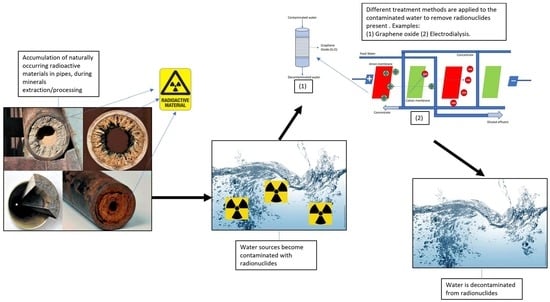
State-of-the-Art Review on Removal of Naturally Occurring Radioactive Materials in Water
Abstract
1. Introduction
2. Background
2.1. Origin and Sources of Natural Radioactivity
2.2. Physicochemical Characteristics of Radon, Radium, Thorium, and Uranium
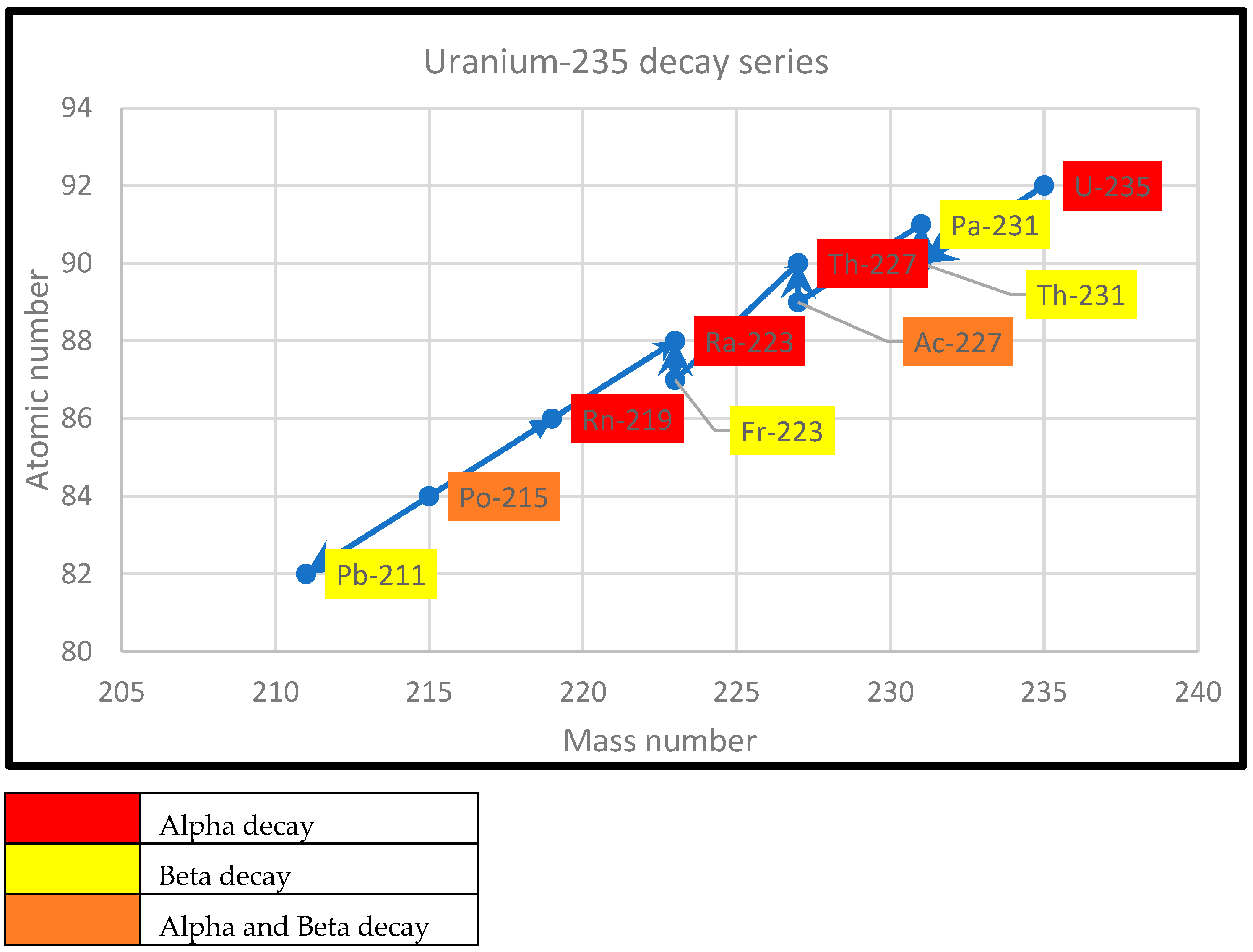
2.3. Decay Series of Uranium-238, Thorium-232, and Uranium-235
2.4. Natural Radionuclides in Water
2.5. Radionuclides in Drinking Water Supplies
2.6. Regulatory Framework for Drinking Water Sources
2.7. Regulatory Framework
3. Materials and Methods
4. Techniques for Treating Radionuclides in Water
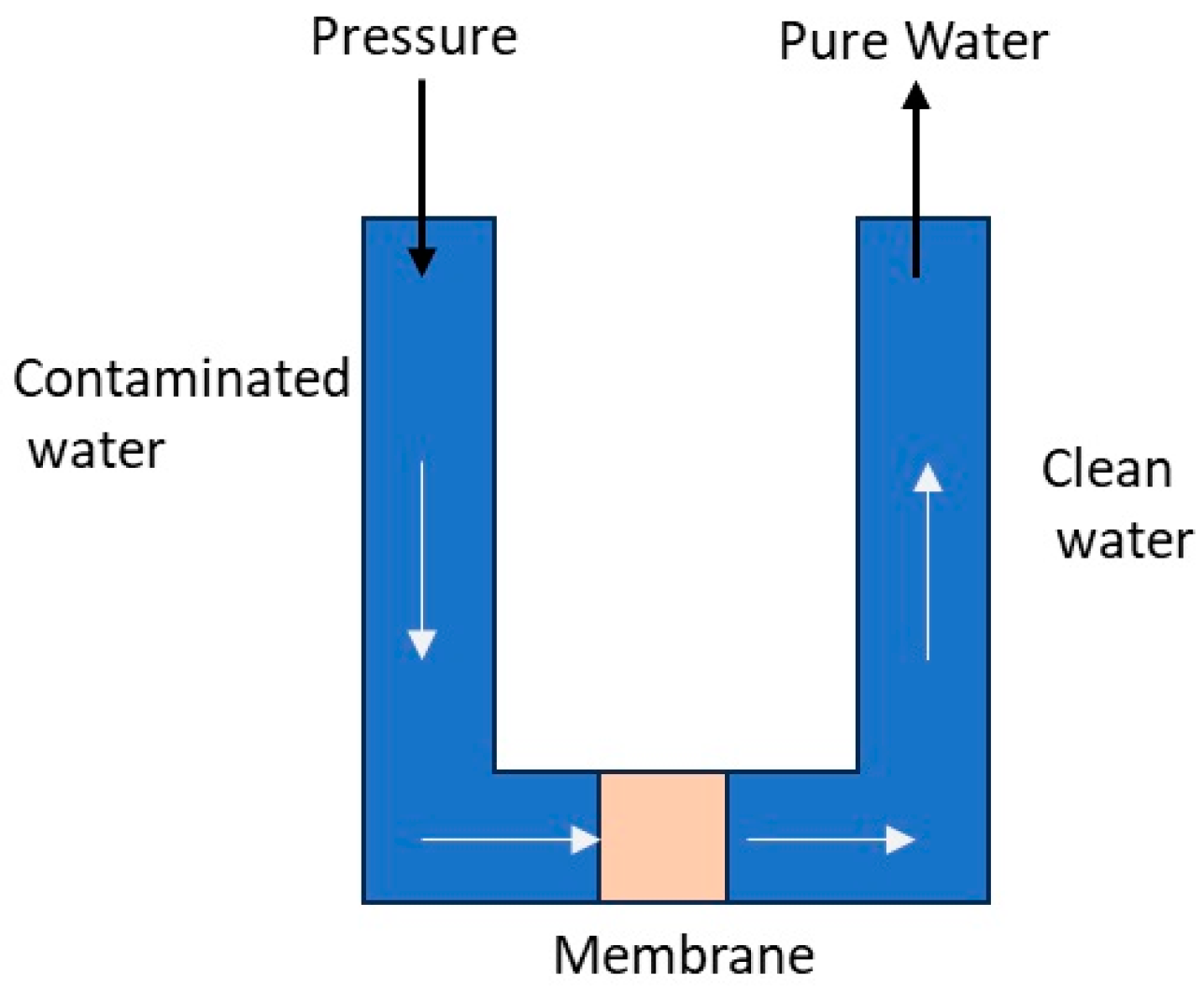
4.1. Membrane Filtration Technology
4.2. Nanotechnology
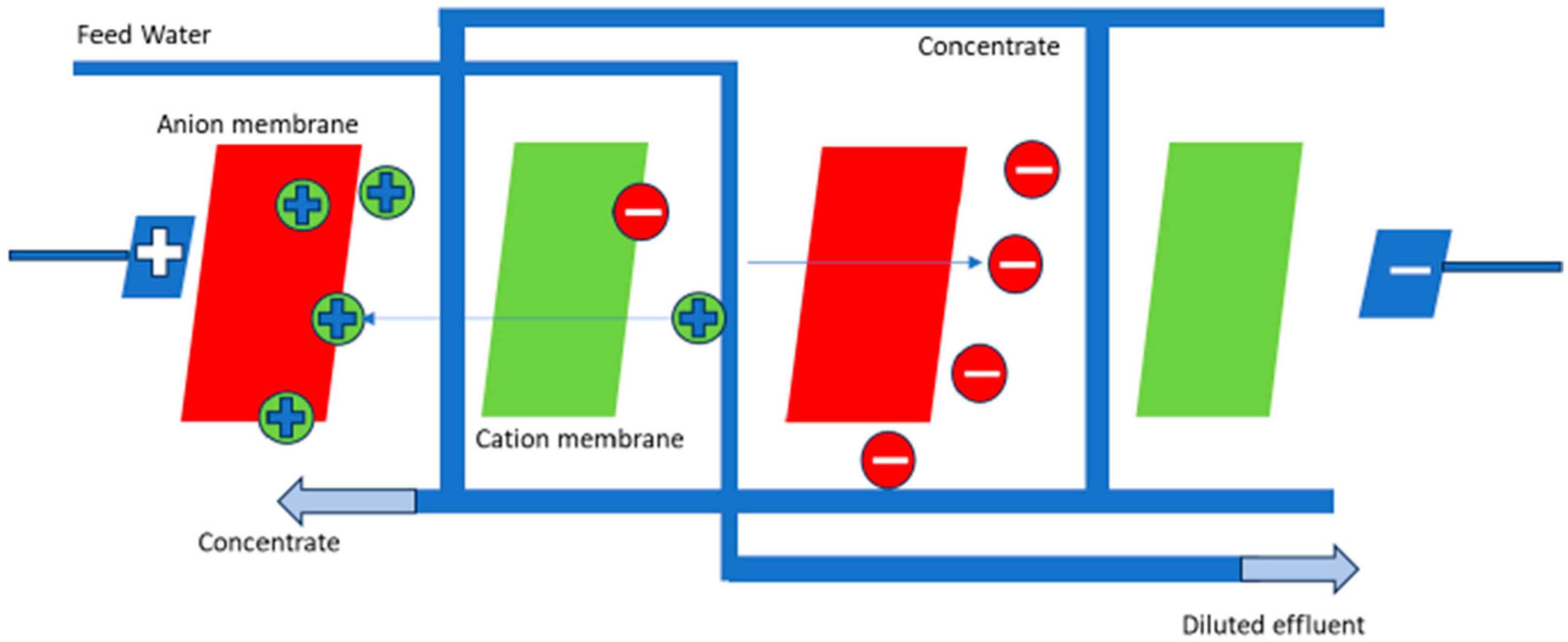
4.3. Electrochemical Methods
4.4. Bioremediation
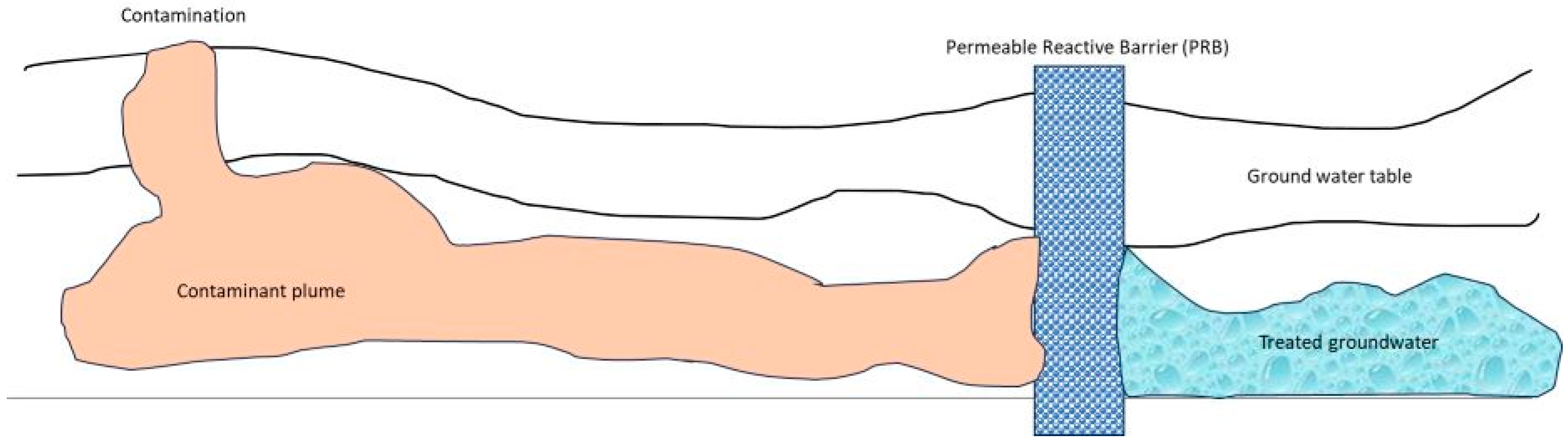
4.5. Permeable Reactive Barrier
4.6. Aeration Technique
4.7. Activated Carbon
4.8. Comparative Costs of the Different Techniques
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richardson, D.B.; Rage, E.; Demers, P.A.; Do, M.T.; Fenske, N.; Deffner, V.; Kreuzer, M.; Samet, J.; Bertke, S.J.; Kelly-Reif, K.; et al. Lung Cancer and 222Rn: Pooled Analysis of Uranium Miners Hired in 1960 or Later. J. Environ. Health 2022, 130, 057010. [Google Scholar]
- World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241565486 (accessed on 4 June 2023).
- US Nuclear Regulatory Commission. Natural Radiation Sources. 2018. Available online: https://www.nrc.gov/docs/ml1827/ml18276a155.pdf (accessed on 6 June 2023).
- Alsharef, S.; Alanazi, M.; Alharthi, F.; Qandil, D.; Qushawy, M. Review About Radiopharmaceuticals: Preparation, Radioactivity, And Applications. J. Appl. Pharm. 2020, 202, 112. [Google Scholar] [CrossRef]
- Malkapur, S.M.; Ghodke, S.S.; Sujatha, P.N.; Singh, Y.; Shivakumar, K.S.; Sen, M.; Pulgur, A.V. Waste-polymer incorporated concrete mixes for neutron and gamma radiation shielding. Prog. Nucl. Energy 2021, 135, 103694. [Google Scholar] [CrossRef]
- Missimer, T.M.; Teaf, C.; Maliva, R.G.; Danley-Thomson, A.; Covert, D.; Hegy, M. Natural Radiation in the Rocks, Soils, and Groundwater of Southern Florida with a Discussion on Potential Health Impacts. Int. J. Environ. Res. Public Health 2019, 16, 1793. [Google Scholar] [CrossRef]
- Konya, J.; Nagy, N.M. Radioactive Decay. In Nuclear and Radiochemistry; Elsevier: Amsterdam, The Netherlands, 2012; Available online: https://shop.elsevier.com/books/nuclear-and-radiochemistry/konya/978-0-12-391430-9 (accessed on 6 June 2023).
- EBSCO. Radon (Rn) Information Services. 2022. Available online: https://www.ebsco.com/research-starters/science/radon-rn (accessed on 21 February 2025).
- Belete, G.D.; Anteneh, Y.A. General Overview of Radon Studies in Health Hazard Perspectives. J. Oncol. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- WHO. WHO Handbook on Indoor Radon: A Public Health Perspective. 2009. Available online: https://www.who.int/publications/i/item/9789241547673 (accessed on 21 February 2025).
- EPA (Environmental Protection Agency). Radon. 2019. Available online: https://www.epa.gov/radon (accessed on 21 February 2025).
- Sicius, H. Handbook of the Chemical Elements; Springer: New York, NY, USA, 2024; pp. 77–139. [Google Scholar]
- Stackelberg, P.E.; Szabo, Z.; Jurgens, B.C. Radium mobility and the age of groundwater in public-drinking-water supplies from the Cambrian-Ordovician aquifer system, north-central USA. J. Appl. Geochem. 2018, 89, 34–48. [Google Scholar] [CrossRef]
- World Nuclear Association. Thorium. 2024. Available online: https://world-nuclear.org/information-library/current-and-future-generation/thorium (accessed on 21 February 2025).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Thorium. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591328 (accessed on 21 February 2025).
- Pidchenko, I.; Salminen-Paatero, S.; Rothe, J.; Suksi, J. Study of uranium oxidation states in geological material. J. Environ. Radioact. 2013, 124, 141–146. [Google Scholar] [CrossRef]
- Bird, G.A. Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2019; pp. 11220–11262. [Google Scholar] [CrossRef]
- Keith, S.; Faroon, O.; Roney, N.; Scinicariello, F.; Wilbur, S.; Ingerman, L.; Llados, F.; Plewak, D.; Wohlers, D.; Diamond, G. Toxicological Profile for Uranium. Agency for Toxic Substances and Disease Registry. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK158804/ (accessed on 21 February 2025).
- US Nuclear Regulatory Commission. Annual Radioactive Effluent Release Report and Radiological Environmental Operating Report. 2023. Available online: https://www.nrc.gov/docs/ML2411/ML24116A119.pdf (accessed on 2 July 2023).
- Sources, Effects and Risks of Ionizing Radiation: United Nations Scientific Committee on the Effects of Atomic Radiation 2016. Report to the General Assembly, with Scientific Annexes. 2016. Available online: https://documents.un.org/doc/undoc/gen/v24/049/32/pdf/v2404932.pdf (accessed on 17 May 2024).
- Hou, X.; Dai, X. Environmental liquid scintillation analysis. In Handbook of Radioactivity Analysis; L’Annunziata, M.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry [ATSDR]. Radium-226. 2016. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/radionuclides.pdf (accessed on 17 May 2024).
- Silva, A.S.; Dinis, M.d.L. Assessment of indoor 222Rn concentration and time-series analysis of gamma dose rate in three thermal spas from Portugal. Environ. Monit. Assess. 2022, 194, 611. [Google Scholar] [CrossRef]
- Silva, A.S.; Dinis, M.d.L.; Pereira, A. Indoor 222Rn Levels in Thermal Spas and the Compliance with the European BSS Directive: A Portuguese Case Study. In Occupational and Environmental Safety and Health II. Studies in Systems, Decision and Control; Arezes, P.M., Baptista, J.S., Barroso, M.P., Carneiro, P., Cordeiro, P., Costa, N., Melo, R.B., Miguel, A.S., Perestrelo, G., Eds.; Springer: Cham, Switzerland, 2020; Volume 277. [Google Scholar] [CrossRef]
- Santamarta, J.C.; Hernández-Gutiérrez, L.E.; Rodríguez-Martín, J.; Lario-Bascones, R.J.; Morales-González-Moro, Á.; Cruz-Pérez, N. 222Rn measurements in groundwater mines in La Palma and El Hierro, Canary Islands (Spain). Arch. Min. Sci. 2020, 65, 864–876. [Google Scholar]
- International Commission on Radiological Protection. 2014 Annual Report. Available online: https://www.icrp.org/docs/ICRP%20Annual%20Report%202014.pdf (accessed on 4 June 2024).
- Przylibski, T.A.; Staśko, S.; Domin, E. 222Rn groundwater in a 222Rn-prone area: Possible uses and problems: An example from SW part of Kłodzko Valley, Sudetes, SW Poland. Environ. Geochem. Health 2022, 44, 4539–4555. [Google Scholar] [CrossRef]
- Kallio, A.; Leikoski, N.; Otaki, M. Natural radioactivity of residues from groundwater treatment facilities in Finland. J. Radiol. Prot. 2023, 43, 031517. Available online: https://iopscience.iop.org/article/10.1088/1361-6498/acf8d1 (accessed on 6 June 2024). [CrossRef]
- US Environmental Protection Agency [EPA]. Radionuclides in Drinking Water: A Small Entity Compliance Guide. 2015. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/compliance-radionuclidesindw.pdf (accessed on 6 June 2023).
- Commission Recommendation of 20 December 2001 on the Protection of the Public Against Exposure to Radon in Drinking Water Supplies (Notified Under Document Number C(2001) 4580). 2001. Available online: http://data.europa.eu/eli/reco/2001/928/oj (accessed on 7 June 2023).
- Sources, Effects and Risks of Ionizing Radiation United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2017 Report to the General Assembly, with Scientific Annexes. Available online: https://www.unscear.org/unscear/uploads/documents/unscear-reports/UNSCEAR_2017_Report-CORR.pdf (accessed on 19 May 2023).
- Technical Regulation by the Board of Directors of the Jordanian Institute of Standards and Metrology (JISM) 2021. Available online: https://www3.dfc.gov/environment/eia/ammanwater/Annex%20C21-Drinking%20Water%20Standards.pdf (accessed on 19 May 2023).
- De Jesus, M. Groundwater Protection for Public Water Supply in Portugal. United Nations Economic Commission for Europe. 2021. Available online: https://unece.org/fileadmin/DAM/env/water/meetings/groundwater01/portugal.pdf (accessed on 28 June 2023).
- Environmental Implementation Review 2022 Country Report–Portugal. Available online: https://environment.ec.europa.eu/system/files/2022-09/Portugal%20-%20EIR%20Country%20Report%202022%20%28EN%29.PDF (accessed on 26 June 2023).
- Jordan Ministry of Water and Irrigation. Jordan Water Sector Facts and Figures 2020. 2020. Available online: https://www.mwi.gov.jo/ebv4.0/root_storage/ar/eb_list_page/facts_and_figures_english_2020.pdf (accessed on 25 June 2023).
- Al-Bedri, M.B.H.; Younis, T.A.J.; Abdulghani, I.J.; Hameed, W.O. Determination of naturally occurring radionuclides in Disi aquifer water of Jordan. IOP Conf. Ser. Mater. Sci. Eng. A 2020, 871, 012066. [Google Scholar] [CrossRef]
- Mundigl, S. International Conference on Radiation Safety: Improving Radiation Protection in Practice. European Commission. 2020. Available online: https://www.iaea.org/sites/default/files/20/11/rasa-exemptionandclearancemundigl.pdf (accessed on 28 June 2023).
- Leier, M.; Suursoo, S.; Madis, K. Cost-benefit analysis of health risks caused by indicative dose from drinking water consumption. J. Environ. Radioact. 2021, 54, 106546. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Safety Reports Series No. 114. 2023. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/PUB1986_web.pdf (accessed on 28 June 2023).
- Alkhadra, M.A.; Conforti, K.M.; Gao, T.; Tian, H.; Bazant, M.Z. Continuous Separation of Radionuclides from Contaminated Water by Shock Electrodialysis. Environ. Sci. Technol. 2019, 54, 527–536. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Su, X.; Suss, M.E.; Tian, H.; Guyes, E.N.; Shocron, A.N.; Conforti, K.M.; de Souza, J.P.; Kim, N.; Tedesco, M.; et al. Electrochemical methods for water purification, ion separations, and energy conversion. Chem. Rev. 2022, 122, 13547–13635. [Google Scholar] [CrossRef] [PubMed]
- Mastropietro, T.F.; Bruno, R.; Pardo, E.; Armentano, D. Reverse osmosis and nanofiltration membranes for highly efficient PFASs removal: Overview, challenges and future perspectives. Dalton Trans. 2021, 50, 5398–5410. [Google Scholar] [CrossRef]
- Montaña, M.; Camacho, A.; Serrano, I.; Devesa, R.; Matia, L.; Vallés, I. Removal of radionuclides in drinking water by membrane treatment using ultrafiltration, reverse osmosis and electrodialysis reversal. J. Environ. Radioact. 2013, 125, 86–92. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents nanoparticles magnetic biosorbents: A review. J. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Kumar, N.; Balamurugan, A.; Shafreen, M.; Rahim, A.; Vats, S.; Vishwakarma, K. Nanomaterials: Emerging Trends and Future Prospects for Economical Agricultural System. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Berlin/Heidelberg, Germany, 2020; pp. 281–305. [Google Scholar] [CrossRef]
- Laver, N.R. The Removal of Radionuclides from Contaminated Water Samples Using Graphene Oxide Nano-Flakes. Ph.D. Thesis, UCL (University College London), London, UK, 2020. [Google Scholar]
- Kim, J.; Cote, L.J.; Huang, J. Two dimensional soft material: New faces of graphene oxide. J. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Akpomie, K.G.; Conradie, J.; Adegoke, K.A.; Oyedotun, K.O.; Ighalo Joshua, O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O. Adsorption mechanism and modeling of radionuclides and heavy metals onto zno nanoparticles. Appl. Water Sci. 2022, 13, 20. [Google Scholar] [CrossRef]
- Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive Wastewater Treatment Technologies: A Review. Molecules 2023, 28, 1935. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.; Thomas-Alyea, K.E. 8.3 Models for electrode kinetics. In Electrochemical Systems; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 207–225. Available online: https://download.e-bookshelf.de/download/0000/7525/33/L-G-0000752533-0002367278.pdf (accessed on 14 July 2023).
- Chung, A.P.; Sousa, T.; Pereira, A.; Morais, P.V. Microorganisms–tools for bioremediation of uranium contaminated environments. Procedia Earth Planet. Sci. 2014, 8, 53–58. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar]
- Chamberlain, J.; Michalczak, L.; Seneca, S. Design and Installation of a Permeable Treatment Wall at the West Valley Demonstration Project to Mitigate Expansion of Strontium-90 Contaminated Groundwater-11138; Waste Management Symposium, Inc.: Phoenix, AZ, USA, 2011. [Google Scholar]
- Jastaniah, S.D.; Shakhreet, B.Z.; Abbas, H.Y. A Mitigating Technique for the Treatment of Small Volumes Drinking Water from 222Rn Gas. Open J. Biophys. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pal, A.; Saha, B.B. A Critical Review of the Removal of Radionuclides from Wastewater Employing Activated Carbon as an Adsorbent. Materials 2022, 15, 8818. [Google Scholar] [CrossRef]
- Alabdula’aly, A.I.; Maghrawy, H.B. Comparative study of different types of granular activated carbon in removing medium level radon from water. J. Radioanal. Nucl. Chem. 2011, 287, 77–85. [Google Scholar] [CrossRef]
- Munter, R. Technology for the Removal of Radionuclides from Natural Water and Waste Management. Available online: https://www.academia.edu/80981582/Technology_for_the_removal_of_radionuclides_from_natural_water_and_waste_management_state_of_the_art?source=swp_share (accessed on 15 April 2023).
- EPA (Environmental Protection Agency). A Regulators’ Guide to the Management of Radioactive Residuals from Drinking Water Treatment Technologies. 2006. Available online: https://www.epa.gov/sites/default/files/2015-05/documents/816-r-05-004.pdf (accessed on 1 June 2023).


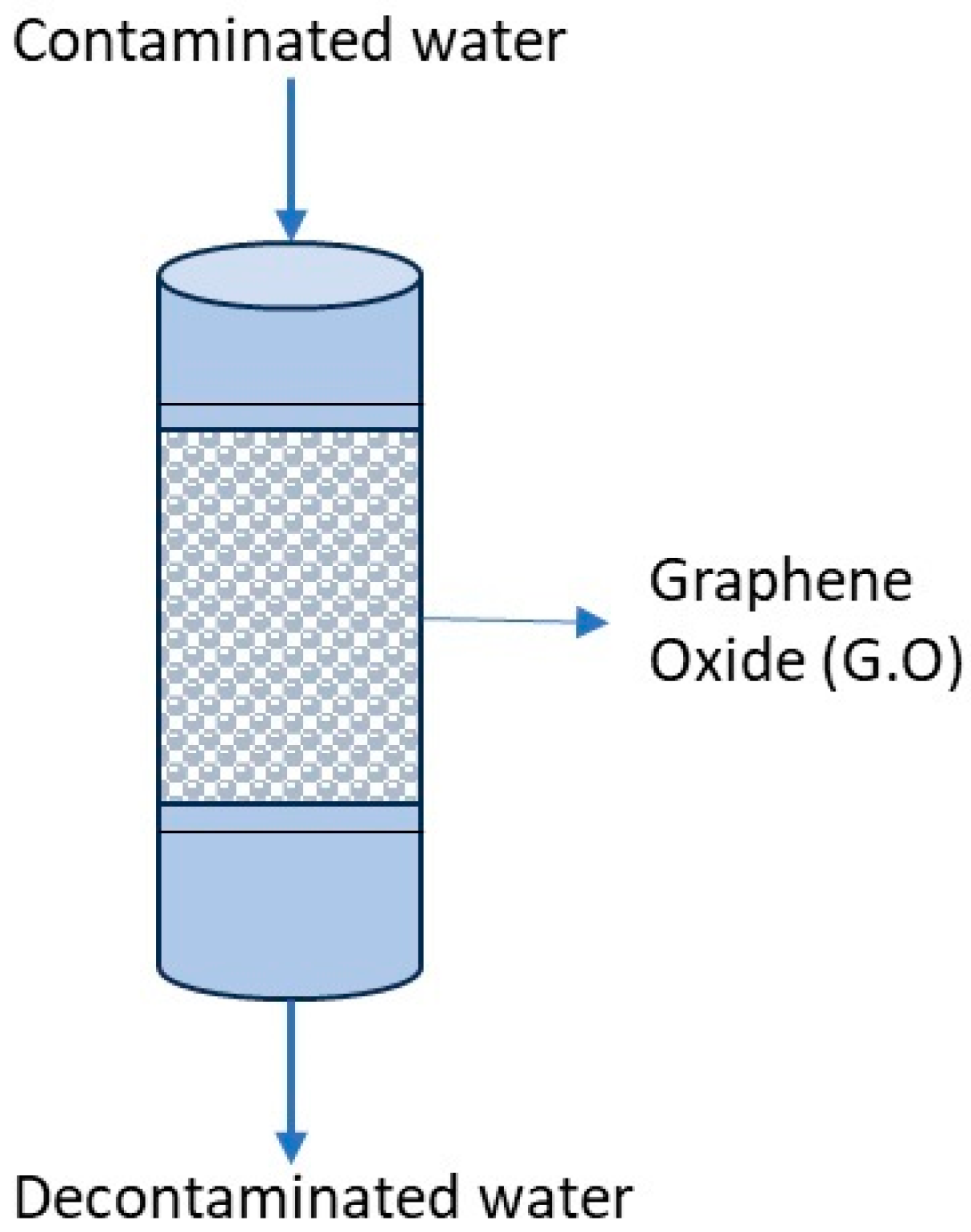
| Material | Reactivity | Solubility | Mobility | References |
|---|---|---|---|---|
| Radon | Chemically inert noble gas. However, its short-lived decay products, like polonium, are chemically reactive and easily attached to aerosols and particulate matter in the air. | Not soluble in water as a gas. Its decay products, mainly polonium-210, may be soluble, deposited on surfaces, or dissolved in water. | Easily dispersed in the gaseous state and can penetrate through different materials such as soil and construction. Its mobility is influenced mainly by diffusion and pressure gradients in the soil or building materials. | [8,9,10,11] |
| Radium | A reactive chemical element within the alkaline earth group that can combine with other elements to form a chemical compound [5]. Its reactivity varies with the chemical form and or condition in which the chemical is present. | Generally insoluble in water at neutral pH levels. It dissolves well in water at ordinary temperatures, except in cases where the pH is low or the salinity is high [6]. | Exists as a free phase in the environment, often in groundwater. Radium mobility can be affected by factors such as the acidity level, temperature, and other chemicals in the water or soil. | [12,13] |
| Thorium | It is capable of actively combining with other elements to form a compound. It also has variable oxidation states and reacts with oxygen, acids, and other chemical reagents. | Its solubility may fluctuate based on the type of form, including in the diet and other situations that characterize one’s environment. Like most elements, thorium can form both insoluble and soluble compounds with water. | Very mobile in some settings, particularly in an acidic environment. Its mobility in groundwater and surface water can contaminate soil and water sources. | [14,15] |
| Uranium | React with other elements and can exist in different oxidation states. It is most relevant in the areas of nuclear energy and pollution. | Solubility of compounds in water can be variable depending on the chemical form and the conditions of the environment. Depending on the pH and redox conditions, uranium can dissolve in water to form soluble and insoluble complexes. | Can be mobile in the environment, especially in groundwater and surface water. Its mobility is influenced by factors such as pH, temperature, and the presence of another chemical. Uranium contamination can also cause the element to be transported over vast distances and affect the environment. | [16,17,18] |
| Element | Half-Life | Emitted Radiation |
|---|---|---|
| Uranium-238 | 4.468 billion years | Alpha |
| Thorium-234 | 24.1 days | Beta |
| Protactinium-234 | 1.17 min | Beta |
| Uranium-234 | 245,500 years | Alpha |
| Thorium-230 | 75,380 years | Alpha |
| Radium-226 | 1600 years | Alpha |
| Radon-222 | 3.823 days | Alpha |
| Polonium-218 | 3.05 min | Alpha |
| Lead-214 | 26.8 min | Beta |
| Bismuth-214 | 19.9 min | Beta |
| Polonium-214 | 164 microseconds | Alpha |
| Lead-210 | 22.2 years | Beta |
| Bismuth-210 | 5102 days | Beta |
| Polonium-210 | 138 days | Alpha |
| Lead-206 (stable) | N/A | N/A |
| Element | Half-Life | Emitted Radiation |
|---|---|---|
| Thorium-232 | 14.05 billion years | Alpha |
| Radium-228 | 5.75 years | Beta |
| Actinium-228 | 6.13 h | Beta |
| Thorium-228 | 1.913 years | Alpha |
| Radium-224 | 3.62 days | Alpha |
| Radon-222 | 55.62 s | Alpha |
| Polonium-216 | 0.146 s | Alpha |
| Lead-212 | 10.463 h | Beta |
| Bismuth-212 | 60.55 min | Alpha, Beta |
| Polonium-212 | 0.298 microseconds | Alpha |
| Thallium-208 | 3.053 min | Beta |
| Lead-208 (stable) | N/A | N/A |
| Element | Half-Life | Emitted Radiation |
|---|---|---|
| Uranium-235 | 0.71 billion years | Alpha |
| Thorium-231 | 25.6 h | Beta |
| Protactinium-231 | 33,000 years | Alpha |
| Actinium-227 | 22 years | Alpha, Beta |
| Thorium-227 | 18.2 days | Alpha |
| Francium-223 | 22 min | Beta |
| Radium-223 | 11.7 days | Alpha |
| Rodon-219 | 3.9 s | Alpha |
| Polonium-215 | 1.8 microseconds | Alpha, Beta |
| Lead-211 | 36 min | Beta |
| Astatine-215 | 1.8 milliseconds | Alpha |
| Bismuth-211 | 2.16 min | Alpha, Beta |
| Thallium-207 | 4.8 min | Beta |
| Polonium-211 | 0.52 s | Beta |
| Lead-207(stable) | - | - |
| United States | |
|---|---|
| Radionuclides | Maximum Contaminant Level 1 |
| Combined Radium-226 and Radium-228 | 185 Bq/m3 |
| Gross Alpha (excluding 222Rn and uranium) | 555 Bq/m3 |
| Uranium | 0.372 Bq/m3 |
| Europe | |
| Parameter | Parametric Value 2 |
| Tritium | 100,000 Bq/m3 |
| Rn-222 | 100,000 Bq/m3 |
| Indicative Dose | 0.1 mSv |
| Nuclide | Derived Concentration 3 |
| Radium-226 | 500 Bq/m3 |
| Radium-228 | 200 Bq/m3 |
| Lead-210 | 200 Bq/m3 |
| Uranium-234 | 2800 Bq/m3 |
| Uranium-238 | 3000 Bq/m3 |
| Polonium-210 | 100 Bq/m3 |
| Jordan | |
| Radioactive Material | Standard level 4 |
| Alpha Particles (excluding Rn-222) * | 500 Bq/m3 |
| Beta Particles (excluding Carbon-14 and Tritium) ** | 1000 Bq/m3 |
| Treatment Technique | Removal Techniques | Removal Efficiency |
|---|---|---|
| Radium | Coagulation/filtration | 65–95% |
| Membrane processes | 90–99% | |
| Lime softening | 80–95% | |
| Uranium | Coagulation/filtration | 80–95% |
| Lime softening | 85–99% | |
| Membrane processes | 90–99% | |
| Radon | Aeration system | 70–99% |
| Packed tower | 90–99% | |
| Granular activated carbon | 80–99% |
| Stage | Component | Function |
|---|---|---|
| 1 | Pre-filter, spun-bounded polypropylene | Removes suspended impurities like particles of rust and dust |
| 2 | Pre-carbon filter, silver-impregnated granular-activated carbon | Removes color, odor, and free chlorine and absorbs organics and pesticides |
| 3 | Sediment filter, spun PP cartridge | Acts as a final filter to remove smaller contaminants and remaining particles. Reduces fine turbidity |
| 4 | Reverse osmosis membrane thin-film composite (TFC) ~0.0001 micron | Removes TDS, hardness, fluoride, pesticides, heavy metals like lead, mercury, cadmium, uranium, and arsenic, etc., and micro-organisms like bacteria, viruses, and protozoan cysts |
| 5 | Post-carbon filter, silver-impregnated fine granular-activated carbon | Imparts bacteriostatic property and helps in reviving the original taste of water |
| Feature | Graphene Oxide (GO) Membranes | Membrane Technology (RO) |
|---|---|---|
| Mechanism | Size-sieving, tunable ion separation | Pressure-driven separation, size, and charge exclusion |
| Selectivity | High, tunable | Lower |
| Water Flux | High in some cases | Generally lower than GO membranes |
| Energy Consumption | Potentially lower | Can be high |
| Fouling | Prone to fouling | Prone to fouling |
| Applications | Separation of radionuclides in complex mixtures and highly acidic or saline conditions | Treatment of radionuclide-containing wastewater, water purification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shomali, Z.; Pereira, A.; Marques, A.C.; Dinis, M.d.L. State-of-the-Art Review on Removal of Naturally Occurring Radioactive Materials in Water. Int. J. Environ. Res. Public Health 2025, 22, 727. https://doi.org/10.3390/ijerph22050727
Al-Shomali Z, Pereira A, Marques AC, Dinis MdL. State-of-the-Art Review on Removal of Naturally Occurring Radioactive Materials in Water. International Journal of Environmental Research and Public Health. 2025; 22(5):727. https://doi.org/10.3390/ijerph22050727
Chicago/Turabian StyleAl-Shomali, Zaid, Alcides Pereira, Ana Clara Marques, and Maria de Lurdes Dinis. 2025. "State-of-the-Art Review on Removal of Naturally Occurring Radioactive Materials in Water" International Journal of Environmental Research and Public Health 22, no. 5: 727. https://doi.org/10.3390/ijerph22050727
APA StyleAl-Shomali, Z., Pereira, A., Marques, A. C., & Dinis, M. d. L. (2025). State-of-the-Art Review on Removal of Naturally Occurring Radioactive Materials in Water. International Journal of Environmental Research and Public Health, 22(5), 727. https://doi.org/10.3390/ijerph22050727