The Colombian Medical Cannabis Paradox: A Scoping Review of Structural Barriers and Health Inequity
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Research Question and PCC (Population, Concept, Context) Framework
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Study Selection and Data Extraction
2.6. Synthesis and Presentation of Results
2.7. Figure Creation
3. Results
3.1. Studies Identified for Review
3.2. Overview of Scientific Research: Multidisciplinary and Critical Approaches
3.3. Gap 1: Access to Health—Between Right on Paper and Patient Reality
3.4. Gap 2: Rural Development—Between Promised Inclusion and Documented Marginalization
3.5. Gap 3: The Regulatory Framework—Between Theoretical Vanguard and Practical Complexity
4. Discussion
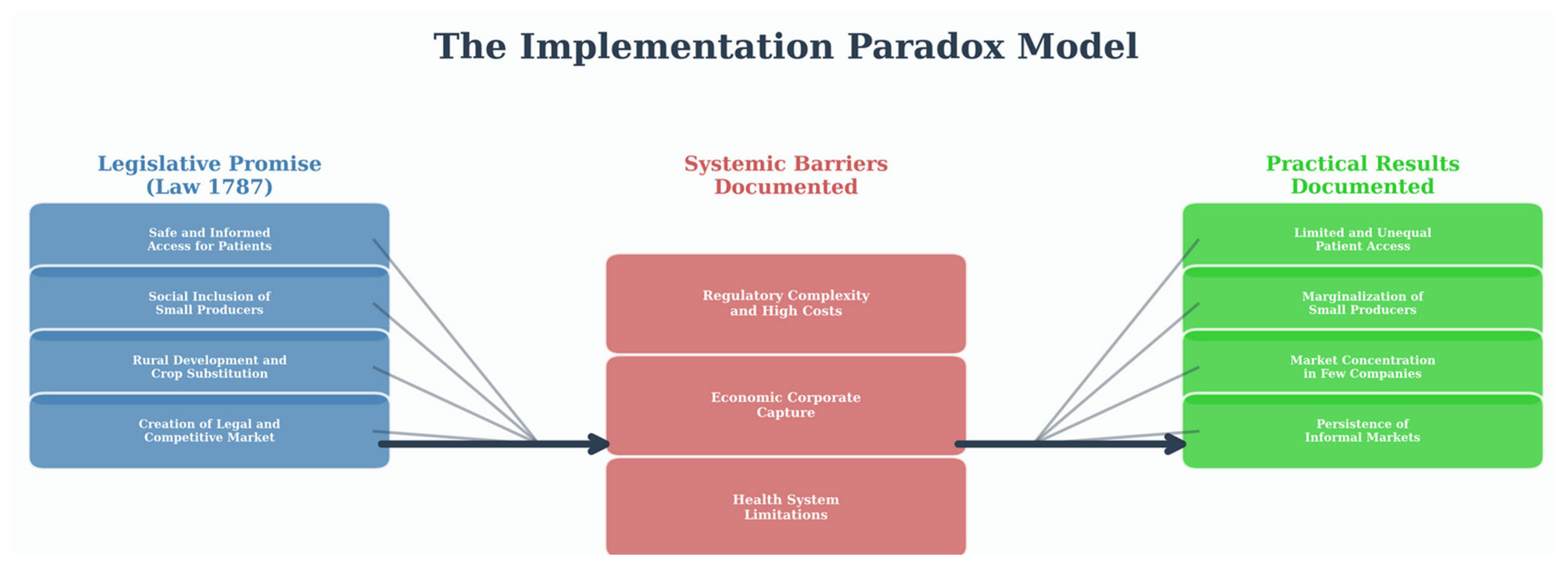
4.1. Main Findings and the Formalization of an Implementation Paradox
4.1.1. The Legislative Promise as the Horizon of Public Policy
4.1.2. Structural Barriers as the Central Mechanism of Inequity
4.1.3. Practical Outcomes: The Evidence of Systemic Inequity
4.2. Comparison with Previous Literature and Contribution of the Study
4.3. Study Limitations
4.4. Implications for Practice and Future Research
4.4.1. Recommendations for Policymakers
- For the Ministry of Justice and the Ministry of Agriculture: It is crucial to review licensing requirements to create differentiated and simplified pathways for small-scale producers. This could include relaxing land tenure requirements, creating associative licensing models, and providing state technical assistance to meet quality standards.
- For the Ministry of Health and Social Protection: A national program of continuing medical education and training is required to equip health professionals with the necessary tools to prescribe with confidence. Likewise, it is essential to ensure the effective implementation of Resolution 2292 of 2021, which already updated the Health Benefits Plan (PBS) to allow for the inclusion and coverage of compounded preparations and cannabis-based medicines. The goal is to ensure that this regulation translates into real and equitable access, so that it ceases to be an economic privilege.
4.4.2. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Case Study |
| CS | Cross-sectional Survey |
| JBI | Joanna Briggs Institute |
| NR | Narrative Review |
| PBS | Health Benefits Plan |
| PCC | Population, Concept, Context |
| PRISMA-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews |
| PT | Process Tracing |
| RA | Regulatory Analysis |
References
- Calderón Vallejo, G.A.; Pareja Hincapié, L.M.; Caicedo Cano, C.; Chica Ríos, R.A. Regulación del uso de marihuana en Colombia con fines medicinales. Hacia Promoc. Salud 2017, 22, 43–55. [Google Scholar] [CrossRef]
- Rivera-Velez, L. Usos estratégicos de la moralidad en la formulación de políticas públicas: El caso del cannabis medicinal en Colombia. En-Claves Del Pensam. 2025, 19, 58–89. [Google Scholar] [CrossRef]
- Lozada-Martinez, I.D.; Neira-Rodado, D.; Martinez-Guevara, D.; Cruz-Soto, H.S.; Sanchez-Echeverry, M.P.; Liscano, Y. Why Is It Important to Implement Meta-Research in Universities and Institutes with Medical Research Activities? Front. Res. Metr. Anal. 2025, 10, 1497280. [Google Scholar] [CrossRef]
- Barry, R.A.; Glantz, S.A. Marijuana Regulatory Frameworks in Four US States: An Analysis Against a Public Health Standard. Am. J. Public Health 2018, 108, 914–923. [Google Scholar] [CrossRef]
- Cox, C. Implications of the 2018 Canadian Cannabis Act: Should Regulation Differ for Medicinal and Non-Medicinal Cannabis Use? Health Policy 2021, 125, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Queirolo, R.; Sotto, B. Conflicting Forces in the Implementation of Medicinal Cannabis Regulation in Uruguay. J. Cannabis Res. 2023, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.R.; Henriques, A.T.; Limberger, R.P. Medical Cannabis Regulation: An Overview of Models around the World with Emphasis on the Brazilian Scenario. J. Cannabis Res. 2022, 4, 33. [Google Scholar] [CrossRef]
- Ruheel, M.A.; Gomes, Z.; Usman, S.; Homayouni, P.; Ng, J.Y. Facilitators and Barriers to the Regulation of Medical Cannabis: A Scoping Review of the Peer-Reviewed Literature. Harm Reduct. J. 2021, 18, 106. [Google Scholar] [CrossRef]
- Vélez-Torres, I.; Hurtado, D.; Bueno, B. Medicinal Marijuana, Inc.: A Critique on the Market-Led Legalization of Cannabis and the Criminalization of Rural Livelihoods in Colombia. Crit. Crim. 2021, 29, 505–526. [Google Scholar] [CrossRef]
- Castaño, G.A.; Carrillo, S.; Malagón, R. Barreras de acceso al cannabis medicinal en Colombia. Una revisión narrativa. Rev. Colomb. Cienc. Quím. Farm. 2025, 54, 44–67. [Google Scholar]
- Orjuela-Rojas, J.M.; García Orjuela, X.; Ocampo Serna, S. Medicinal Cannabis: Knowledge, Beliefs, and Attitudes of Colombian Psychiatrists. J. Cannabis Res. 2021, 3, 26. [Google Scholar] [CrossRef]
- Liscano, Y.; Anillo Arrieta, L.A.; Montenegro, J.F.; Prieto-Alvarado, D.; Ordoñez, J. Early Warning of Infectious Disease Outbreaks Using Social Media and Digital Data: A Scoping Review. Int. J. Environ. Res. Public Health 2025, 22, 1104. [Google Scholar] [CrossRef]
- Santos, W.M.D.; Secoli, S.R.; Püschel, V.A.D.A. The Joanna Briggs Institute Approach for Systematic Reviews. Rev. Lat. -Am. Enferm. 2018, 26, e3074. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Villanueva Parra, I.; Muñoz Diaz, V.; Martinez Guevara, D.; Cruz Mosquera, F.E.; Prieto-Alvarado, D.E.; Liscano, Y. A Scoping Review of Angiostrongyliasis and Other Diseases Associated with Terrestrial Mollusks, Including Lissachatina Fulica: An Overview of Case Reports and Series. Pathogens 2024, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.; Tuckerman, J.; Kaufman, J.; Costa, D.; Durrheim, D.N.; Trevena, L.; Thomas, S.; Danchin, M. Comparing Inductive and Deductive Analysis Techniques to Understand Health Service Implementation Problems: A Case Study of Childhood Vaccination Barriers. Implement. Sci. Commun. 2021, 2, 100. [Google Scholar] [CrossRef]
- Bustmante Matoma, H.A.B.; Murillo Ortega, V.M. Panorama del cannabis medicinal en el contexto rural integral colombiano Overview of medical cannabis in the Colombian comprehensive rural context. Podium 2023, 44, 37–52. [Google Scholar] [CrossRef]
- Ledezma-Morales, M.; Cristina Rodríguez, A.; Amariles, P. Mercado del Cannabis medicinal en Colombia: Una oportunidad para el sector salud que requiere lineamientos estratégicos del gobierno nacional y la academia. Rev. Medicas UIS 2020, 33, 53–58. [Google Scholar] [CrossRef]
- Cubillos Sánchez, P.A. Cannabis for medical and scientific purposes: The Colombian landscape. Colomb. J. Anesthesiol. 2020, 49, e954. [Google Scholar] [CrossRef]
- Riveros Santoya, D.C.; Portilla Mogollón, E.A. Regulación actual del cannabis visto desde los beneficios terapéuticos de los cannabinoides. Rev. La Prop. Inmater. 2021, 31, 195–208. [Google Scholar] [CrossRef]
- Maldonado Agudelo, L.M.M.; Tirado, M.A.; Jiménez, M.P.A. Las Oportunidades del Cannabis Medicinal Colombiano en el Mercado Asiático y Europeo. Punto De Vista 2023, 14, 135. [Google Scholar] [CrossRef]
- Hallinan, C.M.; Gunn, J.M.; Bonomo, Y.A. Implementation of Medicinal Cannabis in Australia: Innovation or Upheaval? Perspectives from Physicians as Key Informants, a Qualitative Analysis. BMJ Open 2021, 11, e054044. [Google Scholar] [CrossRef]
- Rønne, S.T.; Rosenbaek, F.; Pedersen, L.B.; Waldorff, F.B.; Nielsen, J.B.; Riisgaard, H.; Søndergaard, J. Physicians’ Experiences, Attitudes, and Beliefs towards Medical Cannabis: A Systematic Literature Review. BMC Fam. Pract. 2021, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Durán-Martínez, A.; Pennell, C. Change from Above or Pressure from Below? The Diffusion of Cannabis Reform in Latin America. Bull. Lat. Am. Res. 2024, 43, 347–361. [Google Scholar] [CrossRef]
- MacPhail, S.L.; Bedoya-Pérez, M.A.; Cohen, R.; Kotsirilos, V.; McGregor, I.S.; Cairns, E.A. Medicinal Cannabis Prescribing in Australia: An Analysis of Trends Over the First Five Years. Front. Pharmacol. 2022, 13, 885655. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.P.M.; Garzón, B.A.L. Oportunidades Y Riesgos De Inversión En La Industria Del Cannabis En Colombia 2023; Repositorio Institucional Lumieres: Bogotá, Colombia, 2024. [Google Scholar]
- Hoyos, J.S. Producción Y Comercialización De Cannabis Con Fines Medicinales; Universidad de La Salle: Bogotá, Colombia, 2023. [Google Scholar]
- Espitia, J.V. Desafíos Y Condicionantes De La Industria De Cannabis Con Fines Medicinales En Colombia; Universidad de La Salle: Bogotá, Colombia, 2020. [Google Scholar]
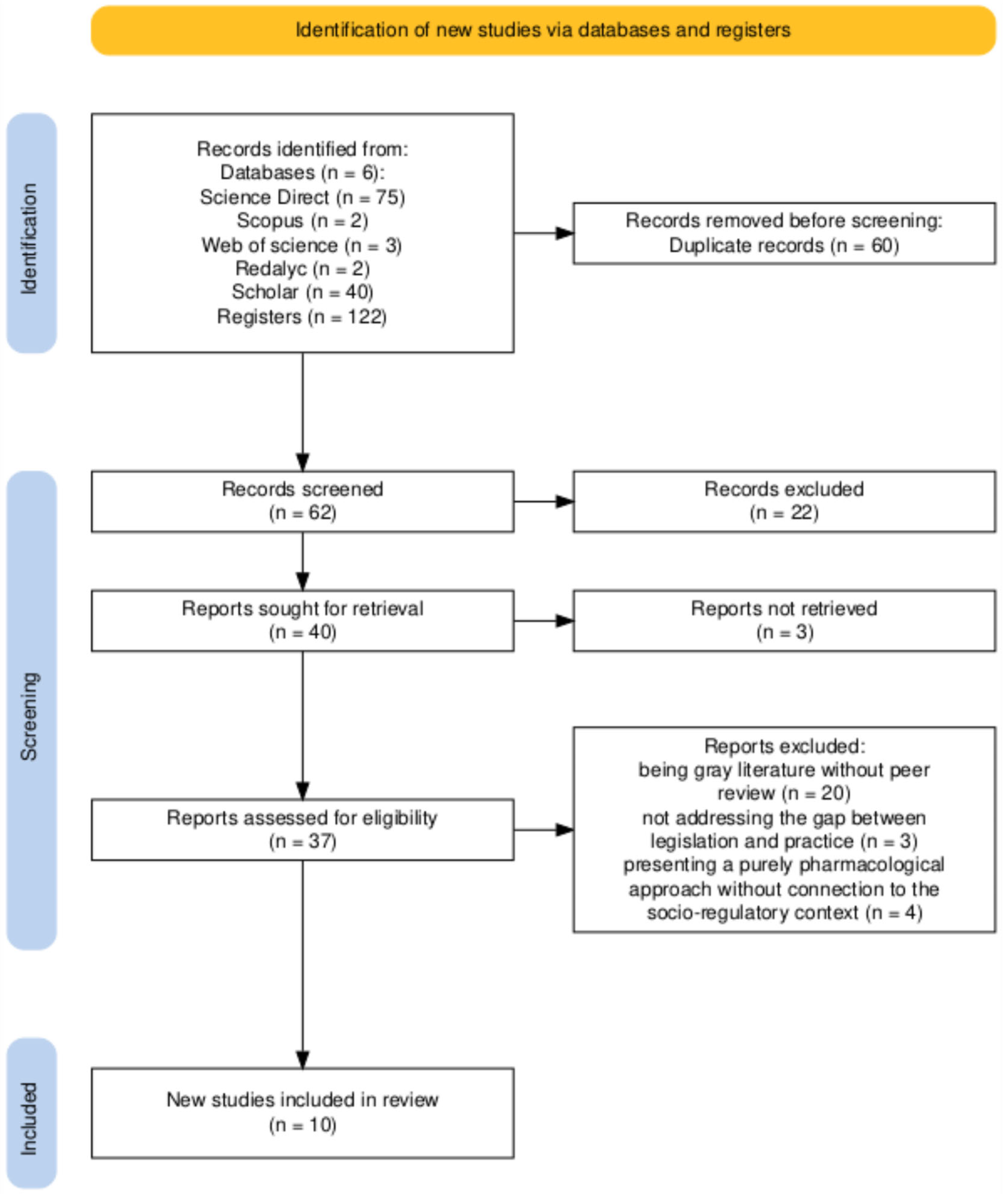
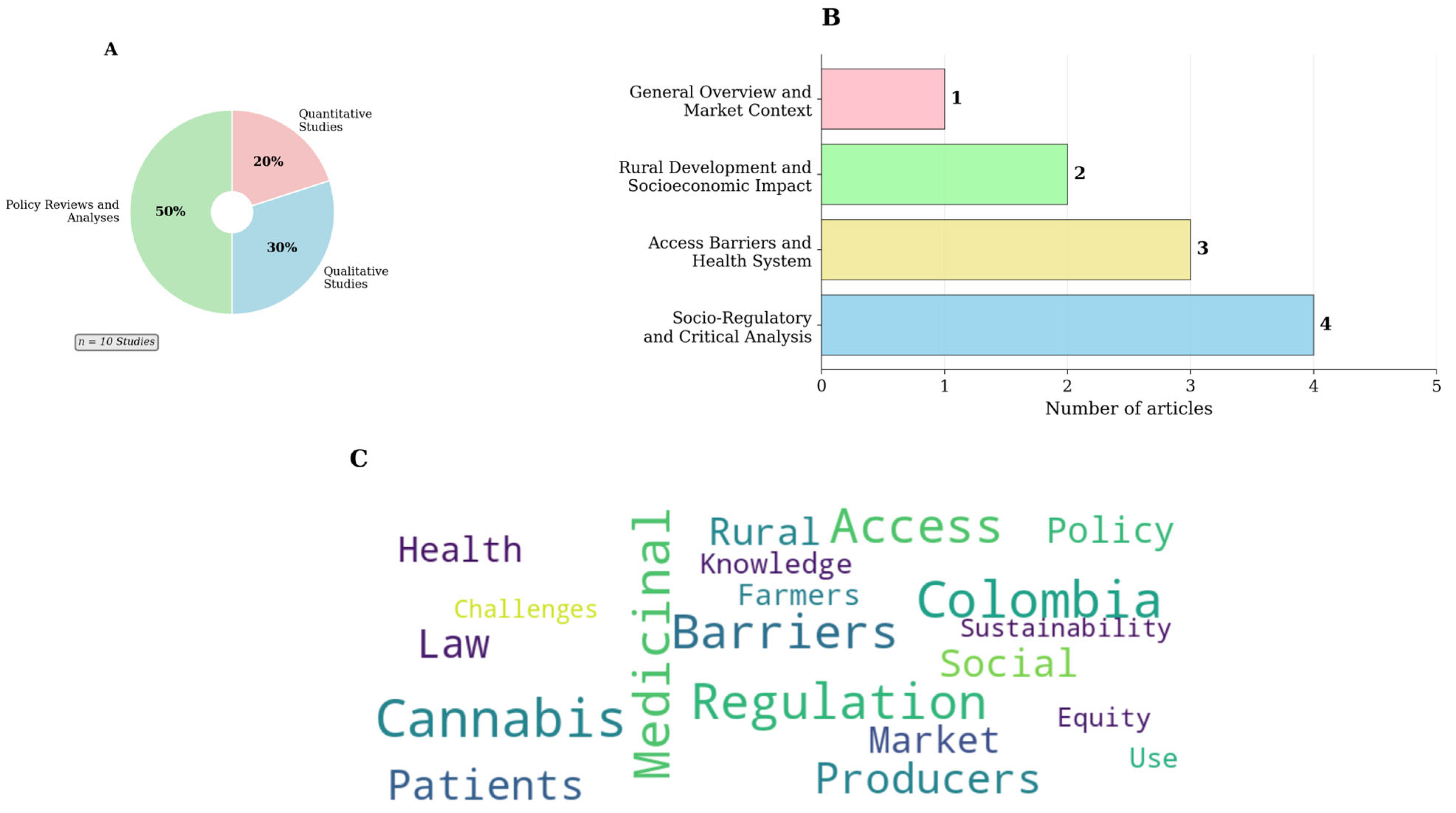
| Authors and Year | Study Type | Main Focus |
|---|---|---|
| Vélez-Torres et al. (2021) [9] | CE (Critical Criminology) | Analysis of the marginalization of peasants and the corporate capture of the market. |
| Castaño et al. (2025) [10] | NR | Identification of barriers to access to medical cannabis in Colombia. |
| Bustamante & Murillo (2023) [17] | CS (Survey) | Overview of medical cannabis for comprehensive rural development. |
| Orjuela-Rojas et al. (2021) [11] | CS (Survey) | Knowledge, beliefs, and attitudes of Colombian psychiatrists. |
| Rivera-Vélez (2025) [2] | PT (Process Tracing) | Strategic use of morality in the formulation of public policy. |
| Ledezma-Morales et al. (2020) [18] | RA | Analysis of gaps between regulations, availability, and access to products. |
| Cubillos Sánchez (2021) [19] | NR (Special Article) | General overview of the industry, international comparison, and therapeutic evidence. |
| Calderón Vallejo et al. (2017) [1] | CE (Interviews) | Interpretation of the initial regulation process and the public debate. |
| Riveros Santoya & Portilla M. (2021) [20] | RA | Detailed analysis of the licensing process and regulatory framework. |
| Maldonado Agudelo et al. (2023) [21] | CE (Descriptive) | Market and export opportunities under the legal framework. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín Arroyave, O.P.; León Cruz, P. The Colombian Medical Cannabis Paradox: A Scoping Review of Structural Barriers and Health Inequity. Int. J. Environ. Res. Public Health 2025, 22, 1792. https://doi.org/10.3390/ijerph22121792
Marín Arroyave OP, León Cruz P. The Colombian Medical Cannabis Paradox: A Scoping Review of Structural Barriers and Health Inequity. International Journal of Environmental Research and Public Health. 2025; 22(12):1792. https://doi.org/10.3390/ijerph22121792
Chicago/Turabian StyleMarín Arroyave, Olga Patricia, and Pedro León Cruz. 2025. "The Colombian Medical Cannabis Paradox: A Scoping Review of Structural Barriers and Health Inequity" International Journal of Environmental Research and Public Health 22, no. 12: 1792. https://doi.org/10.3390/ijerph22121792
APA StyleMarín Arroyave, O. P., & León Cruz, P. (2025). The Colombian Medical Cannabis Paradox: A Scoping Review of Structural Barriers and Health Inequity. International Journal of Environmental Research and Public Health, 22(12), 1792. https://doi.org/10.3390/ijerph22121792





