The Development and Initial Validation of the Memorial Symptom Assessment Scale-Long COVID (MSAS-LC): A Promising Tool for Measuring Long COVID
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument Development
2.2. Application of MSAS-LC
2.2.1. Study Design
2.2.2. Inclusion Criteria
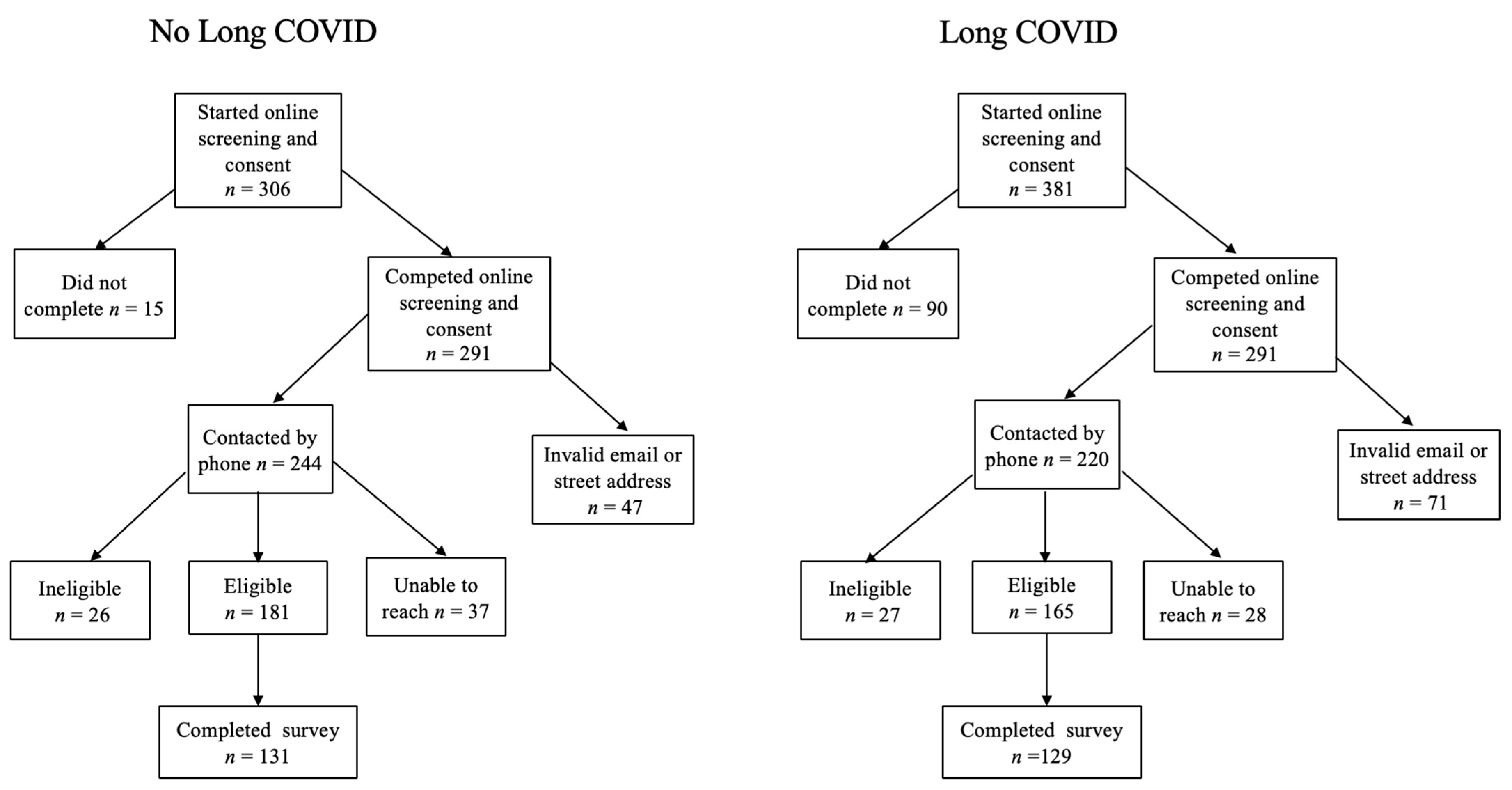
2.2.3. Recruitment Strategy
2.2.4. Sample Size
2.2.5. Screening and Exclusion
2.2.6. Data Collection
2.2.7. Instruments
2.2.8. Data Analysis
3. Results
3.1. Demographic Characteristics
3.2. Data Quality, Face Validity, and Participant Feedback Regarding the MSAS-LC
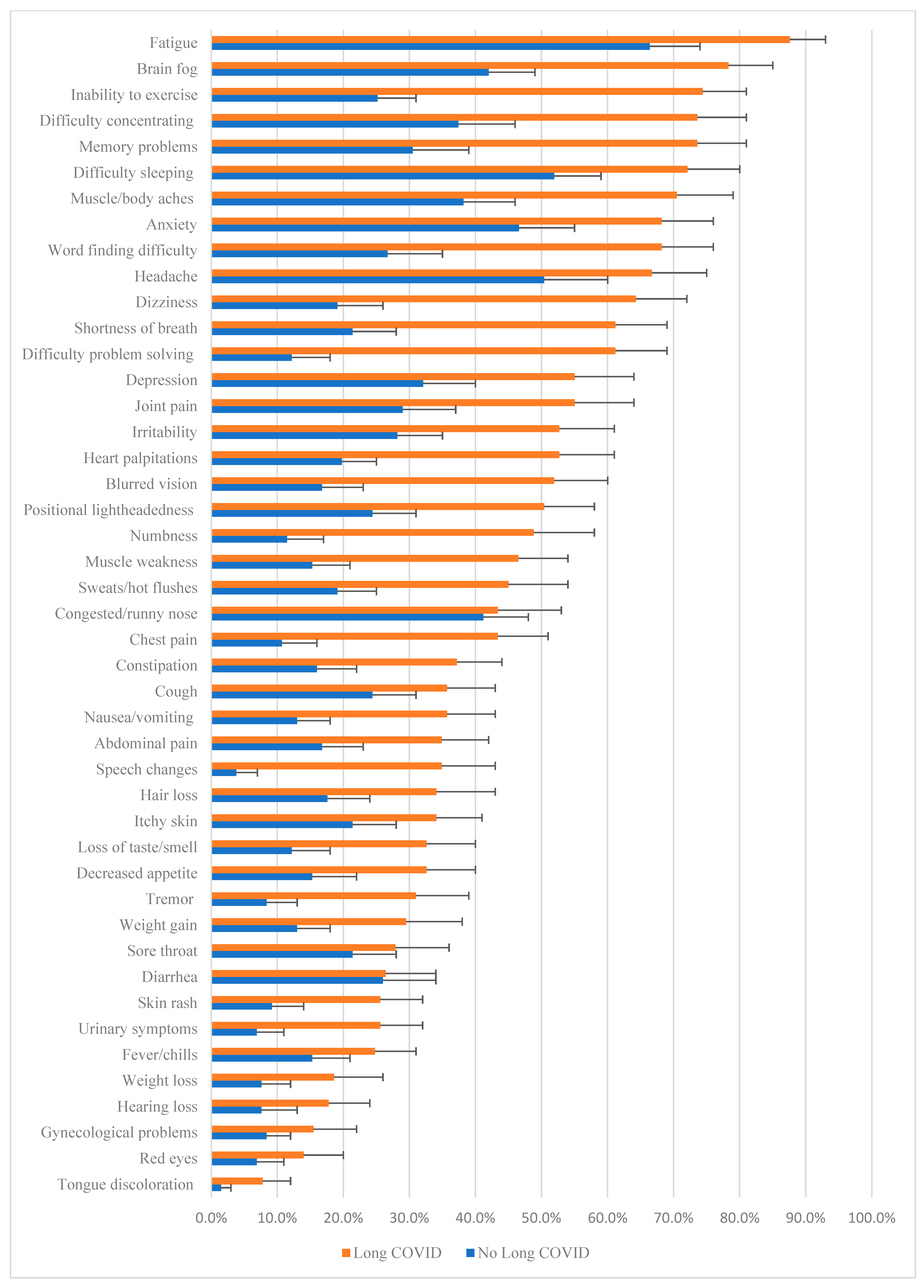
3.3. Prevalence and Burden of MSAS-LC Symptoms in Those Without and with Long COVID
3.4. Neurologic
3.5. Otolaryngologic
3.6. Gastrointestinal
3.7. Psychiatric
3.8. Musculoskeletal
3.9. Unclassified Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSAS-LC | Memorial Symptom Assessment Scale-Long COVID |
| WHO | World Health Organization |
| SBQ-LC | The Symptom Burden Questionnaire for Long COVID |
| PTSD | Post Traumatic Stress Disorder |
| RT-PCR | Reverse transcription polymerase chain reaction |
| REDCap | Research Electronic Data Capture |
| ReCAPTCHA | Revised Completely Automated Public Turing test to Tell Computers and Humans Apart |
References
- WHO. Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 27 April 2024).
- The Lancet. Long COVID: 3 years in. Lancet 2023, 401, 795. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services Office for Civil Rights. Guidance on “Long COVID” as a Disability Under the ADA, Section 504, and Section 1557; US Department of Health and Human Services; US Department of Health and Human Services Office for Civil Rights: Washington, DC, USA, 2021.
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Nearly One in Five American Adults Who Have Had COVID-19 Still Have “Long COVID”. Available online: https://www.cdc.gov/nchs/data/databriefs/db480.pdf (accessed on 28 May 2024).
- Nalbandian, A.; Desai, A.D.; Wan, E.Y. Post-COVID-19 Condition. Annu. Rev. Med. 2023, 74, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Merhavy, Z.I.; Junor, T.; Gonzalez, A.; De Filippis, S.M.; Oveisitork, S.; Rivera, E.; Ndukwu, I.; Bhatara, K. Long COVID: A comprehensive overview of the signs and symptoms across multiple organ systems. Korean J. Fam. Med. 2024, 45, 305. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; Harris, A.M. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years—United States, March 2020–November 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Dennis, A.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Eichert, N.; Mouchti, S.; Pansini, M.; Roca-Fernandez, A.; Thomaides-Brears, H. Multi-organ impairment and long COVID: A 1-year prospective, longitudinal cohort study. J. R. Soc. Med. 2023, 116, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Aghajani Mir, M. Brain fog: A narrative review of the most common mysterious cognitive disorder in COVID-19. Mol. Neurobiol. 2024, 61, 9915–9926. [Google Scholar] [CrossRef]
- Arango-Ibanez, J.P.; Córdoba-Melo, B.D.; Gutiérrez Posso, J.M.; Barbosa-Rengifo, M.M.; Herrera, C.J.; Quintana Da Silva, M.A.; Buitrago, A.F.; Coronel Gilio, M.L.; Pow-Chong-Long, F.; Gómez-Mesa, J.E. Long COVID Clusters of Symptoms Persist beyond Two Years after Infection: Insights from the CARDIO COVID 20–21 Registry. Viruses 2024, 16, 1028. [Google Scholar] [CrossRef]
- Niewolik, J.; Mikuteit, M.; Klawitter, S.; Schröder, D.; Stölting, A.; Vahldiek, K.; Heinemann, S.; Müller, F.; Behrens, G.M.N.; Klawonn, F.; et al. Cluster analysis of long COVID symptoms for deciphering a syndrome and its long-term consequence. Immunol. Res. 2024, 72, 605–613. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Woodrow, M.; Carey, C.; Ziauddeen, N.; Thomas, R.; Akrami, A.; Lutje, V.; Greenwood, D.C.; Alwan, N.A. Systematic Review of the Prevalence of Long COVID. Open Forum Infect. Dis. 2023, 10, ofad233. [Google Scholar] [CrossRef]
- CDC. Long COVID or Post-COVID Conditions. 2023. Available online: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 19 April 2024).
- Cha, C.; Baek, G. Symptoms and management of long COVID: A scoping review. J. Clin. Nurs. 2024, 33, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, E.R.; Madlock-Brown, C.; Baratta, J.M.; Bhatia, A.; Davis, H.; Girvin, A.; Hill, E.; Kelly, E.; Kostka, K.; Loomba, J.; et al. Coding long COVID: Characterizing a new disease through an ICD-10 lens. BMC Med. 2023, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Tak, C.R. The health impact of long COVID: A cross-sectional examination of health-related quality of life, disability, and health status among individuals with self-reported post-acute sequelae of SARS CoV-2 infection at various points of recovery. J. Patient-Rep. Outcomes 2023, 7, 31–39. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Z.; Li, S.; Wang, Y.; Liu, G. Impact of Long COVID on Health-Related Quality of Life Among Patients After Acute COVID-19 Infection: A Cross-Sectional Study. Inquiry 2024, 61, 469580241246461. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.E.; Haroon, S.; Subramanian, A.; McMullan, C.; Aiyegbusi, O.L.; Turner, G.M.; Jackson, L.; Davies, E.H.; Frost, C.; McNamara, G.; et al. Development and validation of the symptom burden questionnaire for long COVID (SBQ-LC): Rasch analysis. BMJ 2022, 377, e070230. [Google Scholar] [CrossRef]
- Sivan, M.; Preston, N.; Parkin, A.; Makower, S.; Gee, J.; Ross, D.; Tarrant, R.; Davison, J.; Halpin, S.; O’Connor, R.J. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long COVID or Post-COVID-19 syndrome. J. Med. Virol. 2022, 94, 4253–4264. [Google Scholar] [CrossRef]
- Ye, G.; Zhu, Y.; Bao, W.; Zhou, H.; Lai, J.; Zhang, Y.; Xie, J.; Ma, Q.; Luo, Z.; Ma, S.; et al. The Long COVID Symptoms and Severity Score: Development, Validation, and Application. Value Health 2024, 27, 1085–1091. [Google Scholar] [CrossRef]
- Chou, R.; Herman, E.; Ahmed, A.; Anderson, J.; Selph, S.; Dana, T.; Williams, L.; Ivlev, I. Long COVID Definitions and Models of Care: A Scoping Review. Ann. Intern. Med. 2024, 177, 929–940. [Google Scholar] [CrossRef]
- Chang, V.T.; Hwang, S.S.; Thaler, H.T.; Kasimis, B.S.; Portenoy, R.K. Memorial Symptom Assessment Scale. Expert Rev. Pharmacoecon. Outcomes Res. 2004, 4, 171–178. [Google Scholar] [CrossRef]
- Tran, V.-T.; Riveros, C.; Clepier, B.; Desvarieux, M.; Collet, C.; Yordanov, Y.; Ravaud, P. Development and Validation of the Long Coronavirus Disease (COVID) Symptom and Impact Tools: A Set of Patient-Reported Instruments Constructed From Patients’ Lived Experience. Clin. Infect. Dis. 2022, 74, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, N.; Gurdasani, D.; O’Hara, M.E.; Hastie, C.; Roderick, P.; Yao, G.; Alwan, N.A. Characteristics and impact of Long Covid: Findings from an online survey. PLoS ONE 2022, 17, e0264331. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Comparability of Control and Comparison Groups in Studies Assessing Long COVID. Am. J. Med. Sci. 2025, 138, 148–152.e3. [Google Scholar] [CrossRef]
- Smith, E.M.; Anderson, E.E.; Deer, R.; Prochaska, J.; Bohn, K.; Croisant, S. Reviewing fair subject selection considerations for the unique case of post sequelae COVID-19 translational studies. J. Clin. Transl. Res. 2022, 6, e91. [Google Scholar] [CrossRef]
- CDC. Prevalence of Symptoms ≤12 Months After Acute Illness, by COVID-19 Testing Status Among Adults—United States, December 2020–March 2023. 2023. Available online: https://www.cdc.gov/mmwr/volumes/72/wr/mm7232a2.htm#:~:text=Approximately%2016%25%20of%20adults%20with,%2DCoV%2D2%20test%20result (accessed on 12 December 2023).
- Høeg, T.B.; Ladhani, S.; Prasad, V. How methodological pitfalls have created widespread misunderstanding about long COVID. BMJ Evid.-Based Med. 2024, 29, 142–146. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Glazer, J.V.; MacDonnell, K.; Frederick, C.; Ingersoll, K.; Ritterband, L.M. Liar! Liar! Identifying eligibility fraud by applicants in digital health research. Internet Interv. 2021, 25, 100401. [Google Scholar] [CrossRef]
- Pozzar, R.; Hammer, M.J.; Underhill-Blazey, M.; Wright, A.A.; Tulsky, J.A.; Hong, F.; Gundersen, D.A.; Berry, D.L. Threats of Bots and Other Bad Actors to Data Quality Following Research Participant Recruitment Through Social Media: Cross-Sectional Questionnaire. J. Med. Internet Res. 2020, 22, e23021. [Google Scholar] [CrossRef]
- Danesh, V.; Arroliga, A.C.; Bourgeois, J.A.; Boehm, L.M.; McNeal, M.J.; Widmer, A.J.; McNeal, T.M.; Kesler, S.R. Symptom Clusters Seen in Adult COVID-19 Recovery Clinic Care Seekers. J. Gen. Intern. Med. 2023, 38, 442–449. [Google Scholar] [CrossRef]
- Moniz, M.; Ruivinho, C.; Goes, A.R.; Soares, P.; Leite, A. Long COVID is not the same for everyone: A hierarchical cluster analysis of Long COVID symptoms 9 and 12 months after SARS-CoV-2 test. BMC Infect. Dis. 2024, 24, 1001. [Google Scholar] [CrossRef] [PubMed]
- Dagliati, A.; Strasser, Z.H.; Hossein Abad, Z.S.; Klann, J.G.; Wagholikar, K.B.; Mesa, R.; Visweswaran, S.; Morris, M.; Luo, Y.; Henderson, D.W.; et al. Characterization of long COVID temporal sub-phenotypes by distributed representation learning from electronic health record data: A cohort study. eClinicalMedicine 2023, 64, 102210. [Google Scholar] [CrossRef] [PubMed]
- Omar, I.M.; Weaver, J.S.; Samet, J.D.; Serhal, A.M.; Mar, W.A.; Taljanovic, M.S. Musculoskeletal Manifestations of COVID-19: Currently Described Clinical Symptoms and Multimodality Imaging Findings. Radiographics 2022, 42, 1415–1432. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Frontera, J.A.A.; Lewis, A.; Melmed, K.; Lin, J.; Kondziella, D.; Helbok, R.; Yaghi, S.; Meropol, S.; Wisniewski, T.; Balcer, L.; et al. Prevalence and Predictors of Prolonged Cognitive and Psychological Symptoms Following COVID-19 in the United States. Front. Aging Neurosci. 2021, 13, 690383. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Joli, J.; Buck, P.; Zipfel, S.; Stengel, A. Post-COVID-19 fatigue: A systematic review. Front. Psychiatry 2022, 13, 947973. [Google Scholar] [CrossRef]
- Thomas, B.; Pattinson, R.; Edwards, D.; Dale, C.; Jenkins, B.; Lande, H.; Bundy, E.; Davies, J.L. Defining and measuring Long COVID fatigue–a scoping review. BMJ Open 2024, 14, e088530. [Google Scholar] [CrossRef]
- Evans, R.; Pick, A.; Lardner, R.; Masey, V.; Smith, N.; Greenhalgh, T. Breathing difficulties after COVID-19: A guide for primary care. BMJ 2023, 381, e074937. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Winter, A.J.; Mills, N.L.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat. Commun. 2022, 13, 5663. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data and Characteristics | |||
|---|---|---|---|
| No Long COVID (n = 131) | Long COVID (n = 128) | p Value * | |
| Age (years, median, IQR) | 41 (32, 54) | 46 (36, 58) | 0.007 |
| n (%) | n (%) | ||
| Age group (years) | 0.06 | ||
| 18–39 | 63 (48.0) | 43 (33.6) | |
| 40–59 | 45 (34.4) | 57 (44.5) | |
| ≥60 | 23 (17.6) | 28 (21.9) | |
| Gender | 0.51 | ||
| Female | 101 (77.1) | 103 (80.5) | |
| Male | 30 (22.9) | 25 (19.5) | |
| Prior COVID | NA | ||
| No | 36 (27.5) | 0 (0.0) | |
| Yes | 95 (72.5) | 128 (100.0) | |
| Of those reporting prior COVID, proof of a COVID Diagnosis | N = 95 | <0.001 | |
| No | 45 (47.4) | 23 (18.0) | |
| Yes | 50 (52.6) | 105 (82.0) | |
| Onset of Long COVID symptoms | |||
| <1 year | ----- | 6 (4.7) | |
| ≥1 year | ----- | 48 (37.5) | |
| ≥2 years | ----- | 29 (22.6) | |
| ≥3 years | ----- | 45 (35.2) | |
| Long COVID (n = 128) | No Long COVID (n = 131) | Total (n = 259) | ||||
|---|---|---|---|---|---|---|
| Survey Question | No n (%) | Yes n (%) | No n (%) | Yes n (%) | No n (%) | Yes n (%) |
| 1. Did you have any difficulty understanding the instructions on the scale? | 121 (94.5) | 7 (5.5) | 129 (98.4) | 2 (1.6) | 250 (96.5) | 9 (3.5) |
| 2. Did you have any difficulty answering the questions on the scale? | 114 (89.1) | 14 (10.9) | 125 (95.3) | 6 (4.7) | 239 (92.2) | 20 (7.8) |
| 3. Were the words large enough to read? | 3 (2.3) | 125 (97.7) | 8 (6.2) | 123 (93.8) | 11 (4.3) | 248 (95.7) |
| 4. There were 3 questions we asked about each symptom: how often you have it, how severe it was, and how much distress or bother it caused you. Did you have any difficulty understanding these aspects of the symptoms? | 122 (95.3) | 6 (4.7) | 131 (100) | 0 (0) | 253 (97.7) | 6 (2.3) |
| 5. Did you have any difficulty understanding the different choices you had for each of the 3 questions about each symptom? | 119 (93) | 9 (7.0) | 131 (100) | 0 (0.0) | 250 (96.5) | 9 (3.5) |
| 6. Were there any symptom words that you did not understand? | 122 (95.3) | 6 (4.7) | 129 (98.4) | 2 (1.6) | 251 (96.9) | 8 (3.1) |
| 7. You had room to add up to 7 additional symptoms that were not listed on the scale. Did you need more than 7 spaces for symptoms? If so, about how many more? | 128 (100.0) | 0 (0.0) | 131 (100.0) | 0 (0.0) | 259 (100.0) | 0 (0.0) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Memory | 40 (30.5) | 94 (73.4) | <0.001 | 6.29 (3.66, 10.80) | 0.0 (0.0, 4.0) 0, 11 | 7.0 (0.0, 10.8) 0, 12 | <0.001 | 4.60 (3.67, 5.52) |
| Brain fog | 55 (42.0) | 100 (78.1) | <0.001 | 4.94 (2.86, 8.51) | 0.0 (0.0, 6.0) 0, 12 | 9.0 (4.0, 11.0) 0, 12 | <0.001 | 4.46 (3.48, 5.43) |
| Concentration | 49 (37.4) | 94 (73.4) | <0.001 | 4.63 (2.72, 7.85) | 0.0 (0.0, 5.0) 0, 12 | 8.0 (0.0, 11.0) 0, 12 | <0.001 | 4.20 (3.22, 5.17) |
| Word finding | 35 (26.7) | 87 (68.0) | <0.001 | 5.82 (3.40, 9.96) | 0.0 (0.0, 3.0) 0, 10 | 6.0 (0.0, 10.0) 0, 12 | <0.001 | 3.96 (3.03, 4.89) |
| Problem solving | 16 (12.2) | 78 (60.9) | <0.001 | 11.21 (5.96, 21.10) | 0.0 (0.0, 0.0) 0, 12 | 5.0 (0.0, 9.0) 0, 12 | <0.001 | 3.89 (3.03, 4.74) |
| Speech | 5 (3.8) | 44 (34.4) | <0.001 | 13.20 (5.03, 34.66) | 0.0 (0.0, 0.0) 0, 9 | 0.0 (0.0, 6.0) 0, 12 | <0.001 | 2.44 (1.72, 3.15) |
| Headache | 66 (50.4) | 85 (66.4) | 0.009 | 1.95 (1.17, 3.22) | 3.0 (0.0, 6.0) 0, 10 | 6.0 (0.0, 8.8) 0, 12 | <0.001 | 1.99 (1.08, 2.90) |
| Tremor | 11 (8.4) | 39 (30.5) | <0.001 | 4.78 (2.32, 9.86) | 0.0 (0.0, 0.0) 0, 12 | 0.0 (0.0, 6.0) 0, 11 | <0.001 | 1.69 (0.98, 2.39) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Dizziness/lightheadedness | 25 (19.1) | 82 (64.1) | <0.001 | 7.56 (4.29, 13.31) | 0.0 (0.0, 0.0) 0, 11 | 5.0 (0.0, 8.0) 0, 12 | <0.001 | 3.42 (2.57, 4.27) |
| Blurred vision | 22 (16.8) | 66 (51.6) | <0.001 | 5.27 (2.96, 9.37) | 0.0 (0.0, 0.0) 0, 10 | 5.0 (0.0, 8.0) 0, 12 | <0.001 | 2.69 (1.86, 3.52) |
| Loss of taste or smell | 16 (12.2) | 41 (32.0) | <0.001 | 3.39 (1.78, 6.44) | 0.0 (0.0, 0.0) 0, 12 | 5.0 (0.0, 8.0) 0, 12 | <0.001 | 1.86 (1.03, 2.69) |
| Hearing loss | 10 (7.6) | 22 (17.2) | 0.02 b | 2.51 (1.13, 5.55) | 0.0 (0.0, 0.0) 0, 10 | 5.0 (0.0, 8.0) 0, 12 | 0.02 | 0.76 (0.14, 1.39) |
| Tongue discoloration/enlargement | 2 (1.5) | 9 (7.0) | 0.05 | 4.88 (1.03, 23.04) | 0.0 (0.0, 0.0) 0, 11 | 5.0 (0.0, 8.0) 0, 6 | 0.03 | 0.41 (0.09, 0.73) |
| Red eyes | 9 (6.9) | 17 (13.3) | 0.09 | 2.08 (0.88, 4.85) | 0.0 (0.0, 0.0) 0, 9 | 5.0 (0.0, 8.0) 0, 12 | 0.08 | 0.49 (−0.02, 1.01) |
| Sore throat | 28 (21.4) | 35 (27.3) | 0.26 | 1.38 (0.78, 2.45) | 0.0 (0.0, 0.0) 0, 8 | 5.0 (0.0, 8.0) 0, 12 | 0.18 | 0.58 (−0.03, 1.19) |
| Congestion/runny nose | 54 (41.2) | 55 (43.0) | 0.78 | 1.07 (0.65, 1.76) | 0.0 (0.0, 5.0) 0, 9 | 5.0 (0.0, 8.0) 0, 12 | 0.28 | 0.61 (−0.21, 1.43) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Constipation | 21 (16.0) | 47 (36.7) | <0.001 | 3.04 (1.68, 5.48) | 0.0 (0.0, 0.0) 0, 9 | 0.0 (0.0, 6.0) 0, 12 | <0.001 | 1.70 (0.90, 2.49) |
| Urinary | 9 (6.9) | 32 (25.0) | <0.001 | 4.52 (2.05, 9.92) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 3.0) 0, 12 | <0.001 | 1.40 (0.70, 2.09) |
| Nausea | 17 (13.0) | 45 (35.2) | <0.001 | 3.64 (1.94, 6.80) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 4.0) 0, 12 | <0.001 | 1.38 (0.73, 2.04) |
| Weight gain | 17 (13.0) | 38 (29.7) | 0.001 | 2.83 (1.50, 5.35) | 0.0 (0.0, 0.0) 0, 9 | 0.0 (0.0, 5.0) 0, 12 | <0.001 | 1.62 (0.84, 2.40) |
| Decreased appetite | 20 (15.3) | 41 (32.0) | 0.002 | 2.62 (1.43, 4.79) | 0.0 (0.0, 0.0) 0, 8 | 0.0 (0.0, 5.0) 0, 12 | <0.001 | 1.17 (0.53, 1.80) |
| Abdominal pain | 22 (16.8) | 44 (34.4) | 0.001 | 2.60 (1.44, 4.67) | 0.0 (0.0, 0.0) 0, 10 | 0.0 (0.0, 5.0) 0, 12 | 0.003 | 1.08 (0.31, 1.86) |
| Weight loss | 10 (7.6) | 23 (18.0) | 0.02 | 2.65 (1.20, 5.83) | 0.0 (0.0, 0.0) 0, 10 | 0.0 (0.0, 0.0) 0, 9 | 0.13 | 0.37 (−0.11, 0.85) |
| Diarrhea | 34 (26.0) | 33 (25.8) | 0.98 | 0.99 (0.56, 1.73) | 0.0 (0.0, 3.0) 0, 9 | 0.0 (0.0, 3.0) 0, 12 | 0.97 | 0.08 (−0.63, 0.78) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Psychiatric | ||||||||
| Sleep | 68 (51.9) | 92 (71.9) | 0.001 | 2.37 (1.41, 3.97) | 3.0 (0.0, 7.0) 0, 12 | 7.0 (0.0, 10.0) 0, 12 | <0.001 | 2.60 (1.60, 3.60) |
| Anxiety | 61 (46.6) | 87 (68.0) | <0.001 | 2.44 (1.47, 4.04) | 0.0 (0.0, 7.0) 0, 12 | 6.0 (4.0, 9.0) 0, 12 | <0.001 | 1.97 (1.01, 2.94) |
| Depression | 42 (32.1) | 70 (54.7) | <0.001 | 2.56 (1.54, 4.25) | 0.0 (0.0, 6.0) 0, 12 | 4.0 (0.0, 8.0) 0, 12 | <0.001 | 1.95 (0.98, 2.91) |
| Irritability | 37 (28.2) | 67 (52.3) | <0.001 | 2.79 (1.66, 4.67) | 0.0 (0.0, 4.0) 0, 12 | 3.0 (0.0, 7.0) 0, 12 | <0.001 | 1.87 (0.98, 2.76) |
| Cardiovascular | ||||||||
| Chest pain | 14 (10.7) | 55 (43.0) | <0.001 | 6.30 (3.26, 12.13) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 6.0) 0, 12 | <0.001 | 2.13 (1.41, 2.86) |
| Heart palpations | 26 (19.8) | 87 (52.3) | 0.009 | 4.44 (2.55, 7.71) | 0.0 (0.0, 0.0) 0, 11 | 3.0 (0.0, 7.0) 0, 12 | <0.001 | 2.67 (1.85, 3.48) |
| Vertigo | 32 (24.4) | 64 (50.0) | <0.001 | 3.09 (1.82, 5.25) | 0.0 (0.0, 0.0) 0, 11 | 1.5 (0.0, 8.0) 0, 12 | <0.001 | 2.27 (1.39, 3.15) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Fatigue | 87 (66.4) | 112 (87.5) | <0.001 | 3.54 (1.87, 6.70) | 5.0 (0.0, 7.0) 0, 11 | 9.0 (6.2, 11.0) 0, 12 | <0.001 | 3.58 (2.71, 4.45) |
| Fatigue (exercise) | 33 (25.2) | 95 (74.2) | <0.001 | 8.55 (4.88, 14.96) | 0.0 (0.0, 4.0) 0, 12 | 8.0 (0.0, 11.0) 0, 12 | <0.001 | 5.13 (4.17, 6.10) |
| Muscle aches | 50 (38.2) | 90 (70.3) | <0.001 | 3.84 (2.28, 6.44) | 0.0 (0.0, 5.0) 0, 11 | 7.0 (0.0, 9.0) 0, 12 | <0.001 | 3.22 (2.31, 4.13) |
| Joint pain | 38 (29.0) | 70 (54.7) | <0.001 | 2.95 (1.76, 4.94) | 0.0 (0.0, 5.0) 0, 12 | 5.0 (0.0, 8.0) 0, 12 | <0.001 | 2.32 (1.37, 3.27) |
| Numbness | 15 (11.5) | 62 (48.4) | <0.001 | 7.27 (3.83, 13.78) | 0.0 (0.0, 0.0) 0, 10 | 0.0 (0.0, 8.0) 0, 12 | <0.001 | 2.90 (2.06, 3.74) |
| Muscle weakness | 20 (15.3) | 59 (46.1) | 0.009 | 4.75 (2.63, 8.56) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 8.0) 0, 12 | <0.001 | 2.91 (2.04, 3.79) |
| Prevalence | Burden * | |||||||
|---|---|---|---|---|---|---|---|---|
| Long COVID | Long COVID | |||||||
| No (n = 131) n % | Yes (n = 128) n % | p Value ** | OR (95% C.I.) | No (n = 131) Median (IQR) Min, Max | Yes (n = 128) Median (IQR) Min, Max | p Value *** | Estimated Mean Difference a (95% C.I.) | |
| Shortness of breath | 28 (21.4) | 78 (60.9) | <0.001 | 5.74 (3.31, 9.93) | 0.0 (0.0, 0.0) 0, 11 | 5.0 (0.0, 8.0) 0, 12 | <0.001 | 3.21 (2.39, 4.03) |
| Sweats/hot flushes | 25 (19.1) | 57 (44.5) | <0.001 | 3.40 (1.94, 5.95) | 0.0 (0.0, 0.0) 0, 12 | 0.0 (0.0, 6.0) 0, 11 | <0.001 | 1.69 (0.92, 2.47) |
| Cough | 32 (24.4) | 45 (35.2) | 0.06 | 1.68 (0.98, 2.88) | 0.0 (0.0, 0.0) 0, 10 | 0.0 (0.0, 5.0) 0, 12 | 0.04 b | 0.82 (0.08, 1.56) |
| Hair loss | 23 (17.6) | 43 (33.6) | 0.003 | 2.38 (1.32, 4.25) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 5.8) 0, 12 | 0.002 | 1.36 (0.50, 2.21) |
| Itchy skin | 28 (21.4) | 43 (33.6) | 0.03 | 1.86 (1.06, 3.25) | 0.0 (0.0, 0.0) 0, 9 | 0.0 (0.0, 4.0) 0, 12 | 0.02 | 0.91 (0.18, 1.65) |
| Skin rash | 12 (9.2) | 32 (25.0) | 0.001 | 3.31 (1.61, 6.77) | 0.0 (0.0, 0.0) 0, 8 | 0.0 (0.0, 2.3) 0, 12 | <0.001 | 1.30 (0.67, 1.94) |
| Fever/chills | 20 (15.3) | 31 (24.2) | 0.07 | 1.77 (0.95, 3.32) | 0.0 (0.0, 0.0) 0, 9 | 0.0 (0.0, 0.0) 0, 11 | 0.05 | 0.61 (0.03, 1.20) |
| Gynecological | 11 (8.4) | 20 (15.6) | 0.08 | 2.02 (0.92, 4.41) | 0.0 (0.0, 0.0) 0, 11 | 0.0 (0.0, 0.0) 0, 12 | 0.09 | 0.51 (−0.09, 1.11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S.B.; Dietrich, M.S.; Barroso, J.V. The Development and Initial Validation of the Memorial Symptom Assessment Scale-Long COVID (MSAS-LC): A Promising Tool for Measuring Long COVID. Int. J. Environ. Res. Public Health 2025, 22, 728. https://doi.org/10.3390/ijerph22050728
Sommer SB, Dietrich MS, Barroso JV. The Development and Initial Validation of the Memorial Symptom Assessment Scale-Long COVID (MSAS-LC): A Promising Tool for Measuring Long COVID. International Journal of Environmental Research and Public Health. 2025; 22(5):728. https://doi.org/10.3390/ijerph22050728
Chicago/Turabian StyleSommer, Sadie B., Mary S. Dietrich, and Julie V. Barroso. 2025. "The Development and Initial Validation of the Memorial Symptom Assessment Scale-Long COVID (MSAS-LC): A Promising Tool for Measuring Long COVID" International Journal of Environmental Research and Public Health 22, no. 5: 728. https://doi.org/10.3390/ijerph22050728
APA StyleSommer, S. B., Dietrich, M. S., & Barroso, J. V. (2025). The Development and Initial Validation of the Memorial Symptom Assessment Scale-Long COVID (MSAS-LC): A Promising Tool for Measuring Long COVID. International Journal of Environmental Research and Public Health, 22(5), 728. https://doi.org/10.3390/ijerph22050728






