Ventilatory Responses to Progressive Treadmill Speeds in Women: A Comparative Analysis of Nasal, Oral, and Oronasal Breathing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Analysis
3. Results
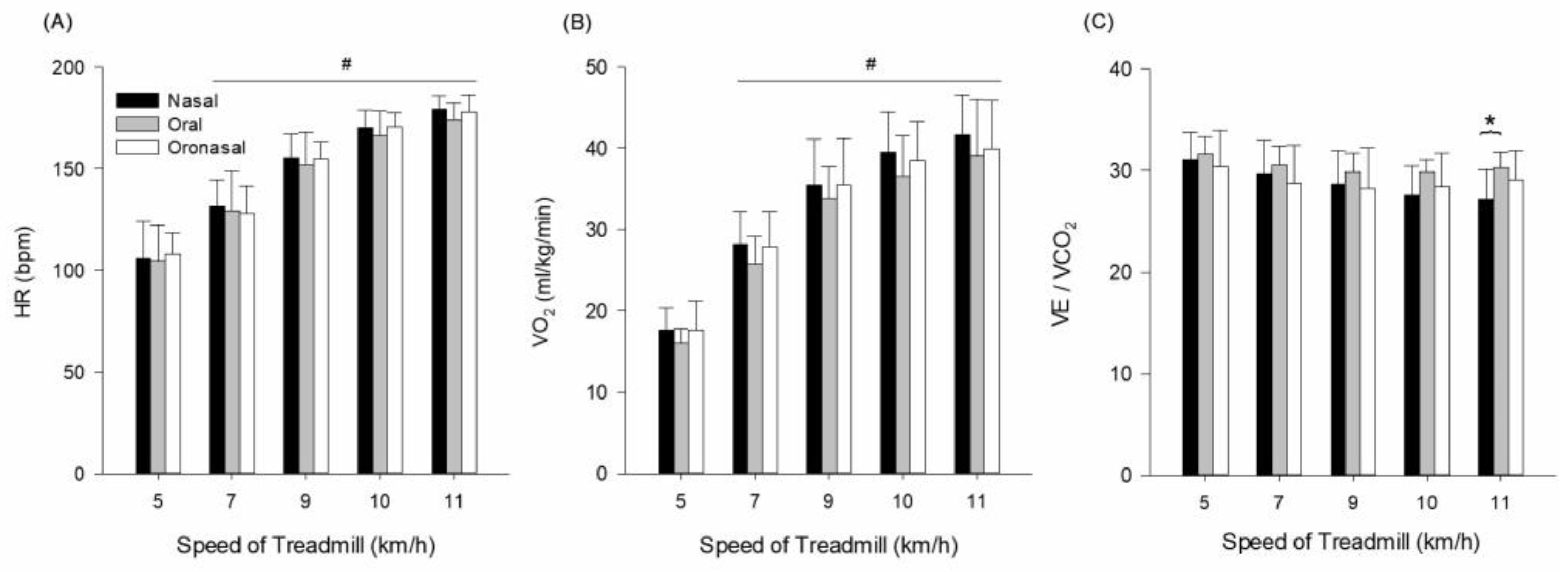
3.1. Cardiopulmonary Responses
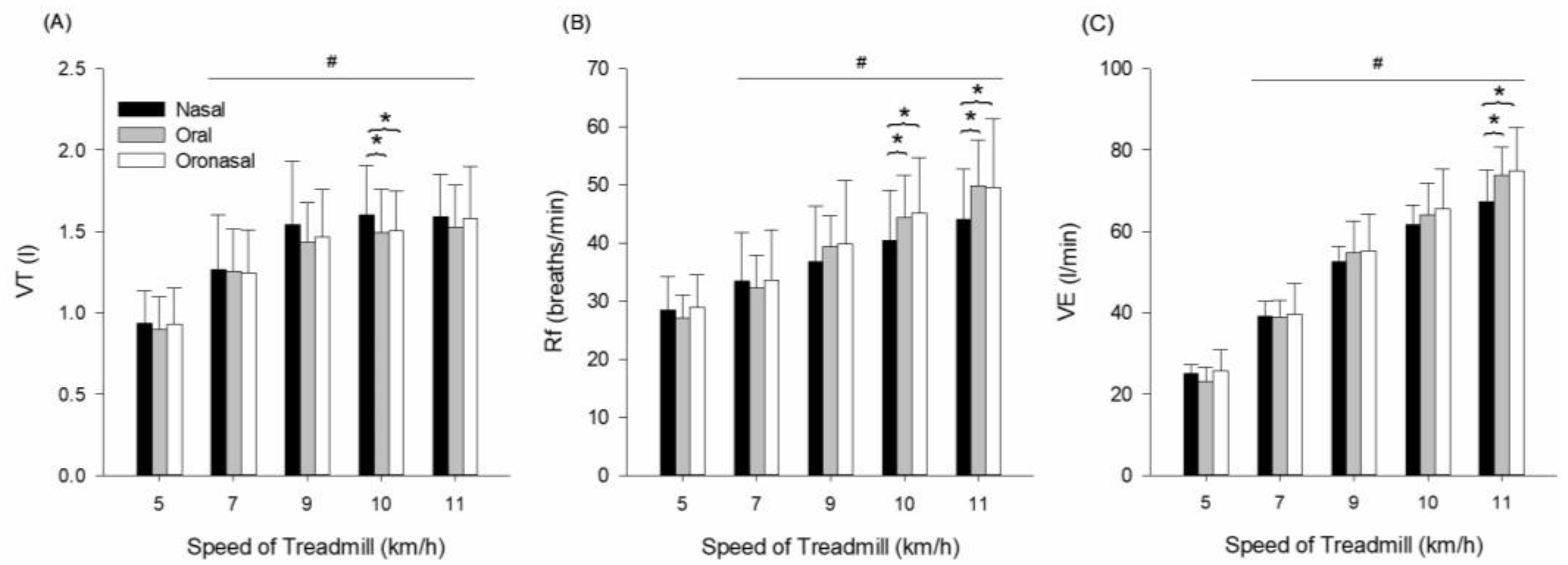
3.2. Pulmonary Ventilatory Responses
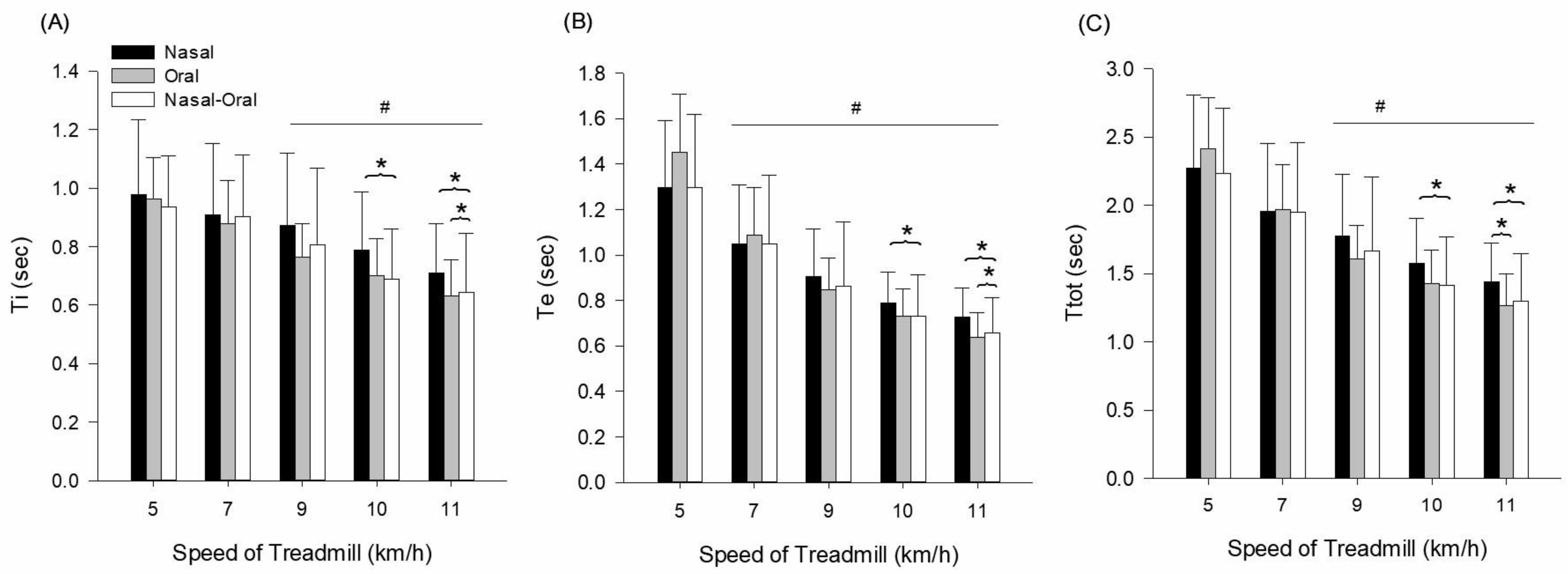
3.3. Breathing Timing Responses
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| HR | Heart rate |
| Rf | Respiratory frequency |
| Te | Expiratory time |
| Ti | Inspiratory time |
| Ttot | Total respiratory cycle time |
| VE | Minute ventilation |
| VE/VCO2 | Minute ventilation/carbon dioxide production |
| VO2 | Oxygen uptake |
| VT | Tidal volume |
References
- Mahtta, R.; Fragkias, M.; Güneralp, B.; Mahendra, A.; Reba, M.; Wentz, E.A.; Seto, K.C. Urban Land Expansion: The Role of Population and Economic Growth for 300+ Cities. Npj Urban Sustain. 2022, 2, 5. [Google Scholar] [CrossRef]
- Araneda, O.F.; Kosche-Cárcamo, F.; Verdugo-Marchese, H.; Tuesta, M. Pulmonary Effects due to Physical Exercise in Polluted air: Evidence from Studies Conducted on Healthy Humans. Appl. Sci. 2021, 11, 2890. [Google Scholar] [CrossRef]
- LaComb, C.O.; Tandy, R.D.; Lee, S.P.; Young, J.C.; Navalta, J.W. Oral Versus Nasal Breathing During Moderate to High Intensity Submaximal Aerobic Exercise. IJKSS 2017, 5, 8–16. [Google Scholar] [CrossRef]
- Recinto, C.; Efthemeou, T.; Boffelli, P.T.; Navalta, J.W. Effects of Nasal or Oral Breathing on Anaerobic Power Output and Metabolic Responses. Int. J. Exerc. Sci. 2017, 10, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Harbour, E.; Stöggl, T.; Schwameder, H.; Finkenzeller, T. Breath Tools: A Synthesis of Evidence-Based Breathing Strategies to Enhance Human Running. Front. Physiol. 2022, 13, 813243. [Google Scholar] [CrossRef]
- Wheatley, J.R.; Amis, T.C.; Engel, L.A. Oronasal Partitioning of Ventilation During Exercise in Humans. J. Appl. Physiol. 1991, 71, 546–551. [Google Scholar] [CrossRef]
- Lizal, F.; Elcner, J.; Jedelsky, J.; Maly, M.; Jicha, M.; Farkas, Á.; Belka, M.; Rehak, Z.; Adam, J.; Brinek, A.; et al. The Effect of Oral and Nasal Breathing on the Deposition of Inhaled Particles in Upper and Tracheobronchial Airways. J. Aerosol. Sci. 2020, 150, 105649. [Google Scholar] [CrossRef]
- Rodenstein, D.O.; Stănescu, D.C. Soft Palate and Oronasal Breathing in Humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 651–657. [Google Scholar] [CrossRef]
- Cole, P.; Niinimaa, V.; Mintz, S.; Silverman, F. Work of Nasal Breathing: Measurement of Each Nostril Independently Using A Split Mask. Acta Otolaryngol. 1979, 88, 148–154. [Google Scholar] [CrossRef]
- LoMauro, A.; Colli, A.; Colombo, L.; Aliverti, A. Breathing Patterns Recognition: A Functional Data Analysis Approach. Comput. Methods Programs Biomed. 2022, 217, 106670. [Google Scholar] [CrossRef]
- Eser, P.; Calamai, P.; Kalberer, A.; Stuetz, L.; Huber, S.; Kaesermann, D.; Guler, S.; Wilhelm, M. Improved Exercise Ventilatory Efficiency with Nasal Compared to Oral Breathing in Cardiac Patients. Front. Physiol. 2024, 15, 1380562. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Gruet, M.; Sacchetti, M. Editorial: Breathing in Sport and Exercise: Physiology, Pathophysiology and Applications. Front. Physiol. 2023, 14, 1347806. [Google Scholar] [CrossRef]
- Tavoian, D.; Craighead, D.H. Deep Breathing Exercise at Work: Potential Applications and Impact. Front. Physiol. 2023, 14, 1040091. [Google Scholar] [CrossRef]
- Lucía, A.; Carvajal, A.; Calderón, F.J.; Alfonso, A.; Chicharro, J.L. Breathing Pattern in Highly Competitive Cyclists During Incremental Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Robinson, D.G.; Verhagen, E.; Fagher, K.; Edouard, P.; Rojas-Valverde, D.; Ahmed, O.H.; Jederström, M.; Usacka, L.; Benoit-Piau, J.; et al. Under-Representation of Women Is Alive and Well in Sport and Exercise Medicine: What It Looks Like and What We Can Do About It. BMJ Open Sport Exerc. Med. 2023, 9, e001606. [Google Scholar] [CrossRef] [PubMed]
- Bhammar, D.M.; Balmain, B.N.; Babb, T.G.; Bernhardt, V. Sex Differences in The Ventilatory Responses to Exercise in Mild to Moderate Obesity. Exp. Physiol. 2022, 107, 965–977. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Molgat-Seon, Y. Sex, Gender and The Pulmonary Physiology of Exercise. Eur. Respir. Rev. 2022, 31, 210074. [Google Scholar] [CrossRef]
- James, J.J.; Klevenow, E.A.; Atkinson, M.A.; Vosters, E.E.; Bueckers, E.P.; Quinn, M.E.; Kindy, S.L.; Mason, A.P.; Nelson, S.K.; Rainwater, K.A.H.; et al. Underrepresentation of Women in Exercise Science and Physiology Research Is Associated with Authorship Gender. J. Appl. Physiol. 2023, 135, 932–942. [Google Scholar] [CrossRef]
- Sheel, A.W.; Richards, J.C.; Foster, G.E.; Guenette, J.A. Sex Differences in Respiratory Exercise Physiology. Sports Med. 2004, 34, 567–579. [Google Scholar] [CrossRef]
- Hunt, K.J.; Saengsuwan, J. Changes in Heart Rate Variability with Respect to Exercise Intensity and Time During Treadmill Running. Biomed. Eng. Online 2018, 17, 128. [Google Scholar] [CrossRef]
- Jang, D.G.; Ko, B.H.; Sunoo, S.; Nam, S.S.; Park, H.Y.; Bae, S.K. A Preliminary Study of A Running Speed Based Heart Rate Prediction During an Incremental Treadmill Exercise. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5323–5326. [Google Scholar] [CrossRef]
- Barnes, K.R.; Kilding, A.E. Running Economy: Measurement, Norms, and Determining Factors. Sports Med. Open 2015, 1, 8. [Google Scholar] [CrossRef]
- Travers, G.; Kippelen, P.; Trangmar, S.J.; González-Alonso, J. Physiological Function During Exercise and Environmental Stress in Humans-An Integrative View of Body Systems and Homeostasis. Cells 2022, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Chinevere, T.D.; Faria, E.W.; Faria, I.E. Nasal Splinting Effects on Breathing Patterns and Cardiorespiratory Responses. J. Sports Sci. 1999, 17, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.R.; King, K.; Papalia, S.; Goodman, C.; Turley, K.R.; Wilmore, J.H. Comparison of Maximal Oxygen Consumption with Oral and Nasal Breathing. Aust. J. Sci. Med. Sport 1995, 27, 51–55. [Google Scholar]
- Moris, J.M.; Cardona, A.; Hinckley, B.; Mendez, A.; Blades, A.; Paidisetty, V.K.; Chang, C.J.; Curtis, R.; Allen, K.; Koh, Y. A Framework of Transient Hypercapnia to Achieve an Increased Cerebral Blood Flow Induced by Nasal Breathing During Aerobic Exercise. Cereb. Circ. Cogn. Behav. 2023, 5, 100183. [Google Scholar] [CrossRef] [PubMed]
- Niinimaa, V.; Cole, P.; Mintz, S.; Shephard, R.J. The Switching Point from Nasal to Oronasal Breathing. Respir. Physiol. 1980, 42, 61–71. [Google Scholar] [CrossRef]
- Mezzani, A.; Giordano, A.; Komici, K.; Corra, U. Different Determinants of Ventilatory Inefficiency at Different Stages of Reduced Ejection Fraction Chronic Heart Failure Natural History. J. Am. Heart Assoc. 2017, 6, 5. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory Efficiency During Exercise in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef]
- Rawat, D.; Modi, P.; Sharma, S. Hypercapnea. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rappelt, L.; Held, S.; Wiedenmann, T.; Deutsch, J.P.; Hochstrate, J.; Wicker, P.; Donath, L. Restricted Nasal-Only Breathing During Self-Selected Low Intensity Training Does not Affect Training Intensity Distribution. Front. Physiol. 2023, 14, 1134778. [Google Scholar] [CrossRef]
- Folinsbee, L.J.; Wallace, E.S.; Bedi, J.F.; Horvath, S.M. Exercise Respiratory Pattern in Elite Cyclists and Sedentary Subjects. Med. Sci. Sports Exerc. 1983, 15, 503–509. [Google Scholar] [CrossRef]
- Dallam, G.M.; McClaran, S.R.; Cox, D.G.; Foust, C.P. Effect of Nasal Versus Oral Breathing on Vo2max and Physiological Economy in Recreational Runners Following an Extended Period Spent Using Nasally Restricted Breathing. IJKSS 2018, 6, 22–29. [Google Scholar] [CrossRef]
| Age (yr) | Height (cm) | Weight (kg) | Physical Activity Level | Athletic Status | Smoking History | Medication Use |
|---|---|---|---|---|---|---|
| 24.1 ± 2.1 | 163.4 ± 3.9 | 62.7 ± 7.1 | Recreationally active (≥2–3 sessions/week) | Non- athlete | None reported | None reported |
| Variable | Speed (km) | Nasal | Oral | Oronasal | Inferential Statistics |
|---|---|---|---|---|---|
| HR (beats/min) | 5 | 105.6 ± 18.5 | 104.4 ± 17.8 | 107.8 ± 10.8 | condition (p ≤ 0.001, = 0.971) speed (p ≤ 0.001, = 0.797) interaction (p ≤ 0.001, = 0.463) |
| 7 | 131.4 ± 13.1 | 129.4 ± 19.4 | 128.0 ± 13.6 | ||
| 9 | 155.2 ± 11.7 | 151.9 ± 16.2 | 154.6 ± 8.4 | ||
| 10 | 170.2 ± 8.7 | 166.0 ± 12.6 | 170.6 ± 6.9 | ||
| 11 | 179.3 ± 6.3 | 174.2 ± 8.1 | 177.9 ± 8.0 | ||
| VO2 (mL/kg/min) | 5 | 17.6 ± 2.8 | 16.0 ± 1.7 | 17.6 ± 3.6 | condition (p ≤ 0.001, = 0.970) speed (p ≤ 0.001, = 0.856) interaction (p ≤ 0.001, = 0.616) |
| 7 | 28.2 ± 4.0 | 25.8 ± 3.3 | 27.8 ± 4.4 | ||
| 9 | 35.5 ± 5.6 | 33.8 ± 3.9 | 35.5 ± 5.7 | ||
| 10 | 39.4 ± 5.1 | 36.5 ± 5.0 | 38.5 ± 4.8 | ||
| 11 | 41.7 ± 4.8 | 39.1 ± 6.9 | 39.9 ± 5.9 | ||
| VE/VCO2 | 5 | 31.1 ± 2.7 | 31.6 ± 1.8 | 30.4 ± 3.6 | condition (p = 0.004, = 0.549) speed (p = 0.115, = 0.226) interaction (p = 0.305, = 0.148) |
| 7 | 29.7 ± 3.3 | 30.6 ± 1.8 | 28.8 ± 3.7 | ||
| 9 | 28.6 ± 3.3 | 29.8 ± 1.8 | 28.2 ± 4.0 | ||
| 10 | 27.6 ± 2.9 | 29.9 ± 1.2 | 28.4 ± 3.3 | ||
| 11 | 27.2 ± 2.9 * | 30.3 ± 1.4 * | 29.1 ± 2.8 |
| Variable | Speed (km) | Nasal | Oral | Oronasal | Inferential Statistics |
|---|---|---|---|---|---|
| VT (L) | 5 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | condition (p ≤ 0.001, = 0.909) speed (p ≤ 0.001, = 0.721) interaction (p ≤ 0.001, = 0.466) |
| 7 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | ||
| 9 | 1.5 ± 0.4 | 1.4 ± 0.2 | 1.5 ± 0.3 | ||
| 10 | 1.6 ± 0.3 | 1.5 ± 0.3 * | 1.5 ± 0.2 * | ||
| 11 | 1.6 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.3 | ||
| Rf (breaths/min) | 5 | 28.4 ± 5.7 | 27.1 ± 4.0 | 29.0 ± 5.6 | condition (p ≤ 0.001, = 0.897) speed (p ≤ 0.001, = 0.757) interaction (p = 0.551, = 0.110) |
| 7 | 33.4 ± 8.4 | 32.2 ± 5.6 | 33.6 ± 8.6 | ||
| 9 | 36.8 ± 9.6 | 39.4 ± 5.3 | 39.9 ± 10.9 | ||
| 10 | 40.4 ± 8.6 | 44.3 ± 7.3 * | 45.2 ± 9.5 * | ||
| 11 | 44.1 ± 8.6 | 49.8 ± 7.9 * | 49.6 ± 11.8 * | ||
| VE (L/min) | 5 | 25.0 ± 2.2 | 23.2 ± 3.4 | 25.6 ± 5.4 | condition (p ≤ 0.001, = 0.980) speed (p ≤ 0.001, = 0.915) interaction (p ≤ 0.001, = 0.427) |
| 7 | 39.0 ± 3.8 | 38.8 ± 4.3 | 39.6 ± 7.4 | ||
| 9 | 52.5 ± 3.6 | 54.8 ± 7.8 | 55.2 ± 9.0 | ||
| 10 | 61.6 ± 4.8 | 64.0 ± 7.8 | 65.5 ± 9.9 | ||
| 11 | 67.2 ± 7.9 | 73.7 ± 7.0 * | 74.8 ± 10.6 * |
| Variable | Speed (km) | Nasal | Oral | Oronasal | Inferential Statistics |
|---|---|---|---|---|---|
| Ti (s) | 5 | 1.0 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.2 | condition (p ≤ 0.001, = 0.794) speed (p ≤ 0.001, = 0.510) interaction (p = 0.740, = 0.084) |
| 7 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.2 | ||
| 9 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.3 | ||
| 10 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.2 * | ||
| 11 | 0.7 ± 0.2 | 0.6 ± 0.1 * | 0.6 ± 0.2 * | ||
| Te (s) | 5 | 1.3 ± 0.3 | 1.5 ± 0.3 | 1.3 ± 0.3 | condition (p ≤ 0.001, = 0.923) speed (p ≤ 0.001, = 0.779) interaction (p = 0.001, = 0.383) |
| 7 | 1.0 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.3 | ||
| 9 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.3 | ||
| 10 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 * | ||
| 11 | 0.7 ± 0.1 | 0.6 ± 0.1 * | 0.7 ± 0.2 * | ||
| Ttot (s) | 5 | 2.3 ± 0.5 | 2.4 ± 0.4 | 2.2 ± 0.5 | condition (p ≤ 0.001, = 0.895) peed (p ≤ 0.001, = 0.739) interaction (p = 0.070, = 0.218) |
| 7 | 2.0 ± 0.5 | 2.0 ± 0.3 | 2.0 ± 0.5 | ||
| 9 | 1.8 ± 0.5 | 1.6 ± 0.2 | 1.7 ± 0.5 | ||
| 10 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.4 * | ||
| 11 | 1.4 ± 0.3 | 1.3 ± 0.2 * | 1.3 ± 0.3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Seo, Y.; Lee, D.T. Ventilatory Responses to Progressive Treadmill Speeds in Women: A Comparative Analysis of Nasal, Oral, and Oronasal Breathing Conditions. Int. J. Environ. Res. Public Health 2025, 22, 718. https://doi.org/10.3390/ijerph22050718
Lee SH, Seo Y, Lee DT. Ventilatory Responses to Progressive Treadmill Speeds in Women: A Comparative Analysis of Nasal, Oral, and Oronasal Breathing Conditions. International Journal of Environmental Research and Public Health. 2025; 22(5):718. https://doi.org/10.3390/ijerph22050718
Chicago/Turabian StyleLee, Seung Hee, Yongsuk Seo, and Dae Taek Lee. 2025. "Ventilatory Responses to Progressive Treadmill Speeds in Women: A Comparative Analysis of Nasal, Oral, and Oronasal Breathing Conditions" International Journal of Environmental Research and Public Health 22, no. 5: 718. https://doi.org/10.3390/ijerph22050718
APA StyleLee, S. H., Seo, Y., & Lee, D. T. (2025). Ventilatory Responses to Progressive Treadmill Speeds in Women: A Comparative Analysis of Nasal, Oral, and Oronasal Breathing Conditions. International Journal of Environmental Research and Public Health, 22(5), 718. https://doi.org/10.3390/ijerph22050718







