The Potential Role of Ecotoxicological Data in National Essential Medicine Lists: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Essential Medicines
2.1.2. Health Expenditures
2.1.3. Ecotoxicological Data on Medicines
2.2. Data Analysis
3. Results
3.1. Reported Ecotoxicological Risks of the Example Medicines
3.2. Inclusion of Example Medicines in Essential Medicine Lists
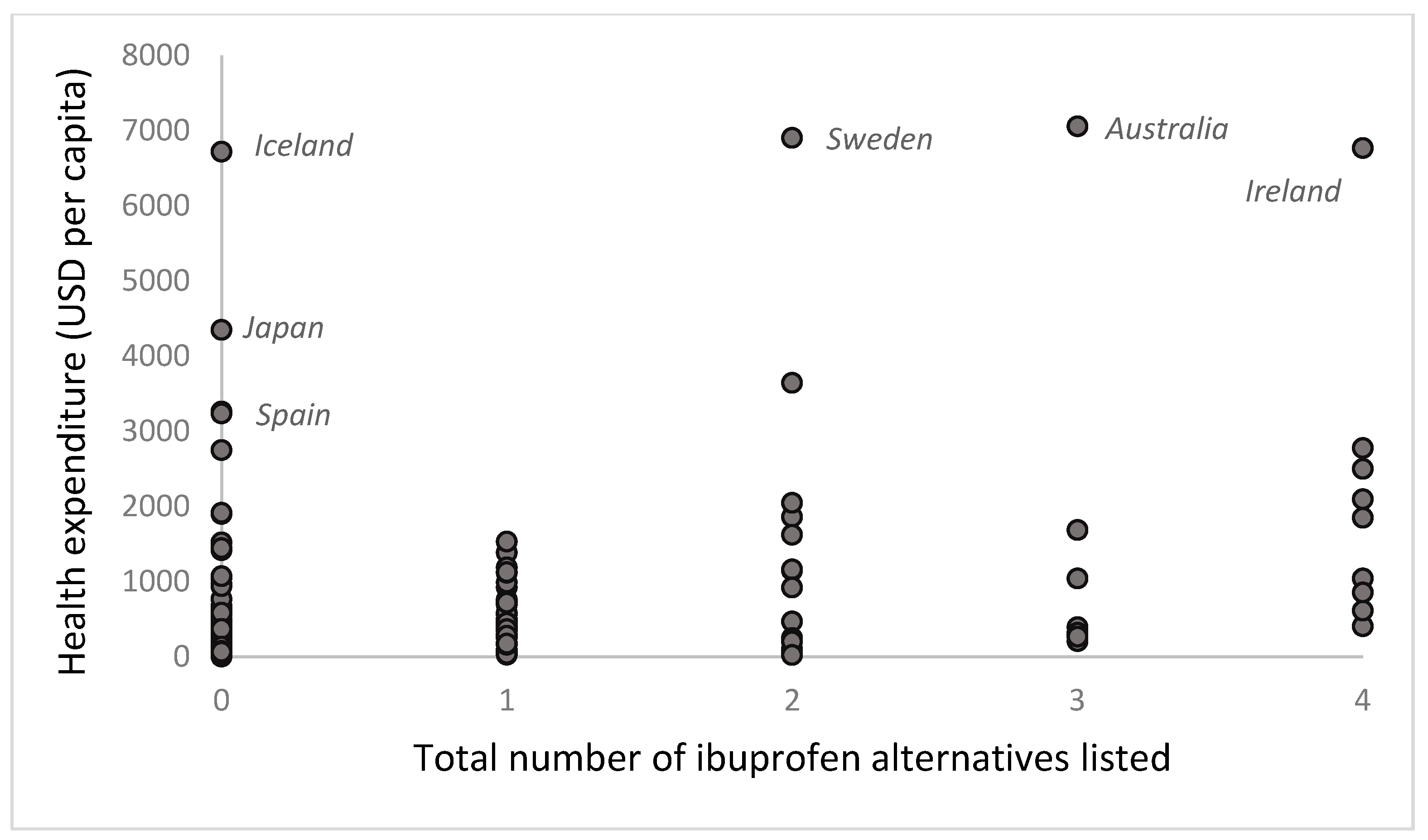
3.3. Healthcare Expenditure for Countries That List the Example Medicines
4. Discussion
4.1. Availability and Quality of Ecotoxicological Data
4.2. Opportunities for EMLs to Support Efforts to Reduce the Environmental Harms from Medicines
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- De Soete, W.; Boone, L.; Willemse, F.; De Meyer, E.; Heirman, B.; Van Langenhove, H.; Dewulf, J. Environmental resource footprinting of drug manufacturing: Effects of scale-up and tablet dosage. Resour. Conserv. Recycl. 2014, 91, 82–88. [Google Scholar] [CrossRef]
- Kookana, R.S.; Williams, M.; Boxall, A.B.A.; Larsson, D.G.J.; Gaw, S.; Choi, K.; Yamamoto, H.; Thatikonda, S.; Zhu, Y.-G.; Carriquiriborde, P. Potential ecological footprints of active pharmaceutical ingredients: An examination of risk factors in low-, middle- and high-income countries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130586. [Google Scholar] [CrossRef]
- Sanusi, I.O.; Olutona, G.O.; Wawata, I.G.; Onohuean, H. Occurrence, environmental impact and fate of pharmaceuticals in groundwater and surface water: A critical review. Environ. Sci. Pollut. Res. Int. 2023, 30, 90595–90614. [Google Scholar] [CrossRef] [PubMed]
- Sammut Bartolo, N.; Azzopardi, L.M.; Serracino-Inglott, A. Pharmaceuticals and the environment. Early Hum. Dev. 2021, 155, 105218. [Google Scholar] [CrossRef]
- Parker, G.; Miller, F.A. Tackling Pharmaceutical Pollution Along the Product Lifecycle: Roles and Responsibilities for Producers, Regulators and Prescribers. Pharmacy 2024, 12, 173. [Google Scholar] [CrossRef]
- Boxall, A.B.A. The environmental side effects of medication: How are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef]
- Mercer, C. How health care contributes to climate change. Can. Med. Assoc. J. 2019, 191, E403–E404. [Google Scholar] [CrossRef]
- Sijm-Eeken, M.; Jaspers, M.; Peute, L. Identifying Environmental Impact Factors for Sustainable Healthcare: A Scoping Review. Int. J. Environ. Res. Public. Health 2023, 20, 6747. [Google Scholar] [CrossRef]
- United Nations United Nations Environment Programme (UNEP). Available online: https://sdgs.un.org/un-system-sdg-implementation/united-nations-environment-programme-unep-44155 (accessed on 23 October 2024).
- WHO. Guidance on Wastewater and Solid Waste Management for Manufacturing of Antibiotics; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses. OECD; 2019. (OECD Studies on Water). Available online: https://www.oecd-ilibrary.org/environment/pharmaceutical-residues-in-freshwater_c936f42d-en (accessed on 22 November 2024).
- Linder, E.; Wettermark, B.; Ovesjö, M.-L.; Sporrong, S.K.; Ramström, H. Knowledge support for environmental information on pharmaceuticals: Experiences among Swedish Drug and Therapeutics Committees. BMC Health Serv. Res. 2023, 23, 618. [Google Scholar] [CrossRef]
- Brhlikova, P.; Deivanayagam, T.A.; Babar, Z.-U.-D.; Osorio-de-Castro, C.G.S.; Caetano, R.; Pollock, A.M. Essential medicines concept and health technology assessment approaches to prioritising medicines: Selection versus incorporation. J. Pharm. Policy Pract. 2023, 16, 88. [Google Scholar] [CrossRef]
- Persaud, N.; Jiang, M.; Shaikh, R.; Bali, A.; Oronsaye, E.; Woods, H.; Drozdzal, G.; Rajakulasingam, Y.; Maraj, D.; Wadhawan, S.; et al. Comparison of essential medicines lists in 137 countries. Bull. World Health Organ. 2019, 97, 394–404C. [Google Scholar] [CrossRef]
- essentialmeds.org. Available online: https://essentialmeds.org/ (accessed on 5 June 2024).
- The World Health Organization. The World Health Organization. WHO|Global Health Observatory (GHO) Data. WHO. World Health Organization. 2023. Available online: https://www.who.int/data/gho (accessed on 10 February 2025).
- Amnesty International Press Release. North Korea’s Crumbling Health System in Dire Need of Aid. July 2010. Available online: https://www.amnesty.org/fr/wp-content/uploads/2021/07/pre012242010en.pdf (accessed on 7 May 2024).
- Brink, S. What Country Spends the Most (and Least) on Health Care per Person? 2017. Available online: https://www.npr.org/sections/goatsandsoda/2017/04/20/524774195/what-country-spends-the-most-and-least-on-health-care-per-person#:~:text=Somalia%20spends%20the%20least%2C%20only,not%20zero%20in%20health%20spending (accessed on 26 March 2025).
- SLL’s Table of Environmentally Hazardous Drug Substances Developed Under the Stockholm County Council’s Environmental Program 2017–2021. Available online: https://janusinfo.se/download/18.7ea3e81f166a3423a9d1b00f/1540468908295/Table-of-environmentally-hazardous-drug-substances-SLL-2017-2021.pdf (accessed on 5 June 2024).
- Janusinfo—Region Stockholm. Available online: https://janusinfo.se/ (accessed on 5 June 2024).
- FASS. Available online: https://www.fass.se/LIF/startpage (accessed on 5 June 2024).
- Janusinfo—Region Stockholm—Vocabulary. Available online: https://janusinfo.se/beslutsstod/lakemedelochmiljo/pharmaceuticalsandenvironment/environment/vocabulary.5.7b57ecc216251fae474883f1.html (accessed on 5 June 2024).
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Geneva, Switzerland, 2012; Volume 35. [Google Scholar]
- Goodpoint. Comparative Assessment of Environmental Risk When Using Diclofenac, Naproxen, Ibuprofen, Ketoprofen, Etoricoxib, Celexocib and Paracetamol. 2019. Available online: https://janusinfo.se/download/18.26bc9b1a16e8972aa5d7eae6/1588834635690/Rapport%20NSAID%20ink%20celecoxib%2020190927_final_databasen.pdf (accessed on 27 November 2024).
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pourebrahimi, S.; Malloum, A.; Ajala, O.J.; AlKafaas, S.S.; Onyeaka, H.; Nnaji, N.D.; Oroke, A.; Bornman, C.; Christian, O.; et al. A review on ciprofloxacin removal from wastewater as a pharmaceutical contaminant: Covering adsorption to advanced oxidation processes to computational studies. Mater. Today Commun. 2023, 37, 107500. [Google Scholar] [CrossRef]
- Diniz, V.; Rath, G.; Rath, S.; Rodrigues-Silva, C.; Guimarães, J.R.; Cunha, D.G.F. Long-term ecotoxicological effects of ciprofloxacin in combination with caffeine on the microalga Raphidocelis subcapitata. Toxicol. Rep. 2021, 8, 429–435. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of Estrogens Present in Environment on Health and Welfare of Animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef]
- Rocha, M.; Rocha, E. Synthetic Progestins in Waste and Surface Waters: Concentrations, Impacts and Ecological Risk. Toxics 2022, 10, 163. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Frankel, T.E.; Meyer, M.T.; Orlando, E.F. Aqueous exposure to the progestin, levonorgestrel, alters anal fin development and reproductive behavior in the eastern mosquitofish (Gambusia holbrooki). Gen. Comp. Endocrinol. 2016, 234, 161–169. [Google Scholar] [CrossRef]
- Torres, N.H.; Santos, G.D.O.S.; Romanholo Ferreira, L.F.; Américo-Pinheiro, J.H.P.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Environmental aspects of hormones estriol, 17β-estradiol and 17α-ethinylestradiol: Electrochemical processes as next-generation technologies for their removal in water matrices. Chemosphere 2021, 267, 128888. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-J.; Ma, D.-D.; Jiang, Y.-X.; Xie, L.; Zhang, J.-N.; Huang, G.-Y.; Chen, H.-X.; Hou, L.-P.; Liu, Y.-S.; Ying, G.-G. Medroxyprogesterone acetate affects sex differentiation and spermatogenesis in zebrafish. Aquat. Toxicol. 2019, 212, 70–76. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Faria, M.; Bellot, M.; Soto, O.; Prats, E.; Montemurro, N.; Manjarrés, D.; Gómez-Canela, C.; Raldúa, D. Developmental exposure to sertraline impaired zebrafish behavioral and neurochemical profiles. Front. Physiol. 2022, 13, 1040598. [Google Scholar] [CrossRef]
- Estévez-Calvar, N.; Canesi, L.; Montagna, M.; Faimali, M.; Piazza, V.; Garaventa, F. Adverse effects of the SSRI antidepressant sertraline on early life stages of marine invertebrates. Mar. Environ. Res. 2017, 128, 88–97. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. WHO Model List of Essential Medicines 23rd Edition. 2023. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed on 29 August 2024).
- WHO. Japan Health Expenditure. Available online: https://data.who.int/countries/392 (accessed on 31 March 2025).
- WHO. Spain Health Expenditure. Available online: https://data.who.int/countries/724 (accessed on 31 March 2025).
- Gildemeister, D.; Moermond, C.T.A.; Berg, C.; Bergstrom, U.; Bielská, L.; Evandri, M.G.; Franceschin, M.; Kolar, B.; Montforts, M.H.M.M.; Vaculik, C. Improving the regulatory environmental risk assessment of human pharmaceuticals: Required changes in the new legislation. Regul. Toxicol. Pharmacol. 2023, 142, 105437. [Google Scholar] [CrossRef]
- Ågerstrand, M.; Berg, C.; Björlenius, B.; Breitholtz, M.; Brunström, B.; Fick, J.; Gunnarsson, L.; Larsson, D.G.J.; Sumpter, J.P.; Tysklind, M.; et al. Improving Environmental Risk Assessment of Human Pharmaceuticals. Environ. Sci. Technol. 2015, 49, 5336–5345. [Google Scholar] [CrossRef]
- Kittery, A.; Miettinen, M. Environmental considerations in the European Union’s pharmaceuticals legislation: Key instruments and their challenges in addressing global manufacturing supply chains. Rev. Eur. Comp. Int. Environ. Law 2023, 32, 77–91. [Google Scholar] [CrossRef]
- Toolan, M.; Walpole, S.; Shah, K.; Kenny, J.; Jónsson, P.; Crabb, N.; Greaves, F. Environmental impact assessment in health technology assessment: Principles, approaches, and challenges. Int. J. Technol. Assess. Health Care 2023, 39, e13. [Google Scholar] [CrossRef]
- Hensher, M. Health technology assessment and healthcare environmental sustainability: Prioritizing effort and maximizing impact. Int. J. Technol. Assess. Health Care 2024, 40, e25. [Google Scholar] [CrossRef]
- Firth, I.; Hitch, J.; Henderson, N.; Cookson, G. Moving towards a more environmentally sustainable pharmaceutical industry: Recommendations for industry and the transition to green HTA. Expert Rev. Pharmacoecon. Outcomes Res. 2023, 23, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Stockholm County Council (Healthcare Region), Sweden. The Wise List 2015. Available online: https://janusinfo.se/download/18.2baa5e3e161e6f2218925d/1535626568380/The-Wise-List-2015.pdf (accessed on 25 September 2024).
- Brodin, T.; Bertram, M.G.; Arnold, K.E.; Boxall, A.B.A.; Brooks, B.W.; Cerveny, D.; Jörg, M.; Kidd, K.A.; Lertxundi, U.; Martin, J.M.; et al. The urgent need for designing greener drugs. Nat. Sustain. 2024, 7, 949–951. [Google Scholar] [CrossRef]

| No. of Countries Listing It | Persistence (According to OECD’s Test Guidelines (Test 301, 308) or Corresponding Other Degradability Tests) [22] | Bioaccumulation [22] | Toxicity [22] | No. of Alternatives (Similar ATC Code) | Clinical Alternatives | |
|---|---|---|---|---|---|---|
| Ciprofloxacin | 149 | Potentially persistent | Low | Very high chronic | 25 | Levofloxacin, nitrofurantoin, norfloxacin, ofloxacin |
| Ethinylestradiol | 137 | Degrades in the environment (the half-life of ethinylestradiol ranges from 4.0 to 5.9 days in water and from 24 to 36 days in sediment or the entire system) | High | Very high chronic | 8 | Estradiol, estriol |
| Ibuprofen | 147 | Degrades in the environment (meets the ready biodegradation test requirements, though there is some uncertainty regarding the 10-day window criterion) | Low | High chronic | 23 | Celecoxib, diclofenac, etoricoxib, ketoprofen, meloxicam, naproxen |
| Levonorgestrel | 135 | Persistent | Below high limit | Very high chronic | 10 | Desogestrel, etonogestrel, medroxyprogesterone, norethisterone |
| Sertraline | 70 | Degrades in the environment (after 45 days of biodegradation using the activated sludge method, 9–32% of sertraline persists) | No potential | Very high acute | 9 | Bupropion, citalopram, duloxetine, escitalopram, fluoxetine, mirtazapine, paroxetine, venlafaxine |
| Total of Five Example Medicines | Total No. of Medicines on List | Health Expenditure USD per Capita (2021) | |
|---|---|---|---|
| Algeria 2023 | 5 | 516 | 205 |
| Antigua and Barbuda 2022 | 5 | 334 | 923 |
| Australia 2023 | 5 | 787 | 7055 |
| Colombia 2019 | 5 | 594 | 558 |
| Cuba 2018 | 5 | 469 | 1186 |
| Dominica 2022 | 5 | 334 | 482 |
| Dominican Republic 2018 | 5 | 386 | 417 |
| Ecuador 2019 | 5 | 424 | 494 |
| El Salvador 2020 | 5 | 272 | 442 |
| Estonia 2012 | 5 | 405 | 2095 |
| Eswatini 2012 | 5 | 312 | 280 |
| Ethiopia 2020 | 5 | 440 | 26 |
| Fiji 2015 | 5 | 291 | 250 |
| Ghana 2017 | 5 | 400 | 100 |
| Greece 2007 | 5 | 918 | 1846 |
| Grenada 2022 | 5 | 334 | 505 |
| Guinea-Bissau 2020 | 5 | 421 | 69 |
| Honduras 2018 | 5 | 351 | 254 |
| Iran (Islamic Republic of) 2017 | 5 | 955 | 393 |
| Ireland 2023 | 5 | 740 | 6764 |
| Jamaica 2015 | 5 | 445 | 372 |
| Lebanon 2018 | 5 | 341 | 307 |
| Libya 2019 | 5 | 538 | 381 |
| Madagascar 2019 | 5 | 414 | 18 |
| Malaysia 2023 | 5 | 428 | 487 |
| Maldives 2021 | 5 | 853 | 1039 |
| Mauritania 2021 | 5 | 326 | 89 |
| Mexico 2017 | 5 | 794 | 611 |
| Mongolia 2020 | 5 | 439 | 316 |
| Montenegro 2020 | 5 | 535 | 985 |
| Morocco 2017 | 5 | 395 | 221 |
| Nauru 2010 | 5 | 230 | 1530 |
| Oman 2020 | 5 | 793 | 853 |
| Pakistan 2021 | 5 | 504 | 43 |
| Palau 2017 | 5 | 278 | 2045 |
| Peru 2018 | 5 | 451 | 412 |
| Philippines 2022 | 5 | 528 | 203 |
| Poland 2017 | 5 | 497 | 1159 |
| Republic of Moldova 2021 | 5 | 506 | 410 |
| Rwanda 2022 | 5 | 393 | 60 |
| Saint Kitts and Nevis 2022 | 5 | 334 | 1114 |
| Saint Lucia 2022 | 5 | 334 | 585 |
| Saint Vincent and Grenadines 2022 | 5 | 334 | 448 |
| Saudi Arabia 2020 | 5 | 525 | 1442 |
| Serbia 2022 | 5 | 697 | 919 |
| Slovenia 2017 + 2023 | 5 | 931 | 2775 |
| Sri Lanka 2019 | 5 | 188 | 166 |
| Sudan 2014 | 5 | 508 | 22 |
| Sweden 2023 | 5 | 309 | 6901 |
| Thailand 2021 | 5 | 583 | 364 |
| Trinidad & Tobago 2019 | 5 | 467 | 1125 |
| Tunisia 2012 | 5 | 642 | 265 |
| Uzbekistan 2021 | 5 | 402 | 157 |
| Zambia 2020 | 5 | 338 | 75 |
| Zimbabwe 2020 | 5 | 301 | 63 |
| Japan 2018 | 0 | 122 | 4347 |
| Spain 2019 | 0 | 39 | 3234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia, C.; Workentin, A.; Parker, G.; Persaud, N. The Potential Role of Ecotoxicological Data in National Essential Medicine Lists: A Cross-Sectional Analysis. Int. J. Environ. Res. Public Health 2025, 22, 632. https://doi.org/10.3390/ijerph22040632
Heredia C, Workentin A, Parker G, Persaud N. The Potential Role of Ecotoxicological Data in National Essential Medicine Lists: A Cross-Sectional Analysis. International Journal of Environmental Research and Public Health. 2025; 22(4):632. https://doi.org/10.3390/ijerph22040632
Chicago/Turabian StyleHeredia, Camila, Aine Workentin, Gillian Parker, and Navindra Persaud. 2025. "The Potential Role of Ecotoxicological Data in National Essential Medicine Lists: A Cross-Sectional Analysis" International Journal of Environmental Research and Public Health 22, no. 4: 632. https://doi.org/10.3390/ijerph22040632
APA StyleHeredia, C., Workentin, A., Parker, G., & Persaud, N. (2025). The Potential Role of Ecotoxicological Data in National Essential Medicine Lists: A Cross-Sectional Analysis. International Journal of Environmental Research and Public Health, 22(4), 632. https://doi.org/10.3390/ijerph22040632







