Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024
Abstract
1. Introduction
2. Method
2.1. Data Source and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
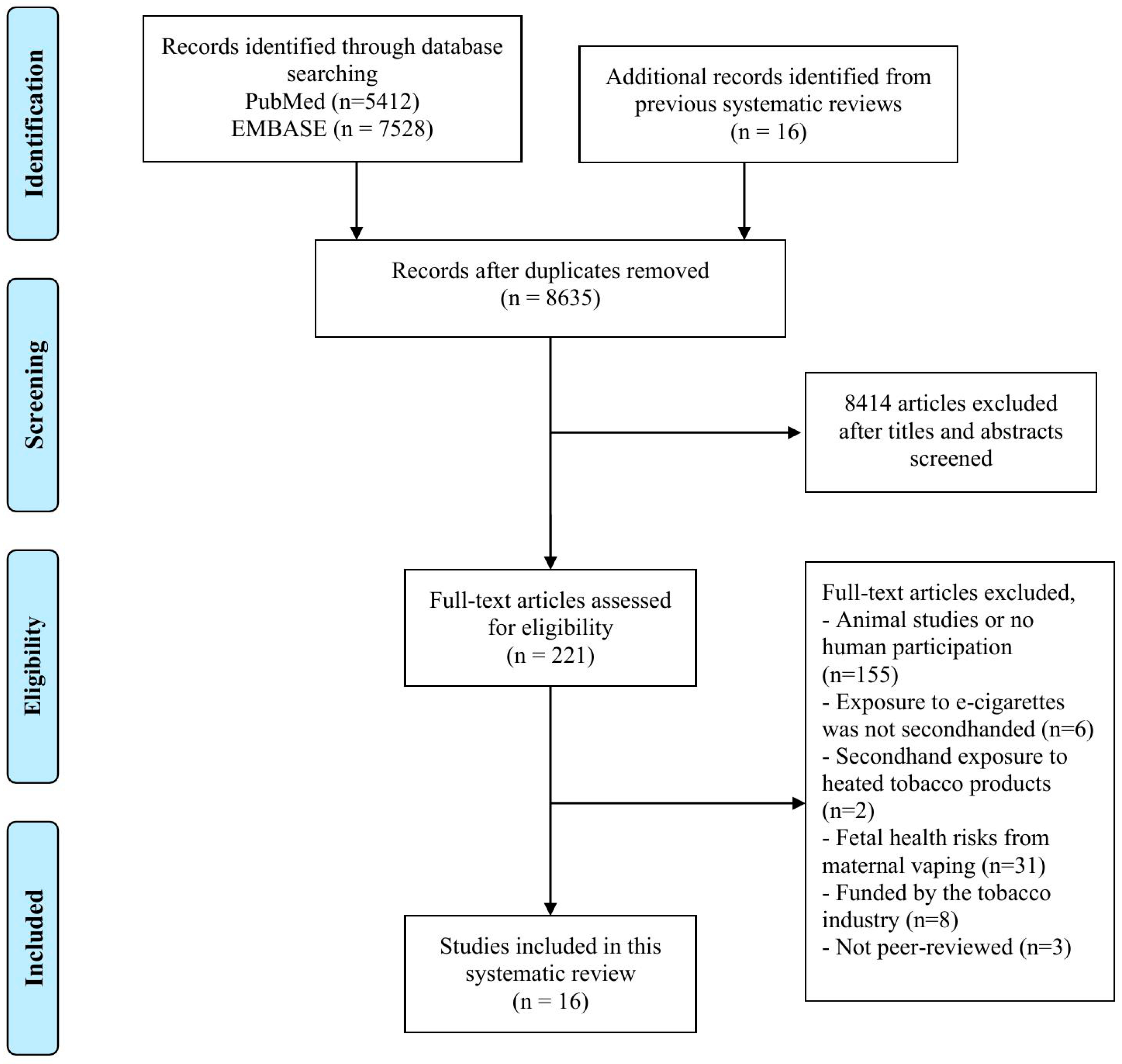
3. Results
3.1. Study Characteristics
3.2. Respiratory Health Effects
3.3. Cardiovascular Health Effects
3.4. Mental Health Effects
3.5. Biomarkers of Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salari, N.; Rahimi, S.; Darvishi, N.; Abdolmaleki, A.; Mohammadi, M. The global prevalence of E-cigarettes in youth: A comprehensive systematic review and meta-analysis. Public Health Pract. 2024, 7, 100506. [Google Scholar] [CrossRef]
- Lapyai, S.; Sritabutra, D. E-cigarettes evolution over the past 10 years: From the generation to the 5th generation or “Toy pod” and cartoon-based marketing strategy targeting younger smokers. J. Public Health Health Sci. Res. 2024, 6, 1–13. [Google Scholar]
- Basáñez, T.; Majmundar, A.; Cruz, T.B.; Unger, J.B. Vaping associated with healthy food words: A content analysis of Twitter. Addict. Behav. Rep. 2018, 8, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Sarin, S.; O’Malley, S.S.; Green, B.G.; Jordt, S.E. The science of flavour in tobacco products. World Health Organ. Tech. Rep. Ser. 2019, 1015, 125–142. [Google Scholar]
- Kassem, N.O.F.; Strongin, R.M.; Stroup, A.M.; Brinkman, M.C.; El-Hellani, A.; Erythropel, H.C.; Etemadi, A.; Exil, V.; Goniewicz, M.L.; Kassem, N.O.; et al. A Review of the Toxicity of Ingredients in e-Cigarettes, Including Those Ingredients Having the FDA’s “Generally Recognized as Safe (GRAS)” Regulatory Status for Use in Food. Nicotine Tob. Res. 2024, 26, 1445–1454. [Google Scholar] [CrossRef]
- Wagoner, K.G.; Berman, M.; Rose, S.W.; Song, E.; Cornacchione Ross, J.; Klein, E.G.; Kelley, D.E.; King, J.L.; Wolfson, M.; Sutfin, E.L. Health claims made in vape shops: An observational study and content analysis. Tob. Control 2019, 28, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Thoonen, K.; Jongenelis, M.I. Perceptions of e-cigarettes among Australian adolescents, young adults, and adults. Addict. Behav. 2023, 144, 107741. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Tong, V.T.; Marynak, K.; King, B.A. Perceptions of Harm to Children Exposed to Secondhand Aerosol From Electronic Vapor Products, Styles Survey, 2015. Prev. Chronic Dis. 2017, 14, E41. [Google Scholar] [CrossRef]
- Gorukanti, A.; Delucchi, K.; Ling, P.; Fisher-Travis, R.; Halpern-Felsher, B. Adolescents’ attitudes towards e-cigarette ingredients, safety, addictive properties, social norms, and regulation. Prev. Med. 2017, 94, 65–71. [Google Scholar] [CrossRef]
- Haggart, K.; Robertson, L.; Blank, M.L.; Popova, L.; Hoek, J. It’s Just Steam: A qualitative analysis of New Zealand ENDS users’ perceptions of secondhand aerosol. Tob. Control 2021, 30, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hess, I.M.; Lachireddy, K.; Capon, A. A systematic review of the health risks from passive exposure to electronic cigarette vapour. Public Health Res. Pract. 2016, 26, 2621617. [Google Scholar] [CrossRef] [PubMed]
- Ballbè, M.; Martínez-Sánchez, J.M.; Sureda, X.; Fu, M.; Pérez-Ortuño, R.; Pascual, J.A.; Saltó, E.; Fernández, E. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res. 2014, 135, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Chorti, M.S.; Poulianiti, K.P.; Jamurtas, A.Z.; Kostikas, K.; Tzatzarakis, M.N.; Wallace Hayes, A.; Tsatsakis, A.M.; Koutedakis, Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal. Toxicol. 2013, 25, 91–101. [Google Scholar] [CrossRef]
- Flouris, A.D.; Poulianiti, K.P.; Chorti, M.S.; Jamurtas, A.Z.; Kouretas, D.; Owolabi, E.O.; Tzatzarakis, M.N.; Tsatsakis, A.M.; Koutedakis, Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012, 50, 3600–3603. [Google Scholar] [CrossRef]
- Alnajem, A.; Redha, A.; Alroumi, D.; Alshammasi, A.; Ali, M.; Alhussaini, M.; Almutairi, W.; Esmaeil, A.; Ziyab, A.H. Use of electronic cigarettes and secondhand exposure to their aerosols are associated with asthma symptoms among adolescents: A cross-sectional study. Respir. Res. 2020, 21, 300. [Google Scholar] [CrossRef]
- Amalia, B.; Fu, M.; Tigova, O.; Ballbè, M.; Castellano, Y.; Semple, S.; Clancy, L.; Vardavas, C.; López, M.J.; Cortés, N.; et al. Environmental and individual exposure to secondhand aerosol of electronic cigarettes in confined spaces: Results from the TackSHS Project. Indoor Air 2021, 31, 1601–1613. [Google Scholar] [CrossRef]
- Amalia, B.; Fu, M.; Tigova, O.; Ballbè, M.; Paniello-Castillo, B.; Castellano, Y.; Vyzikidou, V.K.; O’Donnell, R.; Dobson, R.; Lugo, A.; et al. Exposure to secondhand aerosol from electronic cigarettes at homes: A real-life study in four European countries. Sci. Total Environ. 2023, 854, 158668. [Google Scholar] [CrossRef]
- Ballbè, M.; Fu, M.; Masana, G.; Pérez-Ortuño, R.; Gual, A.; Gil, F.; Olmedo, P.; García-Algar, Ó.; Pascual, J.A.; Fernández, E. Passive exposure to electronic cigarette aerosol in pregnancy: A case study of a family. Environ. Res. 2023, 216 Pt 1, 114490. [Google Scholar] [CrossRef]
- Bayly, J.E.; Bernat, D.; Porter, L.; Choi, K. Secondhand Exposure to Aerosols From Electronic Nicotine Delivery Systems and Asthma Exacerbations Among Youth With Asthma. Chest 2019, 155, 88–93. [Google Scholar] [CrossRef]
- Costantino, S.; Torre, A.; Foti Randazzese, S.; Mollica, S.A.; Motta, F.; Busceti, D.; Ferrante, F.; Caminiti, L.; Crisafulli, G.; Manti, S. Association between Second-Hand Exposure to E-Cigarettes at Home and Exacerbations in Children with Asthma. Children 2024, 11, 356. [Google Scholar] [CrossRef]
- Farrell, K.R.; Weitzman, M.; Karey, E.; Lai, T.K.Y.; Gordon, T.; Xu, S. Passive exposure to e-cigarette emissions is associated with worsened mental health. BMC Public Health 2022, 22, 1138. [Google Scholar] [CrossRef]
- Islam, T.; Braymiller, J.; Eckel, S.P.; Liu, F.; Tackett, A.P.; Rebuli, M.E.; Barrington-Trimis, J.; McConnell, R. Secondhand nicotine vaping at home and respiratory symptoms in young adults. Thorax 2022, 77, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Samuels, T.L.; Yan, K.; Zhang, L.; Adams, J.; Stabenau, K.A.; Kerschner, J.E.; Johnston, N. Association of e-Cigarette Exposure with Pediatric Otitis Media Recurrence. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1018–1025. [Google Scholar] [CrossRef]
- Lee, M.S.; Rees, V.W.; Koutrakis, P.; Wolfson, J.M.; Son, Y.S.; Lawrence, J.; Christiani, D.C. Cardiac Autonomic Effects of Secondhand Exposure to Nicotine from Electronic Cigarettes: An Exploratory Study. Environ. Epidemiol. 2019, 3, e033. [Google Scholar] [CrossRef]
- McClelland, M.L.; Sesoko, C.S.; MacDonald, D.A.; Davis, L.M.; McClelland, S.C. The Immediate Physiological Effects of E-Cigarette Use and Exposure to Secondhand E-Cigarette Vapor. Respir. Care 2021, 66, 943–950. [Google Scholar] [CrossRef]
- Rosenkilde Laursen, K.; Bønløkke, J.H.; Bendstrup, E.; Bilde, M.; Glasius, M.; Heitmann Gutzke, V.; Puthukkadan Moosakutty, S.; Olin, A.C.; Ravn, P.; Østergaard, K.; et al. An RCT of acute health effects in COPD-patients after passive vape exposure from e-cigarettes. Eur. Clin. Respir. J. 2020, 8, 1861580. [Google Scholar] [CrossRef]
- Tzortzi, A.; Teloniatis, S.; Matiampa, G.; Bakelas, G.; Tzavara, C.; Vyzikidou, V.K.; Vardavas, C.; Behrakis, P.; Fernandez, E. Passive exposure of non-smokers to E-Cigarette aerosols: Sensory irritation, timing and association with volatile organic compounds. Environ. Res. 2020, 182, 108963. [Google Scholar] [CrossRef]
- Volesky, K.D.; Maki, A.; Scherf, C.; Watson, L.; Van Ryswyk, K.; Fraser, B.; Weichenthal, S.A.; Cassol, E.; Villeneuve, P.J. The influence of three e-cigarette models on indoor fine and ultrafine particulate matter concentrations under real-world conditions. Environ. Pollut. 2018, 243, 882–889. [Google Scholar] [CrossRef]
- Son, Y.; Giovenco, D.P.; Delnevo, C.; Khlystov, A.; Samburova, V.; Meng, Q. Indoor Air Quality and Passive E-cigarette Aerosol Exposures in Vape-Shops. Nicotine Tob. Res. 2020, 22, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Y.; Xia, T.; Zhu, Y. Effects of Electronic Cigarettes on Indoor Air Quality and Health. Annu. Rev. Public Health 2020, 41, 363–380. [Google Scholar] [CrossRef]
- Cui, T.; Lu, R.; Liu, C.; Wu, Z.; Jiang, X.; Liu, Y.; Pan, S.; Li, Y. Characteristics of second-hand exposure to aerosols from e-cigarettes: A literature review since 2010. Sci. Total Environ. 2024, 926, 171829. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences; Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Eshraghian, E.A.; Al-Delaimy, W.K. A review of constituents identified in e-cigarette liquids and aerosols. Tob. Prev. Cessat. 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- van Drooge, B.L.; Marco, E.; Perez, N.; Grimalt, J.O. Influence of electronic cigarette vaping on the composition of indoor organic pollutants, particles, and exhaled breath of bystanders. Environ. Sci. Pollut. Res. Int. 2019, 26, 4654–4666. [Google Scholar] [CrossRef] [PubMed]
- St Helen, G.; Havel, C.; Dempsey, D.A.; Jacob, P., 3rd; Benowitz, N.L. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016, 111, 535–544. [Google Scholar] [CrossRef]
- Göney, G.; Çok, İ.; Tamer, U.; Burgaz, S.; Şengezer, T. Urinary cotinine levels of electronic cigarette (e-cigarette) users. Toxicol. Mech. Methods 2016, 26, 414–418. [Google Scholar] [CrossRef]
- Hammond, D.; Reid, J.L.; Goniewicz, M.L.; McNeill, A.; O’Connor, R.J.; Corsetti, D.; Block, A.C.; Brose, L.S.; Robson, D. Nicotine Exposure From Smoking Tobacco and Vaping Among Adolescents. JAMA Netw. Open 2025, 8, e2462544. [Google Scholar] [CrossRef]
- St Helen, G.; Bernert, J.T.; Hall, D.B.; Sosnoff, C.S.; Xia, Y.; Balmes, J.R.; Vena, J.E.; Wang, J.S.; Holland, N.T.; Naeher, L.P. Exposure to secondhand smoke outside of a bar and a restaurant and tobacco exposure biomarkers in nonsmokers. Environ. Health Perspect. 2012, 120, 1010–1016. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Eisner, M.D.; Lazcano-Ponce, E.; Zielinska-Danch, W.; Koszowski, B.; Sobczak, A.; Havel, C.; Jacob, P.; Benowitz, N.L. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob. Res. 2011, 13, 202–208. [Google Scholar] [CrossRef]
- Whincup, P.H.; Gilg, J.A.; Emberson, J.R.; Jarvis, M.J.; Feyerabend, C.; Bryant, A.; Walker, M.; Cook, D.G. Passive smoking and risk of coronary heart disease and stroke: Prospective study with cotinine measurement. BMJ 2004, 329, 200–205. [Google Scholar] [CrossRef]
- Chilmonczyk, B.A.; Salmun, L.M.; Megathlin, K.N.; Neveux, L.M.; Palomaki, G.E.; Knight, G.J.; Pulkkinen, A.J.; Haddow, J.E. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N. Engl. J. Med. 1993, 328, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Z.; Luo, D.; Tian, L.; Hu, M.; Xiao, S. Respiratory Symptoms and Urinary Cotinine Levels in Pre-school Children Exposed to Environmental Tobacco Smoke. Front. Public Health 2020, 8, 587193. [Google Scholar] [CrossRef]
- Kim, S. Overview of Cotinine Cutoff Values for Smoking Status Classification. Int. J. Environ. Res. Public Health 2016, 13, 1236. [Google Scholar] [CrossRef]
- Ali, F.R.M.; Diaz, M.C.; Armour, B.S.; Crane, E.; Tynan, M.A.; Marynak, K.L. Trends in U.S. E-cigarette Sales Measured in Milligrams of Nicotine, 2019–2024. Am. J. Prev. Med. 2025, 68, 1173–1178. [Google Scholar] [CrossRef]
- Wang, X.; Ghimire, R.; Shrestha, S.S.; Borowiecki, M.; Emery, S.; Trivers, K.F. Trends in Nicotine Strength in Electronic Cigarettes Sold in the United States by Flavor, Product Type, and Manufacturer, 2017–2022. Nicotine Tob. Res. 2023, 25, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Vogel, E.A.; Benowitz, N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob. Control 2022, 31, e88–e93. [Google Scholar] [CrossRef]
- Diaz, M.C.; Silver, N.A.; Bertrand, A.; Schillo, B.A. Bigger, stronger and cheaper: Growth in e-cigarette market driven by disposable devices with more e-liquid, higher nicotine concentration and declining prices. Tob. Control 2025, 34, 65. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2025: Warning About the Dangers of Tobacco; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/publications/i/item/9789240112063 (accessed on 2 September 2025).
- Tattan-Birch, H.; Jackson, S.E.; Shahab, L.; Brown, J. Are People More Likely to Vape or Smoke Indoors? A Population Survey of Adults in England. Nicotine Tob. Res. 2024, 26, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
| First Author (Publication Year) | Country of Study | Study Design | Sample Size | Age of Participants | System | Health Outcome Measures | Summary of Findings | Limitations |
|---|---|---|---|---|---|---|---|---|
| Alnajem A, et al. (2020) [16] | Kuwait | Cross-sectional study | 1565 | 16–19 | Respiratory | Self-reported asthma symptoms | The frequency of exposure to household SHA from e-cigarettes was associated with asthma symptoms. For example, compared to those with no exposure to household SHA, frequent exposure to household SHA was associated with current wheeze (aPR = 1.30, 95% CI 1.04–1.59), current asthma (aPR = 1.56, 95% CI 1.13–2.16), and current uncontrolled asthma symptoms (aPR = 1.88, 95% CI 1.35–2.62). | Self-reported, and the inability to assess temporal sequence of events |
| Amalia B, et al. (2021) [17] | Spain | Experimental study | 2 | 40–49 | Respiratory, Immunology | Biomarkers included saliva nicotine, cotinine, 3-OH-cotinine, nornicotine, NNN, NNK, NNAL, 1,2-PG, 1,3-PG, and glycerol, and short-term health symptoms: irritation relating to ocular (itchiness, burning, watery eyes and dryness), nasal (nasal drip, itchiness, dryness, sneezing and stuffiness), throat-respiratory (dryness, soreness, cough, phlegm and breathlessness) and general complaints (headache, nausea and fatigue) | All biomarkers collected from nonvaping exposed to e-cigarettes were below the limit of quantification; however, short-term irritation symptoms, including dry throat, nose, eyes, and phlegm were reported. | Self-reported; short duration of exposure, and unrealistic exposure conditions |
| Amalia B, et al. (2023) [18] | Greece, Italy, Spain, and the United Kingdom | Experimental study | 50 | 30–49 | Immunology | Biomarkers included saliva nicotine, cotinine, 3-OH-cotinine, nornicotine, NNN, NNK, NNAL, 1,2-PG, 1,3-PG, and glycerol, and self-reported overall health status | Non-users residing with e-cigarette users had low but significantly higher levels of cotinine, 3′-OH-cotinine, and 1,2-propanediol in saliva than non-users living in control homes | Convenience sampling of participants |
| Ballbè M, et al. (2014) [13] | Spain | Observational study | 54 | n/a | Immunology | Saliva and urine cotinine | Cotinine concentrations in non-smokers exposed to e-cigarette vapour at home were significantly higher than in non-smokers from control homes | Convenience sampling of participants and small sample size; only 5 participants in the e-cigarette exposure group |
| Ballbè M, et al. (2023) [19] | Spain | Case study | 3 | 3, 40, 47 | Immunology | Urine, saliva, hair, cord blood and breast milk for nicotine and metabolites (cotinine, 3 OH-cotinine, nornicotine), TSNAs (NNN, NNK, NNAL), 1,2-PD and 1,3-PD, and glycerol | Nicotine and its metabolites were found in a child exposed to e-cigarettes from parents at home. | Small sample size and short duration of exposure; realistic condition, but of very limited scope |
| Bayly JE, et al. (2018) [20] | United States | Cross-sectional study | 11,830 | 11–17 | Respiratory | Self-reported asthma attack | Secondhand e-cigarette exposure was associated with higher odds of reporting an asthma attack in the past 12 months (adjusted OR, 1.27; 95% CI, 1.11–1.47) | Self-reported, and the inability to assess the temporal sequence of events |
| Costantino S, et al. (2024) [21] | Italy | Observational study | 54 | 5–17 | Respiratory | Asthma exacerbations, need for rescue therapy, need for therapeutic step-up, ACT/c-ACT score | Asthmatic patients exposed to secondhand e-cigarettes at home had higher exacerabtions, needed more rescure therapy, therapeutic step-up, and ACT/c-ACT score; however, all results were not statistically significant | Small sample size and self-reported symptoms |
| Farrell KR, et al. (2022) [22] | United States | Cross-sectional study | 16,173 | 18 and older | Mental health | Internalizing mental health disorders | Secondhand e-cigarette emissions exposure among non-users (AOR = 1.43, 1.03–1.99) was associated with increased odds of moderate-to-severe internalizing mental health problems compared to unexposed non-users | Inability to assess the temporal sequence of events |
| Flouris AD, et al. (2012) [15] | Greece | Experimental study | 15 | 28.87 | Immunology | CBC | CBC indices remained unchanged | Small sample size, short duration of exposure |
| Flouris AD, et al. (2013) [14] | Greece | Experimental study | 15 | 18–57 | Respiratory, Immunology | Serum cotinine, lung function (FVC, FEV1, FEV1/FVC ratio, PEF, FVC (FEF25-75)), exhaled CO, FeNO | Serum cotinine was significantly increased after a passive e-cigarette smoking session (silimar to tobacco cigarette smoking); however, lung function, CO, and FeNo remained unchanged compared with a control session. | Small sample size, short duration of exposure |
| Islam T, et al. (2022) [23] | United States | Cohort study | 2097 | 17.3 (first year), and 21.9 (the follow-up year) | Respiratory | Self-reported bronchitic symptoms, wheezing, and shortness of breath | After additionally adjusting for secondhand smoking, secondhand cannabis, and primary vaping or smoking, the secondhand e-cigarette exposure was associated with bronchitic symptoms (OR 1.40, 95% CI1.06 to 1.84) and shortness of breath (OR 1.53, 95% CI 1.06 to 2.21); however, the association between secondhand vaping and wheezing was not statistically significant. | Measurement error due to self-reported exposure and respiratory outcomes |
| Lam TK, et al. (2023) [24] | United States | Cross-sectional study | 2022 | 6–17 | ENT | Self-reported ear infections | E-cigarette exposure in the last 7 days was associated with ≥3 ear infections (OR = 1.61, 95% CI 1.01–2.58, p = 0.047) | Self-reported, and the inability to assess the temporal sequence of events |
| Lee, et al. (2019) [25] | United States | Experimental study | 5 | 29.4 | Cardiovascular | Heart rate-corrected QT interval | Decreased HRV and shortening of the QTc were found during short-term secondhand exposures to e-cigarette emissions | Small sample size, short duration of exposure |
| McClelland ML, et al. (2021) [26] | United States | Experimental study | 73 | 18–63 | Cardiovascular, Respiratory | Immediate physiological effects (BP, mean arterial pressure, RR, FVC, blood sugar, SpO2, oral temperature) | Oral temperatures of nonvaping participants were significantly higher after passive exposure to e-cigarettes; however, other measures were not significantly affected. | Short duration of exposure |
| Rosenkilde K, et al. (2021) [27] | Denmark | Experimental study | 16 | 56–77 | Respiratory | SP-A and albumin in exhaled air, FEV1, FVC, FEV1/FVC, FeNO, plasma proteins, and self-reported symptoms | Exposure to vapes reduced SP-A in exhaled air while increasing numerous plasma proteins considerably. Throat discomfort increased following passive vape exposure, whereas FVC and FEV1 decreased, but not significantly. | Small sample size, short duration of exposure, unrealistic exposure conditions, and not all participants completed all health examination |
| Tzortzi A, et al. (2020) [28] | Greece | Experimental study | 40 | 18–35 | ENT, Respiratory | Symptoms of irritation relating to ocular (itchiness, burning, watery eyes and dryness), nasal (nasal drip, itchiness, dryness, sneezing and stuffiness), throat-respiratory (dryness, soreness, cough, phlegm and breathlessness) and general complaints (headache, nausea and fatigue) | Ocular, nasal, throat-respiratory symptoms and general complaints also increased significantly and lasted up to 30 min after the exposure and were positively associated with the concentrations of the VOC mixture emitted | Self-reported; short duration of exposure, and unrealistic exposure conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanavanich, R.; Thatasawakul, C.; Youngcharoen, K.; Soponvashira, V.; Pichetsin, P. Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024. Int. J. Environ. Res. Public Health 2025, 22, 1408. https://doi.org/10.3390/ijerph22091408
Patanavanich R, Thatasawakul C, Youngcharoen K, Soponvashira V, Pichetsin P. Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024. International Journal of Environmental Research and Public Health. 2025; 22(9):1408. https://doi.org/10.3390/ijerph22091408
Chicago/Turabian StylePatanavanich, Roengrudee, Chawaphat Thatasawakul, Kamolnut Youngcharoen, Veerapattra Soponvashira, and Panpetch Pichetsin. 2025. "Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024" International Journal of Environmental Research and Public Health 22, no. 9: 1408. https://doi.org/10.3390/ijerph22091408
APA StylePatanavanich, R., Thatasawakul, C., Youngcharoen, K., Soponvashira, V., & Pichetsin, P. (2025). Health Effects from Secondhand Exposure to E-Cigarettes: A Systematic Review of Peer-Reviewed Articles from 2004–2024. International Journal of Environmental Research and Public Health, 22(9), 1408. https://doi.org/10.3390/ijerph22091408





