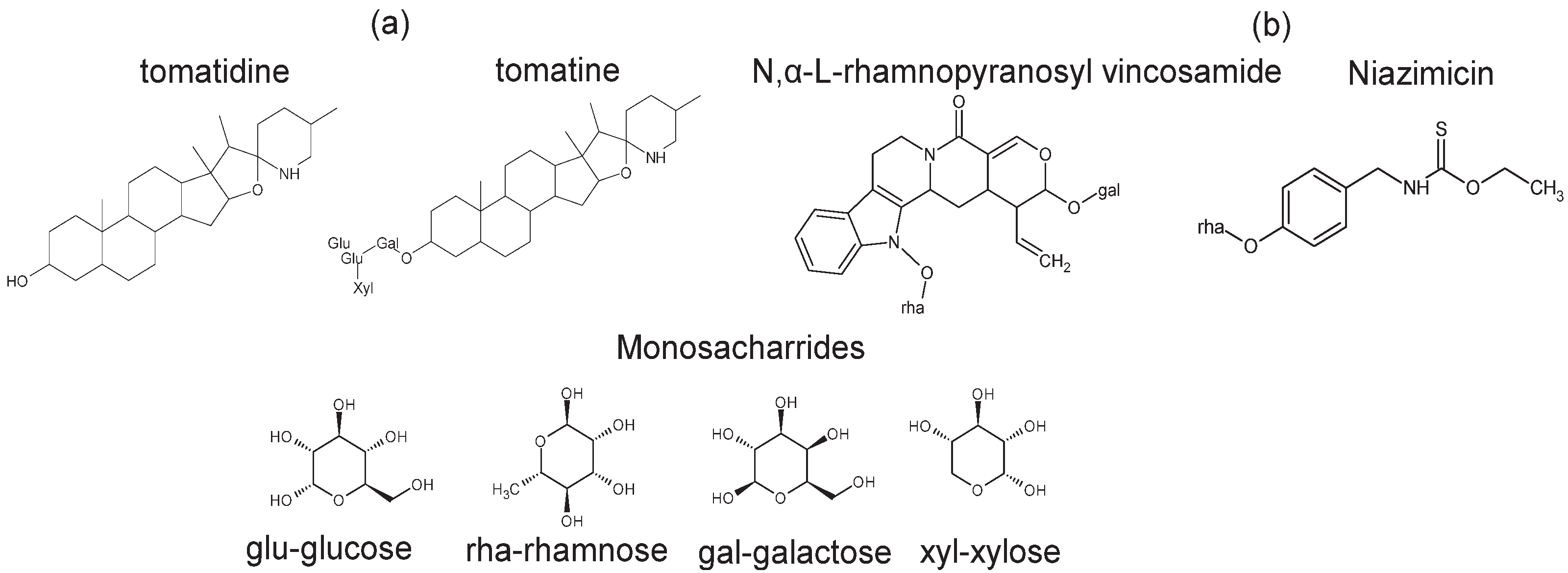
Alkaloids are nitrogen-containing organic compounds present in plants. Steroidal alkaloids (SAs) are derived from steroids and are hence classified as tropanes. Alkaloids of this class are prevalent in a range of plants within the Solanaceae family. The synthesis of SAs originated from glycosylation (addition of mono-/polysaccharides) to sterols, contained in the cell cytosol, yielding steroidal glycoalkaloids, as reported by Okamoto et al. [51]. Steroidal glycoalkaloids derived from Solanum lycopersicum can provide a chemical barrier against a broad range of pathogens [16,52,53]. Some of the SGAs derived from Solanum lycopersicum are shown in Figure 2a, including tomatidine, tomatine, dehydrotomatine, dehyrotomatidine, esculeoside A and esculeoside B, most of which except for the latter two contain spirosolane as the aglycone unit. For instance, α-tomatine, an SGA reported to be obtained from Solanum lycopersicum, can be responsible for the disruption of membranes of cancerous cells, leakage of electrolytes, and depolarization of the membrane potential [54]. Although toxic to humans, the presence of α-tomatine is not toxic to the plant itself possibly due the existence of sterol glycosides and acetylated sterol glycosides in tomato cell membranes [55]. Other SGAs have been shown to inhibit roundworms (nematodes), as described by Kirwa et al. [56], and fungi, as reported by Almadiy et al. [57]. Esculeode A and esculeode B were reported by Zhou et al. [58] to inhibit skin-related cancers.

Figure 2.
Examples of alkaloids reported in (a) Solanum lycopersicum and (b) Moringa oleifera, and common monosaccharides reported to glycosylate the alkaloids.
Alkaloids reported in Moringa oleifera have also been found to reduce blood pressure and treat hypertension [59]. Some of the alkaloids that have been isolated from Moringa oleifera leaves include N,α-l-rhamnopyranosyl vincosamide, phenylacetonitrile pyrrolemarumine,4′-hydroxyphenylethanamide-α-l-rhamnopyranoside, and its glucopyranosyl derivative [39] (Figure 2b). Of these, N,α-l-rhamnopyranosyl vincosamide (VR), as studied by Panda et al. [60], was isolated from Moringa leaves and demonstrated cardio-protective potential in rats. This beneficial action of N,α-l-rhamnopyranosyl vincosamide (VR) was due to its free-radical-scavenging properties [61].
As discussed in this section, both Solanum lycopersicum and Moringa oleifera produce alkaloids. A common trait among the two is that Solanum lycopersicum and Moringa oleifera have both been reported to contain glycosylated alkaloid metabolites with sugars such as glucose, rhamnose, galactose, and xylose, as seen in Figure 2 [16,52,53]. The distinguishing factors between the plants are the aglycone units of the alkaloids, for instance Solanum lycopersicum has been observed to produce alkaloids containing 6-fused rings, such as tomatine, tomatidine, dehydrotomatine, eucleoside A, and eucleoside B, whereas Moringa oleifera generally contains 5-fused rings (Figure 2a).
3.2. Comparison in Mechanism of Alkaloid Bioactivities of Solanum lycopersicum and Moringa oleifera
Alkaloids obtained from Solanum lycopersicum and Moringa oleifera have been shown to be bioactive [21,61,62]. The alkaloid α-tomatine, derived from Solanum lycopersicum, was reported by Yelken et al. [63] to show inhibitory activity on cell proliferation of human breast MCF-7 cancer cells. In the same paper, they further indicated that α-tomatine−cholesterol interactions within the cell membrane of MCF-7 cancer cells played a vital role in the anticarcinogenic effect of α-tomatine [63]. In another study by Friedman et al. [64], animals receiving a tomatine diet had reduced plasma LDL cholesterol levels with increased dietary tomatine content. This was due to the complexation ability of cholesterol to tomatine [64]. Furthermore, dehydrotomatine, the oxidized form of tomatine, was reported by Pinela et al. [65] to inhibit acetylcholinesterase, an enzyme responsible for catalyzing the production of the neurotransmitter acetylcholine, which is also responsible for cancer [21]. Similarly, alkaloids from Moringa oleifera such as niazimicin were reported by Oleg et al. [62] to demonstrate anticancer activity. Panda et al. [60] reported on the cardioprotective behavior of the alkaloid N,α-l-rhamnopyranosyl vincosamide. Moringa oleifera alkaloids were also reported by Kasolo et al. [66] to be efficient in their antimicrobial activity, achieved by the ability of the alkaloids to intercalate with the DNA of microorganisms.
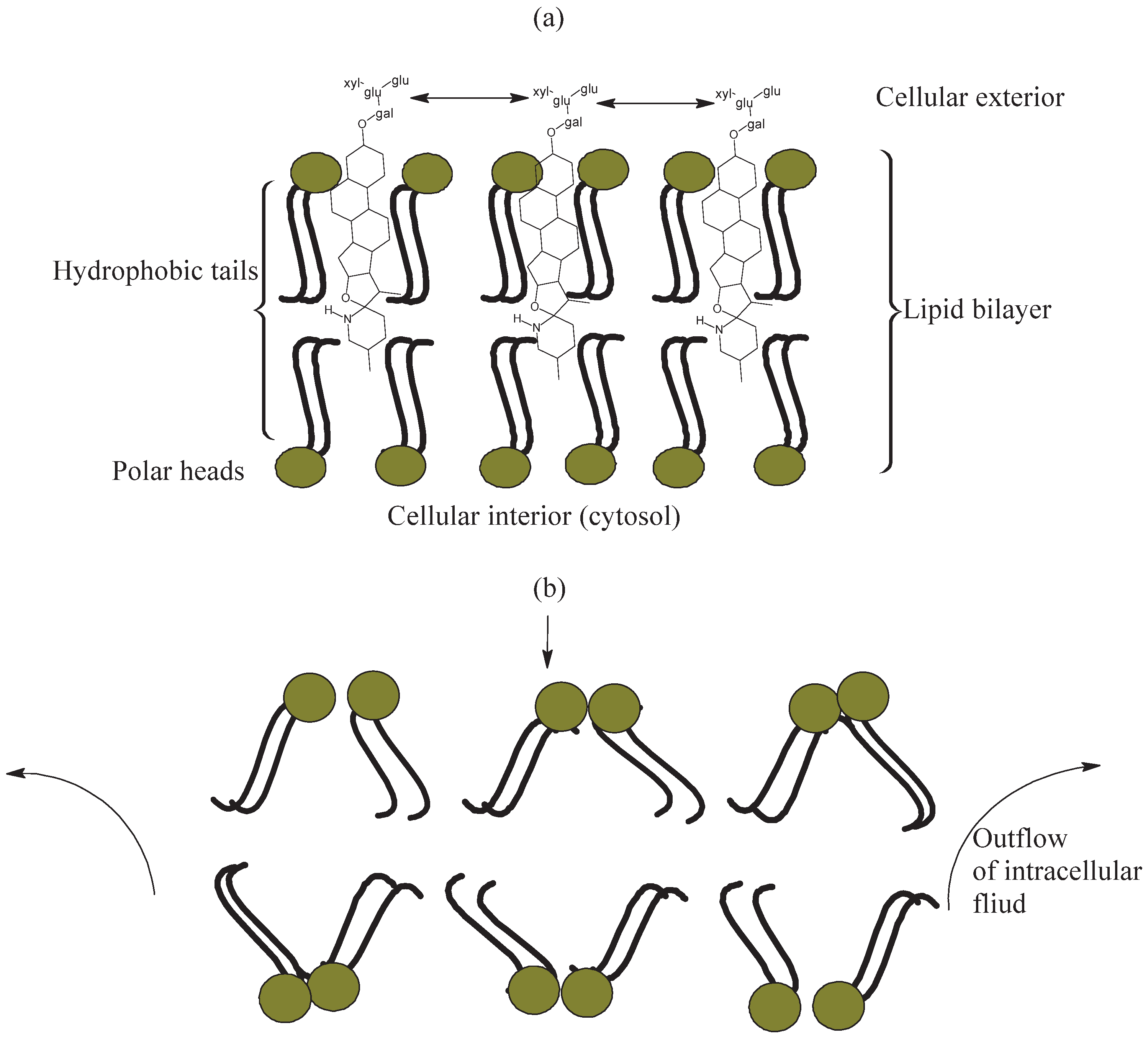
Glycoalkaloids from Solanum lycopersicum have also been reported to disrupt active transport of ions through membranes, proceeding to cause disorders in general body metabolism [67]. For instance, Blankemeyer et al. [55] evaluated the effect of exposure of varying concentrations of α-tomatine and tomatidine on frog embryos and frogs. The study revealed that α-tomatine increased the fluorescence-measured membrane permeability of frog embryos by approximately 600% compared with control values; the corresponding value for tomatidine was about 150%. An illustration of how glycoalkaloids such as α-tomatine incurred damage in the cells of frog embryos is shown in Figure 3a,b. Two phases were involved in the damage caused by α-tomatine in cell membranes. The first involved permeation by α-tomatine through the infected cell membrane by potential hydrogen bond interaction between the carbohydrate side chain of α-tomatine and the polar compartment of the cell-membrane lipid bilayer (Figure 3a) [55]. The alkaloid portion of tomatine interacts with cholesterol, while the sugar groups remain outside the bilipid membrane due to its hydrophilic nature [67]. The extracellular polysaccharides interact with each other through hydrogen bonding, forming a matrix (Figure 3a). Secondly, penetration of the toxic aglycone unit into the cytosol (inner cell compartment) of frog embryos resulted in “loss of barrier function” in the cellular membrane and subsequent changes in ion flux and interstitial currents between neighboring cells (Figure 3b). Therefore, the enhanced activity of α-tomatine relative to tomatidine was due to its sugar moiety, with pores being formed in the lipid bilayer in frog embryos [55]. Thereafter, the increasing porosity of the cell membrane allows permeation of extracellular fluid into the cell, compromising the cytosol’s chemistry [68]. This leads to eventual cell death; examples include inhibition of larval growth of Tribolium castaneum as reported by Weissenberg et al. [69], and inhibition of acetylcholinesterase as described by Pinela et al. [65].

Figure 3.
Mechanism of α-tomatine toxicity in cells of frog embryos: (a) Hydrogen-bond interaction of α-tomatine polysaccharide chains; (b) damage to structural integrity of cell membranes.
Alkaloids have been studied for other bioactivities, as shown in Table 2, which arise from various mechanistic actions. For example, mechanisms of action for alkaloids derived from Solanum lycopersicum involve complexation, disruption of active transport of ions through cell membranes, permeation through the infected cell membrane, and penetration of the toxic aglycone unit into the cytosol [65,66,67]. Alkaloids from Moringa oleifera have a different mode of action to those discussed for Solanum lycopersicum, including intercalation with the DNA of pathogens [62,70]. This probably indicates that the structural design of the alkaloids, such as 6- and 5-fused rings of Solanum lycopersicum and Moringa oleifera respectively, and the nature of the sugars attached, all determine the mode of action and subsequent efficiency of the bioactivity. Table 3 presents the dosage concentrations, dosage period, and type of bioactivity of various flavones and alkaloids. Sun et al. [71] reported on minute concentrations (80 µM) of fisetin that were required for anti-cancer activity.

Table 2.
Some alkaloids reported in Solanum lycopersicum and Moringa oleifera.

Table 3.
Dosage, period, and bioactivity of some flavones and alkaloids.
4. Challenges and Recommendations
Moringa oleifera and Solanum lycopersicum both contain efficient and diverse flavone antioxidants, and have been studied to treat the same ailments including liver damage, detoxification of the body’s digestive system, and reduction of brain inflammation [77,78]. For instance, Buabeid et al. (2022) reported that hepatoprotection from Solanum lycopersisum was induced by isoniazid and rifampicin (INH + RIF), resulting in significant elevation of serum hepatic enzymes including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin, while decreasing the albumin level [79]. Additionally, Fattah et al. (2020) reported that hepatoprotection from Moringa Oleifera occurred through reduction of oxidative stress–induced DNA damage via amelioration of NF-kB and TNF-α, which maintained hepatocyte integrity and reduced hepatic enzyme activity in serum [80]. However, underlying questions remain of interest to metabolomics researchers, particularly which between the two plants may be more potent for treatment of ailments such as liver or brain injury, and what other modes of action may be at play within their antioxidant activity due to polyphenols, or anti-pathogen activity due to alkaloids. Furthermore, taking the view that there are a variety of mechanisms present in Moringa oleifera and Solanum lycopersicum involved in counteracting pathogens and bacteria, there are also questions about what conditions favor one mode of action over another.
The flavones in Moringa oleifera and Solanum lycopersicum contain the (2-(2′-phenyl)-chromen-4-one) backbone. Additionally, variable mono- and polysaccharide chains with unique arrangements are linked to this (2-(2′-phenyl)-chromen-4-one), diversifying the large cluster of related metabolites. Hence, analysis of the similarities and differences in the metabolic profiles of Solanum lycopersicum and Moringa oleifera needs to be scrutinized. Fortunately, advancements in technology have led to the development and availability of instruments such as those for ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectroscopy (UHPLC-QTOF-MS). The appliance of UHPLC-QTOF-MS would permit better isolation from tens of thousands of metabolites and the subsequent characterization of closely related compounds involving geometric and positional isomers, in comparison to conventional instruments such as the 1H-NMR spectrometer. Furthermore, using state-of-the-art analytical tools for UHPLC-QTOF-MS, comprehensive correlations in the metabolic fingerprinting of plants (Moringa oleifera and Solanum lycopersicum) from two distinct families can be identified. For instance, isomers such as quercetin 3-glucoside and quercetin 3-galactoside can conclusively be determined based on ultra-high performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS) rather than conventional H1-NMR. Bearing in mind that these plants can produce a wide range of complex compounds, it is inevitable that racemic mixtures would be obtained that are extremely difficult to separate using chromatographic techniques. Recently, even more advanced techniques have been developed for the isolation of racemic compounds with unique potential for pharmacological applications. This can be achieved by calculating ion-mobility arrival-time distributions and surface collisional cross sections (CCS), using ultra-high-performance liquid chromatography photodiode array detection ion-mobility high-resolution mass spectrometry (UHPLC-PDA-IM-HR-MS).
5. Conclusions
Solanum lycopersicum and Moringa oleifera are two invaluable natural products, due to their metabolite profiles and subsequent nutraceutical potential. Owing to the significant presence of bioactive polyphenols and alkaloid compounds in both plants, it is imperative that the naturally derived metabolites be exploited for various applications. Both Solanum lycopersicum and Moringa oleifera contain a range of polyphenols. For instance, these plants produce flavonols such as kaempferol and quercetin derivatives that can be glycosylated by different sugars. However, the two plants differ in terms of their alkaloid composition, which results in their capability for different biological functions. While these plants can be used to perform the same pharmacological and nutritional functions resulting from the flavonols they have in common, they remain distinguishable by their alkaloid composition.
Author Contributions
T.M.M., D.N., N.T.T. and N.E.M. conceived and drafted the review. N.T.T. and N.E.M. gave corrections to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Research Foundation and Sasol Inzalo for financial support. The funding number is 138442.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Department of Chemistry and Biochemistry at the University of Venda.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Montero-Vargas, J.M.; Castillo, K.C.; Martínez-Gallardo, N.; Ordaz-Ortiz, J.J.; Délano-Frier, J.P.; Winkler, R. Modulation of steroidal glycoalkaloid biosynthesis in tomato (Solanum lycopersicum) by jasmonic acid. Plant Sci. 2018, 277, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Yooying, R.; Jittinandana, S.; Judprasong, K. Effects of cooking methods on antioxidant properties and carotenoids contents of Moringa oleifera Lam. pods. Walailak Procedia 2019, 2019, IC4IR-53. [Google Scholar] [CrossRef]
- Khera, P.; Pandey, M.K.; Wang, H.; Feng, S.; Qiao, L.; Culbreath, A.K.; Kale, A.K.; Wang, S.; Holbrook, C.C.; Zhaung, W.; et al. Mapping quantitative trait loci of resistance to tomato spotted wilt virus and leaf spots in a recombinant inbred line population of peanut (Arachis hypogaea L.) from SunOleic 97R and NC94022. PLoS ONE 2016, 11, e0158452. [Google Scholar] [CrossRef]
- Mvumi, C.; Ngadze, E.; Marais, D.; du Toit, E.S.; Mvumi, B.M. Moringa (Moringa oleifera) leaf extracts inhibit spore germination of Alternaria solani, causal agent of early blight disease of tomato (Solanum lycopersicum). S. Afr. J. Plant Soil 2017, 34, 161–165. [Google Scholar] [CrossRef]
- Elwan, H.A.M.; Elnesr, S.S.; Mohany, M.; Al-Rejaie, S.S. The effects of dietary tomato powder (Solanum lycopersicum L.) supplementation on ther haematological, immunological, serum biochemical antioxidant. J. Anim. Physiol. Anim. Nutr. 2019, 103, 534–546. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S. Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Lee, H.J.; Eom, S.H.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Hyun, T.K. Genome-wide analysis of alternative splicing events during response to drought stress in tomato (Solanum lycopersicum L.). J. Hortic. Sci. Biotechnol. 2020, 95, 286–293. [Google Scholar] [CrossRef]
- Chu, C.; Du, Y.; Yu, X.; Shi, J.; Yuan, X.; Liu, X.; Liu, Y.; Zhang, H.; Zhang, Z.; Yan, N. Dynamics of antioxidant activities, metabolites, phenolic acids, flavonoids, and phenolic biosynthetic genes in germinating Chinese wild rice (Zizania latifolia). Food Chem. 2020, 318, 126483. [Google Scholar] [CrossRef]
- Ojiako, O.A.; Chikezie, P.C. Comparative proximate composition and hypoglycemic properties of three medicinal plants (Verononia amygdalina, Azadirachta indica and Moringa oleifera). Pharmacog. Commun. 2014, 4, 40–48. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Ferreres, F.; Gil, M.I. Antioxidant phenolic metabolites from fruit and vegetables and changes during postharvest storage and processing. Stud. Nat. Prod. Chem. 2000, 23, 739–795. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: Identification of active compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Chutulo, E.C.; Chalannavar, R.K. Endophytic mycoflora and their bioactive compounds from Azadirachta indica: A comprehensive review. J. Fungi 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, A.; Khan, M.A.; Huma, A.L.I. Biotechnological approaches for production of bioactive secondary metabolites in Nigella sativa: An up-to-date review. Int. J. Second. Metab. 2019, 6, 172–195. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kato, Y.; Okabe, A.; Itoi, C.; Ooshiro, A.; Kawaide, H.; Natsume, M. Effect of secondary metabolites of tomato (Solanum lycopersicum) on chemotaxis of Ralstonia solanaceearum, Pathogen of Wilt disease. J. Agric. Food Chem. 2019, 67, 1807–1813. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Comparative metabolic phenotyping of tomato (Solanum lycopersicum) for the identification of metabolic signatures in cultivars differing in resistance to Ralstonia solanacearum. Int. J. Mol. Sci. 2018, 19, 2558. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ncube, B.; Madala, N.E.; Nyakudya, T.T.; Moyo, H.P.; Sibanda, M.; Ndhlala, A.R. Scribbling the cat: A case of the “Miracle” Plant, Moringa oleifera. Plants 2019, 8, 510. [Google Scholar] [CrossRef]
- Ridzuan, N.I.; Abdullah, N.; Vun, Y.L.; Supramaniam, C.V. Micropropagation and defence enzymes assessment of Moringa oleifera L. plantlets using nodal segments as explant. S. Afr. J. Bot. 2020, 129, 56–61. [Google Scholar] [CrossRef]
- Steinbrenner, A.D.; Gómez, S.; Osorio, S.; Fernie, A.R.; Orians, C.M. Herbivore-induced changes in tomato (Solanum lycopersicum) primary metabolism: A whole plant perspective. J. Chem. Ecol. 2011, 37, 1294–1303. [Google Scholar] [CrossRef]
- de Falco, B.; Manzo, D.; Incerti, G.; Garonna, A.P.; Ercolano, M.; Lanzotti, V. Metabolomics approach based on NMR spectroscopy and multivariate data analysis to explore the interaction between the leafminer Tuta absoluta and tomato (Solanum lycopersicum). Phytochem. Anal. 2019, 30, 556–563. [Google Scholar] [CrossRef]
- Ahmadu, T.; Ahmad, K.; Ismail, S.I.; Rashed, O.; Asib, N.; Omar, D. Antifungal efficacy of Moringa oleifera leaf and seed extracts against Botrytis cinerea causing gray mold disease of tomato (Solanum lycopersicum L.). Braz. J. Biol. 2020, 81, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Sicairos-Medina, L.Y.; Luna-Mandujan, A.G.; López-Angulo, G.; Salazar-Salas, N.Y.; Vega-García, M.O.; López-Valenzuela, J.A. Phenolic profiles, antioxidant and antimutagenic activities of Solanum lycopersicum var. cerasiforme accessions from Mexico. CYTA-J. Food. 2018, 16, 715–722. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Hoffmann, T.; Schwab, W. Effects of bio-based coatings on the ripening and quality attributes of tomato (Solanum lycopersicum) fruits. J. Sci. Food Agric. 2019, 99, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Daji, G.; Steenkamp, P.; Madala, N.; Dlamini, B. Phytochemical composition of Solanum retroflexum analysed with the aid of ultra-performance liquid chromatography hyphenated to quadrupole-time-of-flight mass spectrometry (UPLC-qTOF-MS). J. Food Qual. 2018, 2018, 3678795. [Google Scholar] [CrossRef]
- Hamany-Djande, C.Y.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Differential extraction of phytochemicals from the multipurpose tree, Moringa oleifera, using green extraction solvents. S. Afr. J. Bot. 2018, 115, 81–89. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Quirantes-Pine, C.; Fernandez-Gutierrez, R.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Rached, W.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Detailed chemical composition and functional properties of Ammodaucus leucotrichus Cross. & Dur. and Moringa oleifera Lamarck. J. Funct. Foods 2019, 53, 237–247. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. BJBAS 2020, 9, 45. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Bailly, C. The subgroup of 2′-hydroxy-flavonoids: Molecular diversity, mechanism of action, and anticancer properties. Bioorg. Med. Chem. 2021, 32, 116001. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative analysis of chemical constituents of Moringa oleifera leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sadhukhan, P.; Saha, S.; Sil, P.C. Regulation of oxidative stress by different naturally occurring polyphenolic compounds: An emerging anticancer therapeutic approach. React. Oxyg. Species 2017, 3, 81–95. [Google Scholar] [CrossRef]
- Kinger, M.; Kumar, S.; Kumar, V. Some important dietary polyphenolic compounds: An anti-inflammatory and immunoregulatory perspective. Mini-Rev. Med. Chem 2018, 18, 1270–1282. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa Oliefera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Nunthanawanich, P.; Sompong, W.; Sirikwanpong, S.; Mäkynen, K.; Adisakwattana, S.; Dahlan, W.; Ngamukote, S. Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springerplus 2016, 5, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Makita, C.; Madala, N.E.; Cukrowska, E.; Abdelgadir, H.; Chimuka, L.; Steenkamp, P.; Ndhlala, A.R. Variation in pharmacologically potent rutinoside-bearing flavonoids amongst twelve Moringa oleifera Lam. cultivars. S. Afr. J. Bot. 2017, 112, 270–274. [Google Scholar] [CrossRef]
- Dilworth, L.L.; Stennett, D.; Omoruyi, F.O. Effects of Moringa oleifera leaf extract on human promyelocytic leukemia cells subjected to oxidative stress. J. Med. Food 2020, 23, 728–734. [Google Scholar] [CrossRef]
- Akanni, E.O.; Adedeji, A.L.; Adedosu, O.T.; Olaniran, O.I.; Oloke, J.K. Chemopreventive and anti-leukemic effects of ethanol extracts of Moringa oleifera leaves on wistar rats bearing benzene induced leukemia. Curr. Pharm. Biotechnol. 2014, 15, 563–568. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, J.D.; Wang, B.; Lv, Y.J.; Jiang, H.; Liu, G.L.; Qiao, Y.; Ren, M.; Guo, X.F. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS ONE 2013, 8, e72548. [Google Scholar] [CrossRef]
- Kapoor, S.; Dharmesh, S.M. Physiologically induced changes in bound phenolics and antioxidants, DNA/cytoprotective potentials in pectic poly/oligosaccharides of tomato (Solanum lycopersicum). J. Sci. Food Agric. 2016, 96, 4874–4884. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Hashem, A.; Abd, A.E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Afifah, E.N.; Murti, R.H.; Nuringtyas, T.R. Metabolomics approach for the analysis of resistance of four tomato genotypes (Solanum lycopersicum L.) to root-knot nematodes (Meloidogyne incognita). Open Life Sci. 2019, 14, 141–149. [Google Scholar] [CrossRef]
- Filiz, E.; Cetin, D.; Akbudak, M.A. Aromatic amino acids biosynthesis genes identification and expression analysis under salt and drought stresses in Solanum lycopersicum L. Sci. Hortic. 2019, 250, 127–137. [Google Scholar] [CrossRef]
- Atkins, C.A.; Smith, P.M.; Rodriguez-Medina, C. Macromolecules in phloem exudates–a review. Protoplasma 2011, 248, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, R.E. Toxicity of tomatine and tomatidine on weeds, crops and phytopathogenetic fungi. Allelopathy J. 2009, 23, 425–436. [Google Scholar]
- Garcia, P.G.; Neves dos Santos, F.; Zanotta, S.; Eberlin, M.N.; Carazzone, C. Metabolomics of Solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Blankemeyer, J.T.; White, J.B.; Stringer, B.K.; Friedman, M. Effect of α-tomatine and tomatidine on membrane potential of frog embryos and active transport of ions in frog skin. Food Chem. Toxicol. 1997, 35, 639–646. [Google Scholar] [CrossRef]
- Kirwa, K.K.; Murungi, L.K.; Beck, J.K.; Torto, B. Elicidation of different responses in the root knot nematode meloidogyne inconitato tomato root exudate cytokinin, flavonoids, and alkaloids. J. Agric. Food Chem. 2018, 66, 11291–11300. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E. Ecofriendly synthesis of silver nanoparticles using potato steroidal alkaloids and their activity against phytopathogenic fungi. Braz. Arch. Biol. Technol. 2018, 61, 1–14. [Google Scholar] [CrossRef]
- Zhou, J.R.; Kimura, S.; Nohara, T.; Yokomizo, K. Competitive inhibition of mammalian hyaluronidase by tomato saponin, esculeoside A. Nat. Prod. Commun. 2018, 13, 1461–1463. [Google Scholar] [CrossRef]
- Abdulazeez, A.M.; Ajiboye, O.S.; Wudil, A.M.; Abubakar, H. Partial purification and characterization of angiotensin converting enzyme inhibitory alkaloids and flavonoids from the leaves and seeds of Moringa oleifera. J. Adv. Biol. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,α-L-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef]
- Cheraghi, M.; Namdari, M.; Daraee, H.; Negahdari, B. Cardioprotective effect of magnetic hydrogel nanocomposite loaded N, α-L-rhamnopyranosyl vincosamide isolated from Moringa oleifera leaves against doxorubicin-induced cardiac toxicity in rats: In vitro and in vivo studies. J. Microencapsul. 2017, 34, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Oleg, P.; Ilona, G.; Alexey, G.; Irina, S.; Ivan, S.; Vladimir, K. Biological activities of derived bioactive components from Moringa species: An overview. Entomol. Appl. Sci. 2018, 5, 82–87. [Google Scholar] [CrossRef]
- Yelken, B.O.; Balcı, T.; Süslüer, S.Y.; Kayabaşı, C.A.; Kırmızıbayrak., P.B.; Gündüz, C. The effect of tomatine on metastasis related matrix metalloproteinase (MMP) activities in breast cancer cell model. Gene 2017, 627, 408–411. [Google Scholar] [CrossRef]
- Friedman, M.; Fitch, T.E.; Yokoyama, W.E. Lowering of plasma LDL cholesterol in hamsters by the tomato glycoalkaloid tomatine. Food Chem. Toxicol. 2000, 38, 549–553. [Google Scholar] [CrossRef]
- Pinela, J.; Montoya, C.; Carvalho, A.M.; Martins, V.; Rocha, F.; Barata, A.M.; Ferreira, I.C. Phenolic composition and antioxidant properties of ex-situ conserved tomato (Solanum lycopersicum L.) germplasm. Int. Food Res. J. 2019, 125, 108545. [Google Scholar] [CrossRef]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J.W. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plant Res. 2010, 4, 753–757. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M.; Filadelfi-Keszi, M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Noonan, J. Tomatine and Furocoumarins: Toxins in Commonly Consumed Plants. Ph.D. Thesis, Taylor University, Upland, Indiana, 2018. [Google Scholar]
- Weissenberg, M.; Levy, A.; Svoboda, J.A.; Ishaaya, I. The effect of some Solanum steroidal alkaloids and glycoalkaloids on larvae of the red flour beetle, Tribolium castaneum, and the tobacco hornworm, Manduca sexta. Phytochemistry. 1998, 47, 203–209. [Google Scholar] [CrossRef]
- Siddan, A.A.; Abubakar, S.M.; Gadanya, A.M.; Maigari, F.U.; Yahaya, A.S.; Yarima, M. Determination of nutrients and anti-nutrients contents of Moringa oleifera and arachis hypogaea. J. Mol. Microbiol. Biotechnol. 2020, 8, 11–14. [Google Scholar] [CrossRef]
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Wang, X. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef]
- Sharma, D.; Tekade, R.K.; Kalia, K. Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: An in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine 2020, 76, 153235. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, C.; Li, Z.; Shangguan, A.; Jiang, J.; Zeng, W.; Zhang, S.; He, Q. Quercetin as an antiviral agent inhibits the Pseudorabies virus in vitro and in vivo. Virus Res. 2021, 305, 198556. [Google Scholar] [CrossRef] [PubMed]
- Troost, B.; Mulder, L.M.; Diosa-Toro, M.; van de Pol, D.; Rodenhuis-Zybert, I.A.; Smit, J.M. Tomatidine, a natural steroidal alkaloid shows antiviral activity towards chikungunya virus in vitro. Sci. Rep. 2020, 10, 6364. [Google Scholar] [CrossRef] [PubMed]
- Serratì, S.; Porcelli, L.; Guida, S.; Ferretta, A.; Iacobazzi, R.M.; Cocco, T.; Maida, I.; Tamasi, G.; Rossi, C.; Guida, G.; et al. Tomatine displays antitumor potential in in vitro models of metastatic melanoma. Int. J. Mol. Sci. 2020, 21, 5243. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, E.M.; Medhat, D.; Mandour, Y.M.; Hanafi, R.S.; Motaal, A.A. Niazimicin: A thiocarbamate glycoside from Moringa oleifera Lam. seeds with a novel neuroprotective activity. J. Food Biochem. 2021, 45, e13992. [Google Scholar] [CrossRef]
- Kandeil, M.A.; Mohammed, E.T.; Hashem, K.S.; Aleya, L.; Abdel-Daim, M.M. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ. Sci. Pollut. Res. 2019, 27, 19169–19184. [Google Scholar] [CrossRef]
- Amaechi, D.; Ekpe, I.P.; Edet, E.D.; Madu, M.C. Hepatoprotective and hematological effects of solanum melongena (garden egg), Solannum lycopersicum (tomato) and daucus carrots subsp. sativus (carrot) extracts against lead induced toxicity in wistar rats. Asian J. Biochem. Genet. 2021, 7, 10–18. [Google Scholar] [CrossRef]
- Buabeid, M.A.; Arafa, E.S.; Rani, T.; Ahmad, F.U.D.; Ahmed, H.; Hassan, W.; Murtaza, G. Effects of Solanum lycopersicum L.(tomato) against isoniazid and rifampicin induced hepatotoxicity in wistar albino rats. Braz. J. Biol. 2022, 84, e254552. [Google Scholar] [CrossRef]
- Abdel Fattah, M.E.; Sobhy, H.M.; Reda, A.; Abdelrazek, H. Hepatoprotective effect of Moringa oleifera leaves aquatic extract against lead acetate–induced liver injury in male Wistar rats. Environ. Sci. Pollut. Res. 2020, 27, 43028–43043. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).