Determinants of COVID-19 Mortality and Temporal Trends in the Health Regions of the State of São Paulo, Brazil
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBGE | Brazilian Institute of Geography and Statistics |
| GAMLSS | Generalized Additive Model for Location, Scale, and Shape |
| DRS | Departamentos Regionais de Saúde |
| NCDs | Non-communicable diseases |
| SEADE | State System Foundation for Statistical Data Analysis |
| SUS | Sistema Único de Saúde |
| DATASUS | Department of Information and Health Informatics of SUS |
| GAIC | Generalized Akaike Information Criterion |
| AIC | Akaike Information Criterion |
References
- Barbosa, T.P.; Costa, F.B.; Ramos, A.C.; Berra, T.Z.; Arroyo, L.H.; Alves, Y.M.; Santos, F.L.; Arcêncio, R.A. Morbimortalidade por COVID-19 associada a condições crônicas, serviços de saúde e iniquidades: Evidências de sindemia. Rev. Panam. Salud Pública 2022, 46, 1. [Google Scholar] [CrossRef] [PubMed]
- Arcêncio, R. Reiterando o sentido da epidemiologia social na compreensão das desigualdades e avanço da equidade em tempos da COVID-19. An. Inst. Hig. Med. Trop. 2021, 20, 74–77. [Google Scholar] [CrossRef]
- Lima Kubo, H.K.; Campiolo, E.L.; Ochikubo, G.T.; Batista, G. Impacto Da Pandemia do Covid19 no Serviço de Saúde: Uma Revisão de Literatura. Interam. J. Med. Health 2020, 3, 140. [Google Scholar] [CrossRef]
- São Paulo. Secretaria de Estado da Saúde de São Paulo. Plano de Contingência do Estado de São Paulo para a Infecção Humana pelo novo Coronavírus (SARS-CoV-2). 30 April 2020. Available online: https://www.saude.sp.gov.br/resources/ccd/homepage/covid-19/versao_final_finalplano_de_contigencia_03_04_rev_3.pdf (accessed on 15 April 2022).
- São Paulo. Secretaria de Estado da Saúde de São Paulo [Internet]. Estado de São Paulo, Segundo Departamentos de Saúde, 2012. 2012. Available online: https://www.saude.sp.gov.br/ses/institucional/departamentos-regionais-de-saude/regionais-de-saude (accessed on 15 April 2022).
- Mello, G.A.; Pereira, A.P.C.d.M.; Uchimura, L.Y.T.; Iozzi, F.L.; Demarzo, M.M.P.; Viana, A.L.D. A systematic review of the process of regionalization of Brazil’s Unified Health System, SUS. Cienc. Saude Coletiva 2017, 22, 1291–1310. [Google Scholar] [CrossRef]
- São Paulo. Departamentos Regionais de Saúde—Secretaria da Saúde—Governo do Estado de São Paulo [Internet]. saude.sp.gov.br. Available online: https://saude.sp.gov.br/ses/institucional/departamentos-regionais-de-saude/ (accessed on 5 October 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados|IBGE. 2022. Available online: https://www.ibge.gov.br/cidades-e-estados/sp.html (accessed on 15 April 2022).
- Instituto Butantan. Vacinação contra COVID-19 no Brasil Completa 1 ano com Grande Impacto da CoronaVac na Redução de Hospitalizações e Mortes. Portal do Butantan [Internet]. 2022. Available online: https://butantan.gov.br/noticias/vacinacao-contra-covid-19-no-brasil-completa-1-ano-com-grande-impacto-da-coronavac-na-reducao-de-hospitalizacoes-e-mortes (accessed on 30 April 2022).
- Brasil. Fundação Sistema Estadual de Análise de Dados (SEADE). Dados Abertos. April 2022. Available online: https://github.com/seade-R/dados-covid-sp (accessed on 15 April 2022).
- Brasil. Ministério da Saúde. DATASUS. April 2022. Available online: https://opendatasus.saude.gov.br/dataset/covid-19-vacinacao (accessed on 15 April 2022).
- Cleveland, W.S.; Grosse, E. Computational methods for local regression. Stat. Comput. 1991, 1, 47–62. [Google Scholar] [CrossRef]
- Box, G.E.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Heller, G.Z.; Stasinopoulos, M.D.; Rigby, R.A.; Bastiani, F.D. Distributions for Modeling Location, Scale, and Shape. In Using GAMLSS in R; Taylor & Francis Group: Abingdon, UK, 2019; 560p. [Google Scholar] [CrossRef]
- Akaike, H. A new look at statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Azzalini, A.; Capitanio, A. Distributions generated by perturbation of symmetry with emphasis on a multivariate skew t-distribution. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2003, 65, 367–389. [Google Scholar] [CrossRef]
- Baqui, P.; Bica, I.; Marra, V.; Ercole, A.; van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil. Lancet Glob. Health 2020, 8, e1018–e1026. [Google Scholar] [CrossRef]
- Rocha, R.; Atun, R.; Massuda, A.; Rache, B.; Spinola, P.; Nunes, L.; Lago, M.; Castro, M.C. Effect of socioeconomic inequalities and vulnerabilities on health-system preparedness and response to COVID-19 in Brazil: A comprehensive analysis. Lancet Glob. Health 2021, 9, e782–e792. [Google Scholar] [CrossRef]
- Castro, M.C.; Kim, S.; Barberia, L.; Ribeiro, A.F.; Gurzenda, S.; Ribeiro, K.B.; Abbott, E.; Blossom, J.; Rache, B.; Singer, B.H. Spatiotemporal pattern of COVID-19 spread in Brazil. Science 2021, 372, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ahmed, N.; Pissarides, C.; Stiglitz, J. Why inequality could spread COVID-19. Lancet Public Health 2020, 5, e240. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Nourbakhsh, S.; Sah, P.; Fitzpatrick, M.C.; Galvani, A.P. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021, 19, e3001211. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Kumar, A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur. J. Epidemiol. 2021, 36, 1237–1240. [Google Scholar] [CrossRef]
- Barberia, L.G.; Gómez, E.J. Political and institutional perils of Brazil’s COVID-19 crisis. Lancet 2022, 399, 730–732. [Google Scholar] [CrossRef]
- Betsch, C.; Böhm, R.; Korn, L.; Holtmann, C. On the benefits of explaining herd immunity in vaccine advocacy. Nat. Hum. Behav. 2017, 1, 56. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; Villela, E.F.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015, Erratum in BMJ 2021, 374, n2091. https://doi.org/10.1136/bmj.n2091. [Google Scholar] [CrossRef]
- Santos Silva, L.; da Conceição Barbosa, R.B.; Lima, J.P.; Castro-Alves, J.; Ribeiro-Alves, M. Racial Inequalities in the Health Establishment Access to the Treatment of COVID-19 in Brazil in 2020. J. Racial. Ethn. Health Disparities 2025, 12, 222–233. [Google Scholar] [CrossRef]
- Lotta, G.; Nunes, J.; Fernandez, M.; Correa, M.G. The impact of the pandemic on frontline health workers in Brazil: Inequalities and challenges. Rev. Bras. Enferm. 2021, 74 (Suppl. S1), e20210036. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. OpenSAFELY: Factors associated with COVID-19 death in 17 million patients. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Jordan, R.E.; Adab, P.; Cheng, K.K. COVID-19: Risk factors for severe disease and death. BMJ 2020, 368, m1198. [Google Scholar] [CrossRef] [PubMed]
- Dowd, J.B.; Andriano, L.; Brazel, D.M.; Rotondi, V.; Block, P.; Ding, X.; Liu, Y.; Mills, M.C. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 9696–9698. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.M.D.; Souza, A.A.; Castro, M.S.M.; Miranda, W.D.; Jardim, L.L.; Sousa, R.P. Influence of socioeconomic inequality on the distribution of COVID-19 hospitalizations and deaths in Brazilian municipalities, 2020: An ecological study. Epidemiol. Serv. Saude 2023, 32, e2022303. [Google Scholar] [CrossRef] [PubMed]
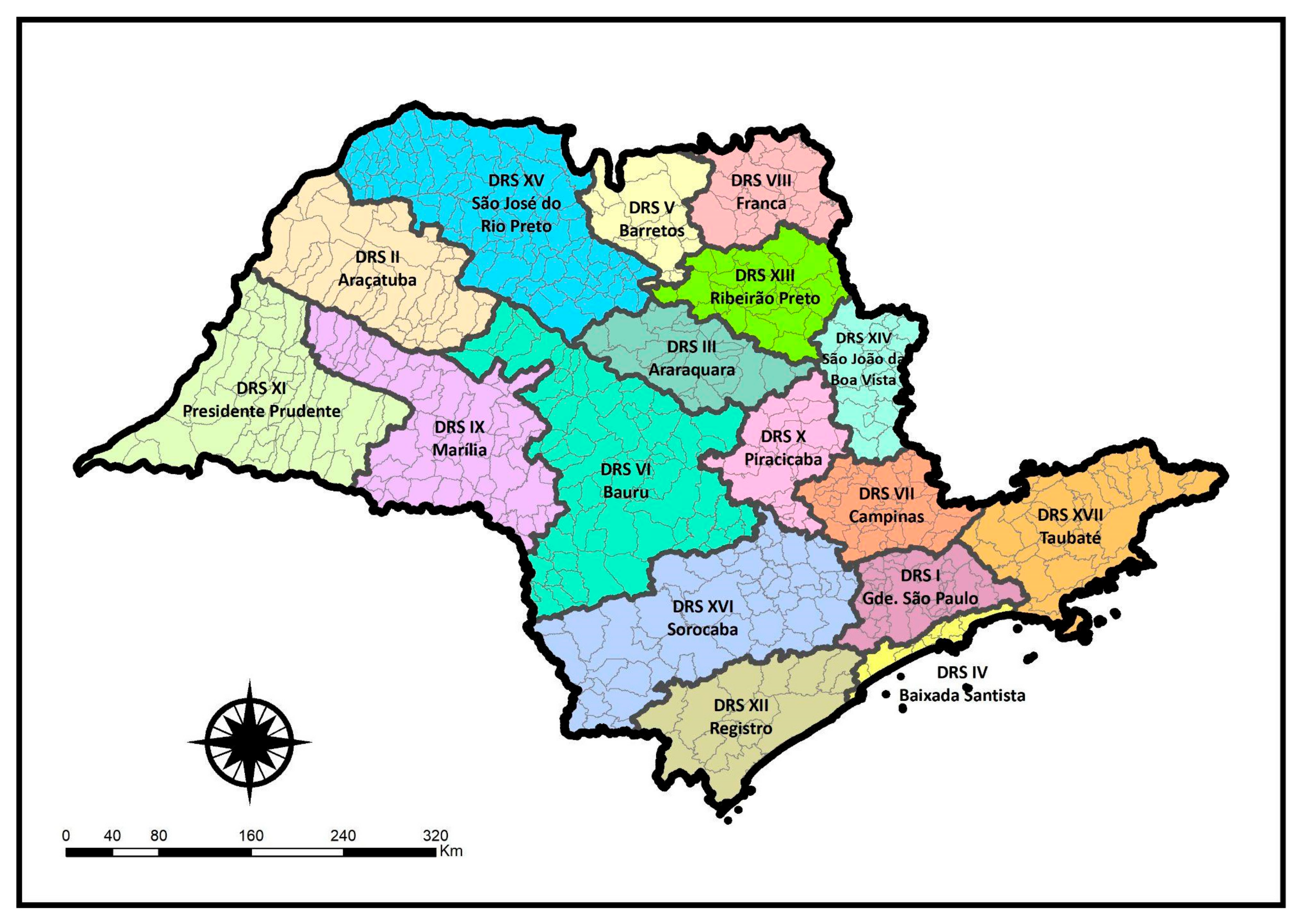
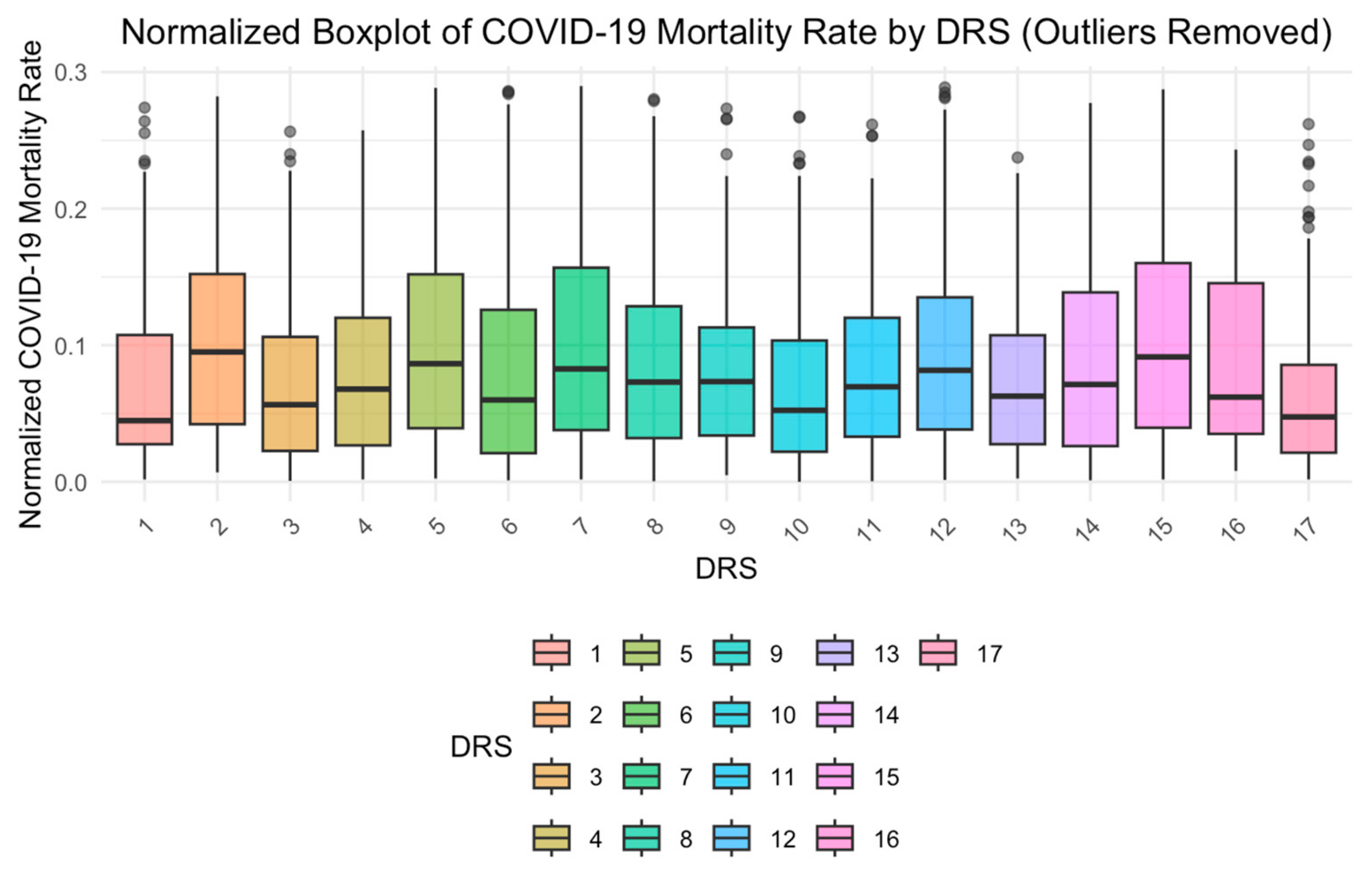
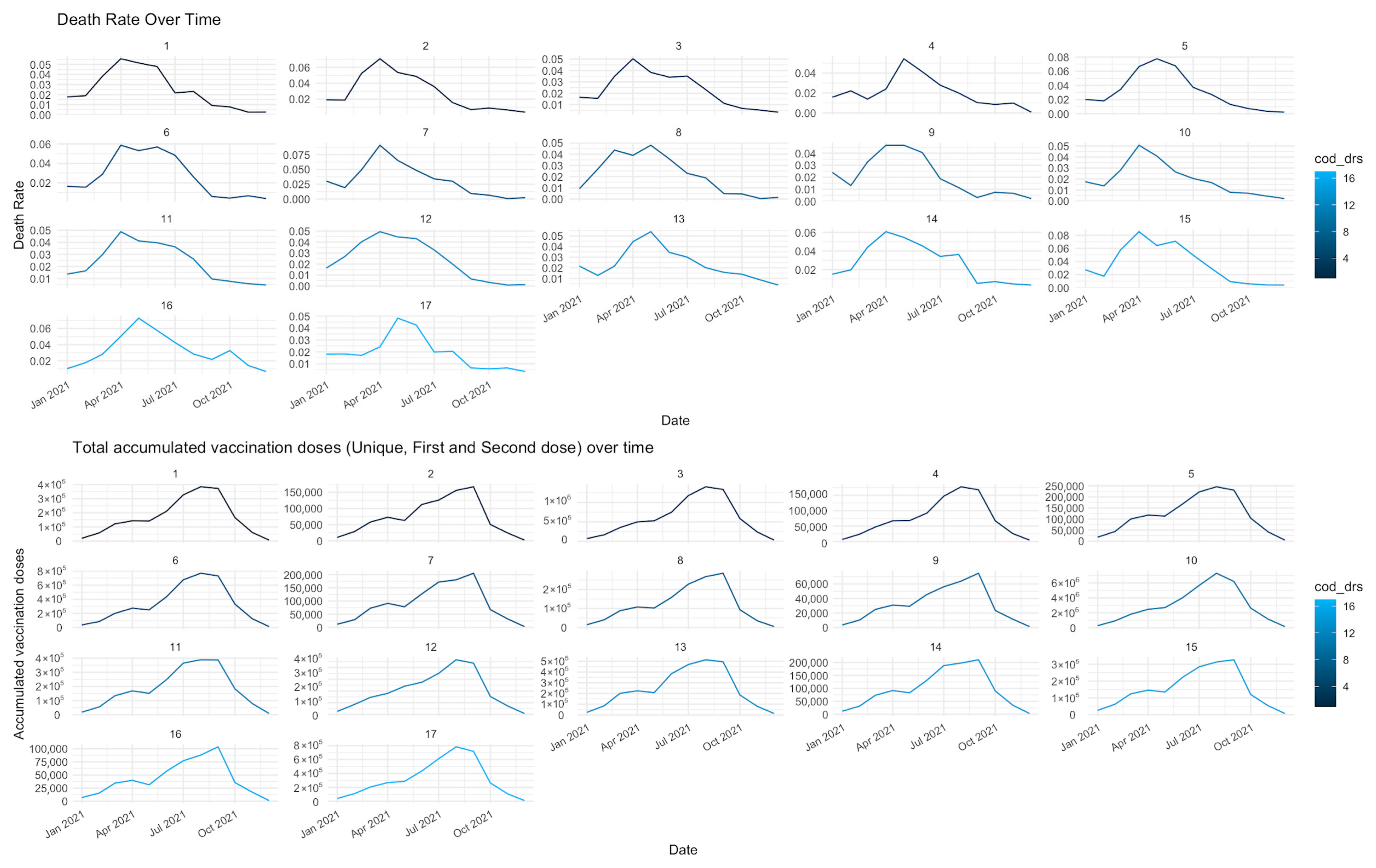
| Dimension | Variables | Date | Data Source |
|---|---|---|---|
| COVID-19 | COVID-19 mortality rate per 100,000 inhabitants | 2020–2021 | SEADE |
| Health indicators | Applied doses (number of COVID-19 vaccines applied in the Population including unique, 1st and 2nd doses) | 2021 | DATASUS |
| Deaths from non-communicable diseases (NCDs) | 2020–2021 | DATASUS | |
| Social Indicators | General population | 2020–2021 | IBGE |
| Population by age group | 2020–2021 | SEADE | |
| Urban population | 2020–2021 | IBGE | |
| Gini index | 2020–2021 | DATASUS |
| Min | Median | Average | Max | |
|---|---|---|---|---|
| Mortality rate per 100,000 inhabitants | 0 | 7.00 | 15.10 | 290.60 |
| Deaths from chronic non-communicable diseases (% of total population) | 0 | 311.00 | 306.47 | 996.00 |
| Percentage of population between 0 and 14 years old (% of total population) | 7.40 | 19.84 | 21.31 | 30.49 |
| Percentage of population aged 15–60 (% of total population) | 57.09 | 65.38 | 68.91 | 80.43 |
| Percentage of population aged 61 or older (% of total population) | 9.30 | 16.22 | 16.42 | 31.47 |
| Percentage of urban population (% of total population) | 24.91 | 93.54 | 89.33 | 100 |
| Gini index (range 0–1) | 0.005 | 0.473 | 0.4586 | 0.6858 |
| COVID-19 Vaccination | 0.10 | 3747.00 | 18,023.61 | 4,314,565.00 |
| Μ | Estimate | Std. Error | t Value | Pr (>|t|) * | Relative Increase (%) |
|---|---|---|---|---|---|
| (Intercept) | 3.128 | 0.14455 | 21.64 | <0.0001 | - |
| V2 | 0.08273 | 0.03094 | 2.674 | <0.0001 | 8.62 |
| V4 | −0.19341 | 0.08952 | −2.161 | 0.0309 | −17.59 |
| V5 | −0.05353 | 0.06969 | −0.768 | 0.4426 | - |
| V9 | 0.01876 | 0.05132 | 0.365 | 0.7148 | - |
| V10 | −0.06153 | 0.0336 | −1.831 | 0.0674 | - |
| V11 | 0.13559 | 0.14247 | 0.952 | 0.3414 | - |
| cod_drs_2 | 0.41721 | 0.24499 | 1.703 | 0.0889 | - |
| cod_drs_3 | 0.04929 | 0.17025 | 0.29 | 0.7722 | - |
| cod_drs_5 | 0.29798 | 0.19087 | 1.561 | 0.1188 | - |
| cod_drs_6 | 0.34907 | 0.17454 | 2 | 0.0457 | 41.77 |
| cod_drs_7 | 0.51329 | 0.1794 | 2.861 | 0.0043 | 67.08 |
| cod_drs_8 | 0.12737 | 0.20243 | 0.629 | 0.5293 | - |
| cod_drs_9 | −0.36026 | 0.26183 | −1.376 | 0.1691 | - |
| cod_drs_10 | 0.01763 | 0.16375 | 0.108 | 0.9143 | - |
| cod_drs_11 | 0.04877 | 0.19551 | 0.249 | 0.8030 | - |
| cod_drs_12 | 0.15125 | 0.16585 | 0.912 | 0.3620 | - |
| cod_drs_13 | −0.13574 | 0.23555 | −0.576 | 0.5645 | - |
| cod_drs_14 | 0.27366 | 0.21084 | 1.298 | 0.1946 | - |
| cod_drs_15 | 0.42503 | 0.19895 | 2.136 | 0.0329 | 52.96 |
| cod_drs_16 | 0.36509 | 0.23625 | 1.545 | 0.1226 | - |
| cod_drs_17 | −0.08403 | 0.18163 | −0.463 | 0.6437 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, T.P.; Berra, T.Z.; Ramos, A.C.V.; Alves, Y.M.; Tavares, R.B.V.; de Paiva, F.S.J.; Alonso, J.B.; Teibo, T.K.A.; de Araújo, J.S.T.; Tártaro, A.F.; et al. Determinants of COVID-19 Mortality and Temporal Trends in the Health Regions of the State of São Paulo, Brazil. Int. J. Environ. Res. Public Health 2025, 22, 772. https://doi.org/10.3390/ijerph22050772
Barbosa TP, Berra TZ, Ramos ACV, Alves YM, Tavares RBV, de Paiva FSJ, Alonso JB, Teibo TKA, de Araújo JST, Tártaro AF, et al. Determinants of COVID-19 Mortality and Temporal Trends in the Health Regions of the State of São Paulo, Brazil. International Journal of Environmental Research and Public Health. 2025; 22(5):772. https://doi.org/10.3390/ijerph22050772
Chicago/Turabian StyleBarbosa, Tatiana Pestana, Thais Zamboni Berra, Antônio Carlos Vieira Ramos, Yan Mathias Alves, Reginaldo Bazon Vaz Tavares, Fernando Spanó Junqueira de Paiva, Jonas Bodini Alonso, Titilade Kehinde Ayandeyi Teibo, Juliana Soares Tenório de Araújo, Ariela Fehr Tártaro, and et al. 2025. "Determinants of COVID-19 Mortality and Temporal Trends in the Health Regions of the State of São Paulo, Brazil" International Journal of Environmental Research and Public Health 22, no. 5: 772. https://doi.org/10.3390/ijerph22050772
APA StyleBarbosa, T. P., Berra, T. Z., Ramos, A. C. V., Alves, Y. M., Tavares, R. B. V., de Paiva, F. S. J., Alonso, J. B., Teibo, T. K. A., de Araújo, J. S. T., Tártaro, A. F., & Arcêncio, R. A. (2025). Determinants of COVID-19 Mortality and Temporal Trends in the Health Regions of the State of São Paulo, Brazil. International Journal of Environmental Research and Public Health, 22(5), 772. https://doi.org/10.3390/ijerph22050772







