Homozygous AA Genotype of IL-17A and 14-bp Insertion Polymorphism in HLA-G 3′UTR Are Associated with Increased Risk of Gestational Diabetes Mellitus
Abstract
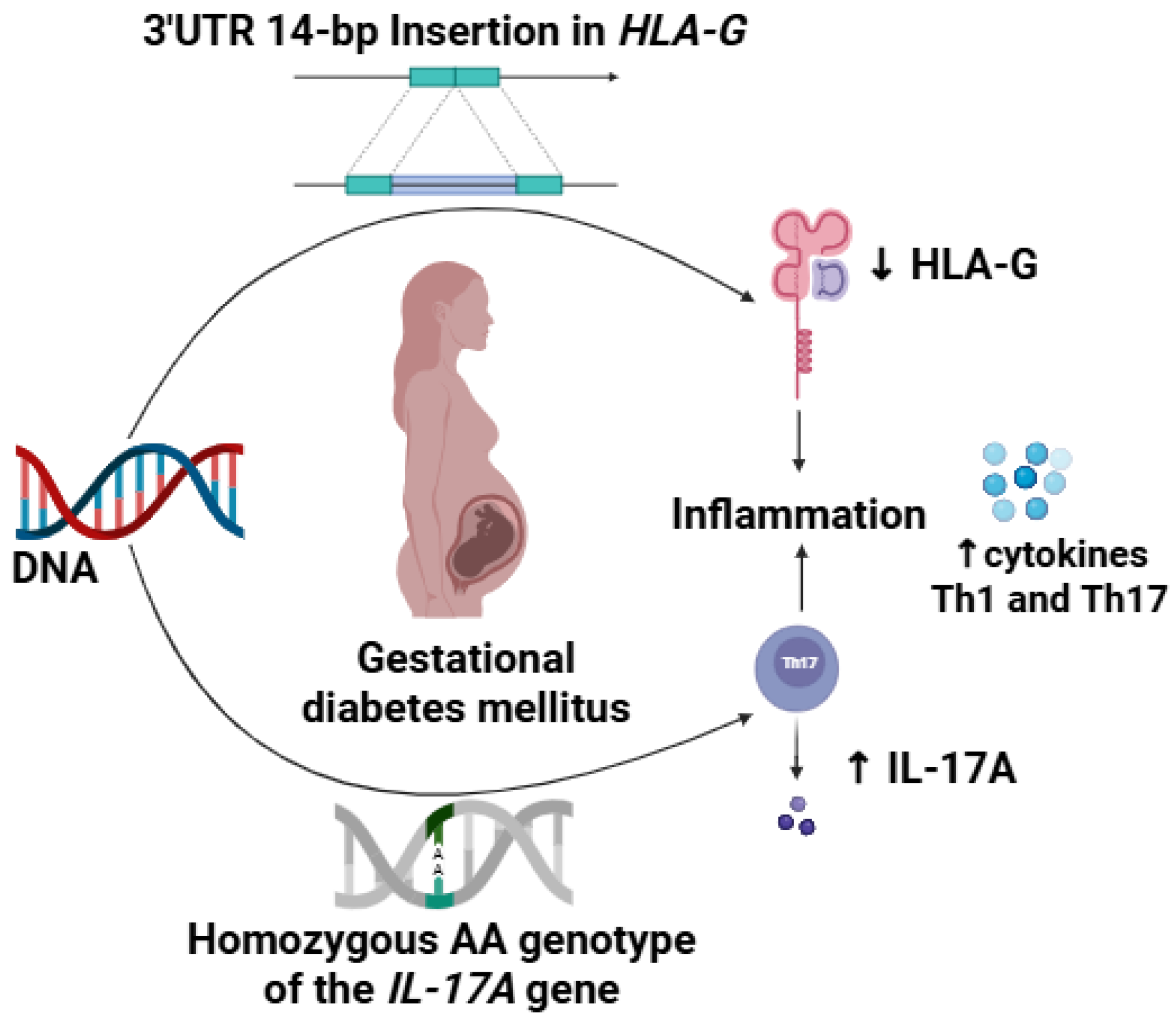
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Samples and DNA Extraction
2.3. HLA-G 14pb Gene Polymorphisms
2.4. IL-17A/RA Gene Polymorphisms
2.5. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Genotype and Allelic Frequency
3.3. Distribution Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | Gestational Diabetes Mellitus |
| HLA-G | Human leukocyte antigen |
| MHC | Major histocompatibility complex |
| UTR | Untranslated region |
| sHLA-G | Soluble human leukocyte antigen |
| indel | Insertion/Deletion polymorphism |
| Ins | Insertion |
| Del | Deletion |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin-6 |
| IL-17 | Interleukin-17 |
| SNP | Single Nucleotide Polymorphism |
| OGTT | Oral glucose tolerance test |
| ADA | American Diabetes Association |
| gDNA | Genomic DNA |
| PCR–RFLP | Polymerase chain reaction-restriction fragment length polymorphism |
| BMI | Body mass index |
| HWE | Hardy-Weinberg Equilibrium |
| NFAT | Nuclear factor of activated T cells |
| AP-1 | Activator protein 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| AhR | Aryl hydrocarbon receptor |
| IRF4 | Interferon regulatory factor 4 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| ILT | Immunoglobulin-like transcript 2 |
| Tregs | Regulatory T cells |
References
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed Pharmacother 2021, 143, 112183. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, L.P.; Gomes, H.A.; Sona, L.; Giacomini, G.M.; Pizzuti, E.P.; Nunes, G.B.; Zanchet, T.M.; Macedo, J.L. Gestational diabetes mellitus prevalence in Brazil: A systematic review and meta-analysis. Cad. Saude Publica 2024, 40, e00064919. [Google Scholar] [PubMed]
- Oztekin, O. New insights into the pathophysiology of gestational diabetes mellitus: Possible role of human leukocyte antigen-G. Med. Hypotheses 2007, 69, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gomes Fagundes, D.L.; França, E.L.; da Silva Fernandes, R.T.; Hara Cde, C.; Morceli, G.; Honorio-França, A.C.; Calderon Ide, M. Changes in T-cell phenotype and cytokines profile in maternal blood, cord blood and colostrum of diabetic mothers. J. Matern. Fetal Neonatal Med. 2016, 29, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Terzieva, A.; Alexandrova, M.; Manchorova, D.; Slavov, S.; Djerov, L.; Dimova, T. HLA-G Expression/Secretion and T-Cell Cytotoxicity in Missed Abortion in Comparison to Normal Pregnancy. Int. J. Mol. Sci. 2024, 25, 2643. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G.; Inversetti, A.; Cristodoro, M.; Ticconi, C.; Scambia, G.; Di Simone, N. HLA-G and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2023, 24, 2557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Shen, L.; Wang, A.; Wang, R. Correlation between inflammatory markers (hs-CRP, TNF-α, IL-1β, IL-6, IL-18), glucose intolerance, and gestational diabetes mellitus in pregnant women. Int. J. Clin. Exp. Med. 2018, 11, 8310–8316. [Google Scholar]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Oğlak, S.C.; Yılmaz, E.Z.; Budak, M.Ş. Abdominal subcutaneous fat thickness combined with a 50-g glucose challenge test at 24–28 weeks of pregnancy in predicting gestational diabetes mellitus. J. Obstet. Gynaecol. 2024, 44, 2329880. [Google Scholar] [CrossRef] [PubMed]
- Oğlak, S.C.; Yavuz, A.; Olmez, F.; Gedik Özköse, Z.; Süzen Çaypınar, S. The reduced serum concentrations of β-arrestin-1 and β-arrestin-2 in pregnancies complicated with gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2022, 35, 10017–10024. [Google Scholar] [CrossRef] [PubMed]
- Abediankenari, S.; Ghasemi, M.; Kim, Y.J. Human leukocyte antigen-G expression on dendritic cells induced by transforming growth factor-beta1 and CD4+ T cells proliferation. Iran. Biomed. J. 2011, 15, 1–5. [Google Scholar] [PubMed]
- van der Ven, K.; Pfeiffer, K.; Skrablin, S. HLA-G polymorphisms and molecule function--questions and more questions--a review. Placenta 2000, 21 (Suppl. A), S86–S92. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S. HLA-G-mediated NK cell senescence promotes vascular remodeling: Implications for reproduction. Cell Mol. Immunol. 2014, 11, 460–466. [Google Scholar] [CrossRef]
- Wedenoja, S.; Yoshihara, M.; Teder, H.; Sariola, H.; Gissler, M.; Katayama, S.; Wedenoja, J.; Häkkinen, I.M.; Ezer, S.; Linder, N.; et al. Fetal HLA-G mediated immune tolerance and interferon response in preeclampsia. eBioMedicine 2020, 59, 102872. [Google Scholar] [CrossRef]
- Krop, J.; Van Der Keur, C.; Kapsenberg, J.M.; Den Hollander, F.; Van Der Hoorn, M.L.P.; Heidt, S.; Claas, F.H.J.; Eikmans, M. Soluble HLA-G blood levels are not increased during ongoing pregnancy in women with a history of recurrent pregnancy loss. J. Reprod. Immunol. 2022, 153, 103665. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, P.; Le Discorde, M.; Mouillot, G.; Marcou, C.; Carosella, E.D.; Moreau, P. The 14 bp deletion-insertion polymorphism in the 3′ UT region of the HLA-G gene influences HLA-G mRNA stability. Hum. Immunol. 2003, 64, 1005–1010. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Seow, K.M.; Chen, K.H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef]
- Oztekin, O.; Cabus, U.; Enli, Y. Decreased serum human leukocyte antigen-G levels are associated with gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2021, 47, 2329–2337. [Google Scholar] [CrossRef]
- Marozio, L.; Garofalo, A.; Berchialla, P.; Tavella, A.M.; Salton, L.; Cavallo, F.; Benedetto, C. Low expression of soluble human leukocyte antigen G in early gestation and subsequent placenta-mediated complications of pregnancy. J. Obstet. Gynaecol. Res. 2017, 43, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Maghsudlu, S.; Ghazanfari Hashemi, M.; Talebi, V.; Vahabi, E.; Sharifpour, S.; Hajibeygi, R.; Maleki, F. Association between Serum HLA-G Levels in The First Trimester of Pregnancy and The Onset of Preeclampsia: A Systematic Review and Meta-analysis Study. Int. J. Fertil. Steril. 2023, 17, 231–235. [Google Scholar]
- La Verde, M.; Luciano, M.; Fordellone, M.; Sampogna, G.; Lettieri, D.; Palma, M.; Torella, D.; Marrapodi, M.M.; Di Vincenzo, M.; Torella, M. Postpartum Depression and Inflammatory Biomarkers of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Monocyte-Lymphocyte Ratio: A Prospective Observational Study. Gynecol. Obstet. Invest. 2024, 89, 140–149. [Google Scholar] [CrossRef]
- Martinetti, M.; Beneventi, F.; Capittini, C.; Locatelli, E.; Simonetta, M.; Cavagnoli, C.; De Maggio, I.; De Silvestri, A.; Pasi, A.; Spinillo, A. The Immunosignature of Mother/Fetus Couples in Gestational Diabetes Mellitus: Role of HLA-G 14 bp ins/del and PAPP-A A/C Polymorphisms in the Uterine Inflammatory Milieu. Dis. Markers 2017, 2017, 4254750. [Google Scholar] [CrossRef]
- Ali, S.; Majid, S.; Ali, M.N.; Taing, S.; Rehman, M.U.; Arafah, A. Cytokine imbalance at materno-embryonic interface as a potential immune mechanism for recurrent pregnancy loss. Int. Immunopharmacol. 2021, 90, 107118. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, X.; Chen, T.; Xu, W.; Feng, F.; Zhao, S.; Wang, Z.; Hu, Y.; Xie, B. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp. Ther. Med. 2018, 16, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Toljic, M.; Nikolic, N.; Joksic, I.; Carkic, J.; Munjas, J.; Karadzov Orlic, N.; Milasin, J. Expression of miRNAs and proinflammatory cytokines in pregnant women with gestational diabetes mellitus. J. Reprod. Immunol. 2024, 162, 104211. [Google Scholar] [CrossRef] [PubMed]
- Tangjittipokin, W.; Thanatummatis, B.; Wardati, F.; Narkdontri, T.; Teerawattanapong, N.; Boriboonhirunsarn, D. The genetic polymorphisms and levels of adipokines and adipocytokines that influence the risk of developing gestational diabetes mellitus in Thai pregnant women. Gene 2023, 860, 147228. [Google Scholar] [CrossRef]
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2003, 26 (Suppl. S1), S103–S105. [Google Scholar] [CrossRef]
- Salazar, L.A.; Hirata, M.H.; Cavalli, S.A.; Machado, M.O.; Hirata, R.D. Optimized procedure for DNA isolation from fresh and cryopreserved clotted human blood useful in clinical molecular testing. Clin. Chem. 1998, 44, 1748–1750. [Google Scholar] [CrossRef] [PubMed]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024. [Google Scholar]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R Package to Perform Whole Genome Association Studies. Bioinformatics 2007, 23, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Champely, S. pwr: Basic Functions for Power Analysis. R Package Version 1.3-0. CRAN. 2020. Available online: https://CRAN.R-project.org/package=pwr (accessed on 18 February 2025).
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Ahmad, F.; Mandava, S.; Das, B.R. Association of Genetic Variants in ARID5B, IKZF1 and CEBPE with Risk of Childhood de novo B-Lineage Acute Lymphoblastic Leukemia in India. Asian Pac. J. Cancer Prev. 2016, 17, 3989–3995. [Google Scholar] [PubMed]
- Li, J.; Xu, L.; Zhao, W.; Pan, J.; Lu, J.; Lu, H.; Yan, J.; Weng, J.; Liu, F. Serum IL-17A concentration and a IL17RA single nucleotide polymorphism contribute to the risk of autoimmune type 1 diabetes. Diabetes Metab. Res. Rev. 2022, 38, e3547. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, Q.A.; Rasool, K.H.; Naji, E.N. Evaluation of IL-6 and IL-17A gene polymorphisms in Iraqi patients infected with COVID-19 and type 2 diabetes mellitus. Hum. Antibodies 2023, 31, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Borilova Linhartova, P.; Kastovsky, J.; Lucanova, S.; Bartova, J.; Poskerova, H.; Vokurka, J.; Fassmann, A.; Kankova, K.; Izakovicova Holla, L. Interleukin-17A Gene Variability in Patients with Type 1 Diabetes Mellitus and Chronic Periodontitis: Its Correlation with IL-17 Levels and the Occurrence of Periodontopathic Bacteria. Mediators Inflamm. 2016, 2016, 2979846. [Google Scholar] [CrossRef]
- Romanowski, M.; Domanski, L.; Pawlik, A.; Osekowska, B.; Dziedziejko, V.; Safranow, K.; Ciechanowski, K. Interleukin-17 gene polymorphisms in patients with post-transplant diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3152–3156. [Google Scholar]
- Ciraci, E.; Elgun, T.; Gok Yurttas, A.; Cagin Kuzey, H.; Ekenoglu Merdan, Y.; Sait Toprak, M.; Tetik, S. Evaluation of serum placenta-specific gene 8 protein, total antioxidant capacity, interleukin-10, interleukin-17A, interleukin-21 and interleukin-33 levels in Turkish women with gestational diabetes mellitus. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2024, 71, 12–18. [Google Scholar]
- Chen, H.; Wang, W.; Xie, H.; Xu, X.; Wu, J.; Jiang, Z.; Zhang, M.; Zhou, L.; Zheng, S. A pathogenic role of IL-17 at the early stage of corneal allograft rejection. Transpl. Immunol. 2009, 21, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Nakamura, M.; Yoshioka, D.; Arima, Y.; et al. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J. Clin. Immunol. 2008, 28, 44–49. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Nakata, K.; Onizuka, M.; Kawase, T.; Akiyama, H.; Miyamura, K.; Morishima, Y.; Fukuda, T.; Kodera, Y.; et al. A genetic variant in the IL-17 promoter is functionally associated with acute graft-versus-host disease after unrelated bone marrow transplantation. PLoS ONE 2011, 6, e26229. [Google Scholar] [CrossRef]
- Hermann-Kleiter, N.; Baier, G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 2010, 115, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Lin, X.; Gaffen, S.L. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J. Biol. Chem. 2004, 279, 52762–52771. [Google Scholar] [CrossRef]
- Tang, H.; Pei, H.; Xia, Q.; Tang, Y.; Huang, J.; Huang, J.; Pei, F. Role of gene polymorphisms/haplotypes and serum levels of interleukin-17A in susceptibility to viral myocarditis. Exp. Mol. Pathol. 2018, 104, 140–145. [Google Scholar] [CrossRef]
- Konuma, T.; Hamatani-Asakura, M.; Monna-Oiwa, M.; Kato, S.; Andoh, S.; Yokoyama, K.; Nannya, Y.; Takahashi, S. Recipient IL-17A polymorphism rs2275913 is associated with acute graft-versus-host disease after single-unit cord blood transplantation. Transpl. Immunol. 2024, 86, 102096. [Google Scholar] [CrossRef]
- Möttönen, M.; Teräsjärvi, J.; Rahikkala, H.; Kvist, S.; Mertsola, J.; He, Q. Association of IL-17A and IL-10 Polymorphisms with Juvenile Idiopathic Arthritis in Finnish Children. Int. J. Mol. Sci. 2024, 25, 8323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, S.; Chen, Q.; Wang, P.; Xu, C.; Tu, X.; Zhang, L.; Wang, Q.K.; Zha, L. Gene polymorphism in IL17A and gene-gene interaction in the IL23R/IL17A axis are associated with susceptibility to coronary artery disease. Cytokine 2023, 164, 156142. [Google Scholar] [CrossRef]
- Gu, Y.; Hu, X.; Liu, C.; Qv, X.; Xu, C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br. J. Haematol. 2008, 142, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, L.A.; Shen, W.-J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Dungu, A.M.; Lundgaard, A.T.; Ryrsø, C.K.; Hegelund, M.H.; Jensen, A.V.; Kristensen, P.L.; Krogh-Madsen, R.; Faurholt-Jepsen, D.; Ostrowski, S.R.; Banasik, K.; et al. Inflammatory and endothelial host responses in community-acquired pneumonia: Exploring the relationships with HbA1c, admission plasma glucose, and glycaemic gap-a cross-sectional study. Front. Immunol. 2024, 15, 1372300. [Google Scholar] [CrossRef] [PubMed]
- Elmadbouly, A.A.; Abdul-Mohymen, A.M.; Eltrawy, H.H.; Elhasan, H.A.A.; Althoqapy, A.A.; Amin, D.R. The association of IL-17A rs2275913 single nucleotide polymorphism with anti-tuberculous drug resistance in patients with pulmonary tuberculosis. J. Genet. Eng. Biotechnol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Kim, E.Y.; Ihm, C.G.; Lee, T.W.; Lee, S.H.; Jeong, K.H.; Moon, J.Y.; Chung, J.H.; Kim, Y.H. Gene polymorphisms of interleukin-17 and interleukin-17 receptor are associated with end-stage kidney disease. Am. J. Nephrol. 2012, 36, 472–477. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Mancilla-Herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, V.; Zalatan, J.; McMaster, M.; Prinsen, R.; Salomon, D.R.; Ricordi, C.; Torbett, B.E.; Meda, P.; Crisa, L. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes 2006, 55, 1214–1222. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yan, W.H.; Lin, A.; Xu, H.H.; Zhang, J.G.; Wang, X.X. The 14 bp deletion polymorphisms in HLA-G gene play an important role in the expression of soluble HLA-G in plasma. Tissue Antigens 2008, 72, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Knabl, J.; Hüttenbrenner, R.; Mahner, S.; Kainer, F.; Desoye, G.; Jeschke, U. Lower HLA-G levels in extravillous trophoblasts of human term placenta in gestational diabetes mellitus than in normal controls. Histochem. Cell Biol. 2023, 159, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Gunavathy, N.; Asirvatham, A.; Chitra, A.; Jayalakshmi, M. Evaluation of HLA-G 14bp Ins/Del and +3142 C/G Polymorphisms in Type 1 Diabetes among South Indian Population. Indian J. Endocrinol. Metab. 2023, 27, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.P.V.; Ururahy, M.A.G.; Souza, K.S.C.; Loureiro, M.B.; Oliveira, Y.M.C.; Oliveira, G.H.M.; Luchessi, A.D.; Carvalho, K.T.C.; Freitas, J.C.O.C.; Donadi, E.A.; et al. The association between the HLA-G 14-bp insertion/deletion polymorphism and type 1 diabetes. Genes. Immun. 2016, 17, 13–18. [Google Scholar] [CrossRef]
- Huang, J.; Burke, P.; Yang, Y.; Seiss, K.; Beamon, J.; Cung, T.; Toth, I.; Pereyra, F.; Lichterfeld, M.; Yu, X.G. Soluble HLA-G inhibits myeloid dendritic cell function in HIV-1 infection by interacting with leukocyte immunoglobulin-like receptor B2. J. Virol. 2010, 84, 10784–10791. [Google Scholar] [CrossRef] [PubMed]
- Schwich, E.; Hò, G.T.; LeMaoult, J.; Bade-Döding, C.; Carosella, E.D.; Horn, P.A.; Rebmann, V. Soluble HLA-G and HLA-G Bearing Extracellular Vesicles Affect ILT-2 Positive and ILT-2 Negative CD8 T Cells Complementary. Front. Immunol. 2020, 11, 2046. [Google Scholar] [CrossRef]
- Jasinski-Bergner, S.; Eckstein, M.; Taubert, H.; Wach, S.; Fiebig, C.; Strick, R.; Hartmann, A.; Seliger, B. The Human Leukocyte Antigen G as an Immune Escape Mechanism and Novel Therapeutic Target in Urological Tumors. Front. Immunol. 2022, 13, 811200. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences (Forward, Reverse) | Cycles | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|---|
| IL-17A | F: AGGTACATGACACCAGAAGACC | 35 | 60 | 514 |
| R: TGCCCACGGTCCAGAAATAC | ||||
| IL-17RA | F: GGAAGAGAGGAGAGGCGAAT | 35 | 60 | 430 |
| R: CACCCCTTTGCCTGGTTCTG | ||||
| HLA-G | F: TGTGAAACAGCTGCCCTGTGT | 30 | 56 | 345 (Del) |
| R: GTCTTCCATTTATTTTGTCTCT | 359 (Ins) |
| Variables | Group | p Value 1 | Total | |
|---|---|---|---|---|
| GDM | Control | |||
| N, % | 79 (50.0%) | 79 (50.0%) | 158 (100.0%) | |
| Current pregnancy | ||||
| Age, years | 31 (26–35) | 30 (25–35) | 0.240 | 31 (25–35) |
| Age, >25 years | 61 (77.2%) | 57 (70.4%) | 0.325 | 116 (73.4%) |
| Gestational age, we. | 32 (27–36) | 33 (24–36) | 0.980 | 32 (25–36) |
| Fasting glucose, mg/dL | 97 (90–106) | 85 (79–90) | 0.001 | 90 (82–98) |
| Glucose, ≥95 mg/dL | 45 (57.0%) | 9 (10.1%) | 0.000 | 53 (33.5%) |
| BMI, kg/m2 | 31.3 (28–35) | 28 (26–31) | 0.001 | 29.5 (27.1–33.2) |
| Gestational BMI, n (%) | ||||
| Low weight | 4 (5.1%) | 8 (10.1%) | 0.003 | 12 (7.6%) |
| Suitable | 15 (19.0%) | 23 (29.1%) | 38 (24.0%) | |
| Overweight | 25 (31.6%) | 34 (43.0%) | 59 (37.3%) | |
| Obesity | 35 (44.3%) | 14 (17.7%) | 49 (31.0%) | |
| Obesity/Overweight, n (%) | 60 (75.9%) | 48 (60.7%) | 0.060 | 108 (68.3%) |
| Obesity, n (%) | 35 (44.3%) | 14 (17.7%) | 0.000 | 49 (31.0%) |
| Obstetric background | ||||
| Children | 63 (79.7%) | 48 (60.7%) | 0.014 | 111 (70.2%) |
| Abortion | 29 (36.7%) | 31 (39.2%) | 0.870 | 60 (38.0%) |
| GDM diagnosis | 6 (7.6%) | 0 (0.0%) | 0.028 | 8 (3.8%) |
| Family history of DM | 59 (75.6%) | 66 (84.6%) | 0.228 | 125 (79.1%) |
| Gene | SNP | HWE | Allele/Genotype | Group | |
|---|---|---|---|---|---|
| 0.0223031 | GDM | Control | |||
| A | 38 (24.1%) | 25 (15.8%) | |||
| G | 120 (75.9%) | 133 (84.2%) | |||
| IL-17A (-197G>A) | rs2275913 | G/G | 50 (63.3%) | 56 (70.9%) | |
| G/A | 20 (25.3%) | 21 (26.6%) | |||
| A/A | 9 (11.4%) | 2 (2.5%) | |||
| 0.03841204 | G | 50 (31.6%) | 45 (28.5%) | ||
| A | 108 (68.4%) | 113 (71.5%) | |||
| IL-17RA (-947A>G) | rs4819554 | A/A | 39 (49.4%) | 44 (55.7%) | |
| A/G | 30 (38.0%) | 25 (31.6%) | |||
| G/G | 10 (12.7%) | 10 (12.7%) | |||
| 0.6210301 | Del | 93 (58.9%) | 98 (62.0%) | ||
| Ins | 65 (41.1%) | 60 (38.0%) | |||
| HLA-G 14-bp | rs66554220 | Ins/Ins | 15 (19.0%) | 8 (10.1%) | |
| Ins/Del | 35 (44.3%) | 44 (55.7%) | |||
| Del/Del | 29 (36.7%) | 27 (34.2%) | |||
| Genetic Model | Genotype | Patients (N = 79) | Control (N = 79) | OR (95% CI) | p Value | FDR |
|---|---|---|---|---|---|---|
| HLA-G 14-bp indel | ||||||
| Codominant | DD | 29 (36.7%) | 27 (34.2%) | 1 | 0.196 | 0.440 |
| DI | 35 (44.3%) | 44 (55.7%) | 0.74 (0.37–1.47) | - | ||
| II | 15 (19.0%) | 8 (10.1%) | 1.75 (0.64–4.77) | - | ||
| Dominant | DD | 29 (36.7%) | 27 (34.2%) | 1 | 0.739 | 0.8321 |
| DI-II | 50 (63.3%) | 52 (65.8%) | 0.90 (0.47–1.72) | - | ||
| Recessive | DD-II | 64 (81.0%) | 71 (89.9%) | 1 | 0.112 | 0.335 |
| II | 15 (19.0%) | 8 (10.1%) | 2.08 (0.83–5.23) | - | ||
| IL-17A -197G>A rs2275913 | ||||||
| Codominant | GG | 50 (63.3%) | 56 (70.9%) | 1 | 0.075 | 0.335 |
| GA | 20 (25.3%) | 21 (26.6%) | 1.07 (0.52–2.19) | - | ||
| AA | 9 (11.4%) | 2 (2.5%) | 5.04 (1.04–24.44) | - | ||
| Dominant | GG | 50 (63.3%) | 56 (70.9%) | 1 | 0.310 | 0.557 |
| GA-AA | 29 (36.7%) | 23 (29.1%) | 1.41 (0.72–2.75) | - | ||
| Recessive | GG-GA | 70 (88.6%) | 77 (97.5%) | 1 | 0.023 | 0.209 |
| AA | 9 (11.4%) | 2 (2.5%) | 4.95 (1.03–23.69) | - | ||
| IL-17RA -947A>G rs4819554 | ||||||
| Codominant | AA | 39 (49.4%) | 44 (55.7%) | 1 | 0.685 | 0.832 |
| AG | 30 (38.0%) | 25 (31.6%) | 1.35 (0.68–2.68) | - | ||
| GG | 10 (12.7%) | 10 (12.7%) | 1.13 (0.42–3.00) | - | ||
| Dominant | AA | 39 (49.4%) | 44 (55.7%) | 1 | 0.425 | 0.638 |
| AG-GG | 40 (50.6%) | 35 (44.3%) | 1.29 (0.69–2.41) | - | ||
| Recessive | AA-AG | 69 (87.3%) | 69 (87.3%) | 1 | 1.00 | 1.000 |
| GG | 10 (12.7%) | 10 (12.7%) | 1.00 (0.39–2.55) | - | ||
| Genetic Model | Genotype | Patients (N = 78) | Control (N = 78) | OR (95% CI) | p Value | FDR |
|---|---|---|---|---|---|---|
| HLA-G 14-bp indel | ||||||
| Codominant | DD | 29 (37.2%) | 27 (34.6%) | 1 | 0.026 | 0.059 |
| DI | 35 (44.9%) | 44 (55.1%) | 0.67 (0.28–1.61) | - | ||
| II | 14 (17.9%) | 8 (10.0%) | 3.34 (0.98–11.41) | - | ||
| Dominant | DD | 29 (37.2%) | 27 (34.6%) | 1 | 0.938 | 0.938 |
| DI-II | 49 (62.8%) | 51 (65.4%) | 0.97 (0.43–2.16) | - | ||
| Recessive | DD-II | 64 (82.1%) | 70 (89.7%) | 1 | 0.010 | 0.048 |
| II | 14 (17.9%) | 8 (10.3%) | 4.20 (1.36–12.96) | - | ||
| IL-17A -197G>A rs2275913 | ||||||
| Codominant | GG | 50 (64.1%) | 55 (70.5%) | 1 | 0.021 | 0.059 |
| GA | 19 (24.4%) | 21 (26.9%) | 1.22 (0.49–3.07) | - | ||
| AA | 9 (11.5%) | 2 (2.6%) | 9.89 (1.63–59.92) | - | ||
| Dominant | GG | 50 (64.1%) | 55 (70.5%) | 1 | 0.140 | 0.253 |
| GA-AA | 28 (35.9%) | 23 (29.5%) | 1.86 (0.81–4.30) | - | ||
| Recessive | GG-GA | 69 (88.5%) | 76 (97.4%) | 1 | 0.006 | 0.048 |
| AA | 9 (11.5%) | 2 (2.6%) | 9.33 (1.57–55.38) | - | ||
| IL-17RA -947A>G rs4819554 | ||||||
| Codominant | AA | 38 (48.7%) | 43 (55.1%) | 1 | 0.733 | 0.825 |
| AG | 30 (38.5%) | 25 (32.1%) | 0.86 (0.37–2.00) | - | ||
| GG | 10 (12.8%) | 10 (12.8%) | 0.62 (0.18–2.09) | - | ||
| Dominant | AA | 38 (48.7%) | 43 (55.1%) | 1 | 0.551 | 0.708 |
| AG-GG | 40 (51.3%) | 35 (44.09) | 0.79 (0.36–1.73) | - | ||
| Recessive | AA-AG | 68 (87.2%) | 68 (87.2%) | 1 | 0.484 | 0.708 |
| GG | 10 (12.8%) | 10 (12.8%) | 0.66 (0.21–2.09) | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.T.B.; Lucas, C.R.; de Carvalho, K.T.C.; Neta, A.P.R.; Bernardes-Oliveira, E.; Camargo, J.D.d.A.S.; Luchessi, A.D.; Cobucci, R.N.; Crispim, J.C.d.O. Homozygous AA Genotype of IL-17A and 14-bp Insertion Polymorphism in HLA-G 3′UTR Are Associated with Increased Risk of Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2025, 22, 327. https://doi.org/10.3390/ijerph22030327
de Souza ATB, Lucas CR, de Carvalho KTC, Neta APR, Bernardes-Oliveira E, Camargo JDdAS, Luchessi AD, Cobucci RN, Crispim JCdO. Homozygous AA Genotype of IL-17A and 14-bp Insertion Polymorphism in HLA-G 3′UTR Are Associated with Increased Risk of Gestational Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2025; 22(3):327. https://doi.org/10.3390/ijerph22030327
Chicago/Turabian Stylede Souza, Amaxsell Thiago Barros, Cecília Rodrigues Lucas, Kleyton Thiago Costa de Carvalho, Antonia Pereira Rosa Neta, Emanuelly Bernardes-Oliveira, Juliana Dantas de Araújo Santos Camargo, André Ducati Luchessi, Ricardo Ney Cobucci, and Janaina Cristiana de Oliveira Crispim. 2025. "Homozygous AA Genotype of IL-17A and 14-bp Insertion Polymorphism in HLA-G 3′UTR Are Associated with Increased Risk of Gestational Diabetes Mellitus" International Journal of Environmental Research and Public Health 22, no. 3: 327. https://doi.org/10.3390/ijerph22030327
APA Stylede Souza, A. T. B., Lucas, C. R., de Carvalho, K. T. C., Neta, A. P. R., Bernardes-Oliveira, E., Camargo, J. D. d. A. S., Luchessi, A. D., Cobucci, R. N., & Crispim, J. C. d. O. (2025). Homozygous AA Genotype of IL-17A and 14-bp Insertion Polymorphism in HLA-G 3′UTR Are Associated with Increased Risk of Gestational Diabetes Mellitus. International Journal of Environmental Research and Public Health, 22(3), 327. https://doi.org/10.3390/ijerph22030327







