Hospital Readmission in Stroke Survivors in Social Vulnerability: Predictive Modeling with Machine Learning from the Perspective of the Chronic Conditions Care Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Sample Characterization
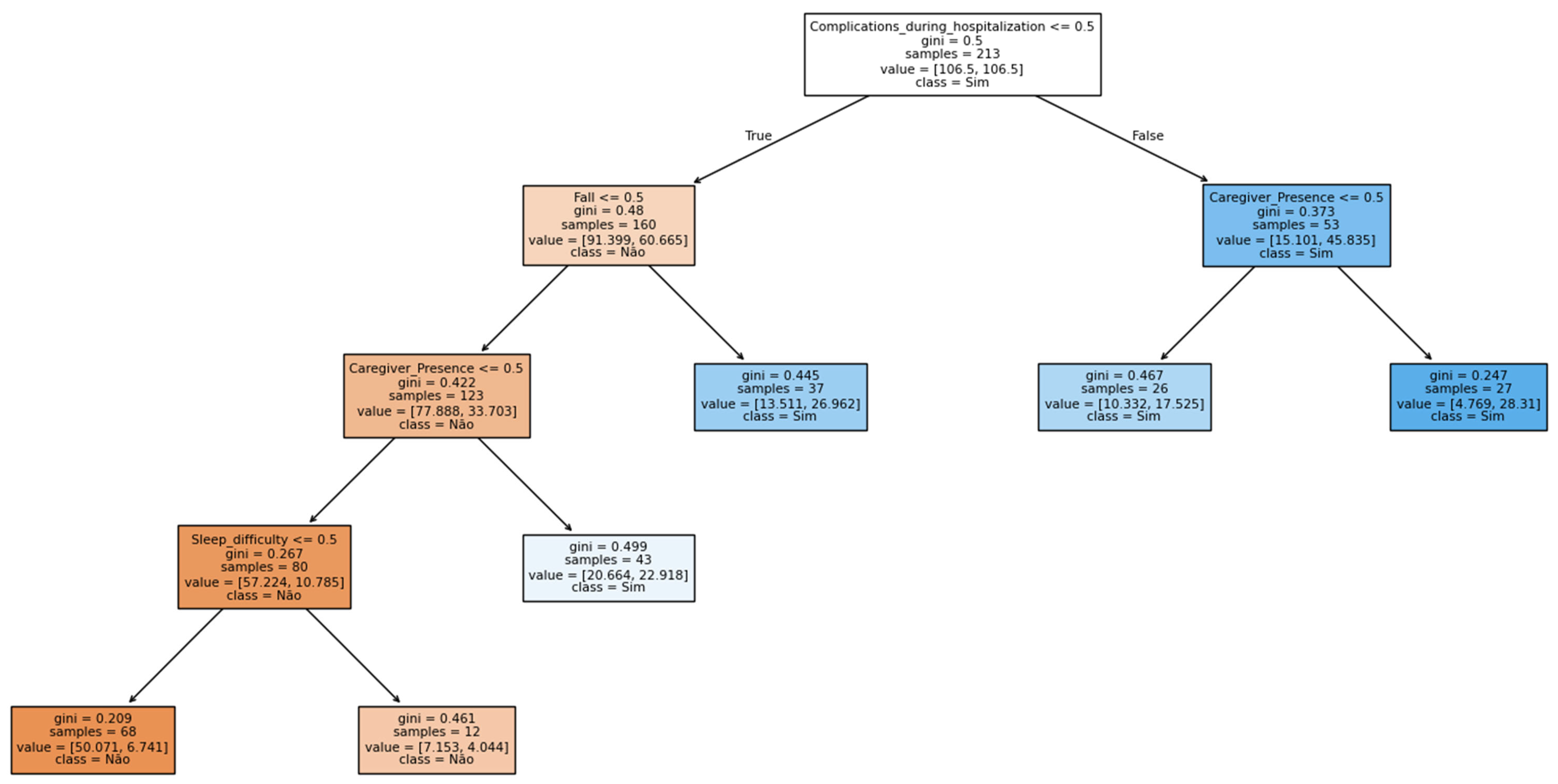
3.2. Construction of ML Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunstmann, F.S.; Lira, C.T.; Icaza, N.G.; Núñez, F.L.; Grazia, R.D. Estratificación de riesgo cardiovascular en la población chilena. Rev. Médica Clínica Las Condes 2012, 23, 657–665. [Google Scholar] [CrossRef][Green Version]
- Monroy, B.; Sanchez, K.; Arguello, P.; Estupiñán, J.; Bacca, J.; Correa, C.V.; Valencia, L.; Castillo, J.C.; Mieles, O.; Arguello, H.; et al. Automated Chronic Wounds Medical Assessment and Tracking Framework Based on Deep Learning. Comput. Biol. Med. 2023, 165, 107335. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K. Current Forecast of COVID-19 in Mexico: A Bayesian and Machine Learning Approaches. PLoS ONE 2022, 17, e0259958. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.D.; Nascimento, C.F.D.; Izbicki, R.; Duarte, Y.A.D.O.; Porto Chiavegatto Filho, A.D. Machine Learning Para Análises Preditivas Em Saúde: Exemplo de Aplicação Para Predizer Óbito Em Idosos de São Paulo, Brasil. Cad. Saúde Pública 2019, 35, e00050818. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020; Available online: https://vizhub.healthdata.org/gbd-compare (accessed on 25 October 2025).
- Rissetti, J.; Feistauer, J.B.; Luiz, J.M.; Da Silveira, L.D.S.; Ovando, A.C. Independência Funcional e Comprometimento Motor Em Indivíduos Pós-AVE Da Comunidade. Acta Fisiátr. 2020, 27, 27–33. [Google Scholar] [CrossRef]
- Gonzalez-Aquines, A.; Rosales, J.; De Souza, A.C.; Corredor-Quintero, A.; Barboza, M.A.; Navia-Gonzalez, V.; Brunet-Perez, F.; Lagos-Servellon, J.; Novarro-Escudero, N.; Ortega-Moreno, D.A.; et al. Availability and Barriers to Access Post-Stroke Rehabilitation in Latin America. J. Stroke Cerebrovasc. Dis. 2024, 33, 107917. [Google Scholar] [CrossRef]
- Ang, S.H.; Hwong, W.Y.; Bots, M.L.; Sivasampu, S.; Abdul Aziz, A.F.; Hoo, F.K.; Vaartjes, I. Risk of 28-Day Readmissions among Stroke Patients in Malaysia (2008–2015): Trends, Causes and Its Associated Factors. PLoS ONE 2021, 16, e0245448. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, X.; Hu, L.; Li, M.; Zhou, M.; Zhao, L.; Yang, R. Risk Factors for 30-Day Readmission in Patients with Ischemic Stroke: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2021, 10, 11083–11105. [Google Scholar] [CrossRef]
- Kilkenny, M.F.; Dalli, L.L.; Kim, J.; Sundararajan, V.; Andrew, N.E.; Dewey, H.M.; Johnston, T.; Alif, S.M.; Lindley, R.I.; Jude, M.; et al. Factors Associated With 90-Day Readmission After Stroke or Transient Ischemic Attack: Linked Data From the Australian Stroke Clinical Registry. Stroke 2020, 51, 571–578. [Google Scholar] [CrossRef]
- Marques, J.C.; Silva, F.A.R.; Martins, A.N.; Oliveira Perdigão, F.S.; Martins Prudente, C.O.; Fagundes, R.R. Perfil de Pacientes Com Sequelas de Acidente Vascular Cerebral Internados Em Um Centro de Reabilitação. Acta Fisiátr. 2019, 26, 144–148. [Google Scholar] [CrossRef]
- Qiu, X.; Xue, X.; Xu, R.; Wang, J.; Zhang, L.; Zhang, L.; Zhao, W.; He, L. Predictors, Causes and Outcome of 30-Day Readmission among Acute Ischemic Stroke. Neurol. Res. 2021, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.R.J. Hospital Readmission in Stroke Survivors One Year versus Three Years after Discharge from Inpatient Rehabilitation: Prevalence and Associations in an Asian Cohort. J. Rehabil. Med. 2021, 53, jrm00208. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Liu, B.; Wan, X.; Zhang, X.; Zhang, J.; Zhou, X.; Lau, A.Y.L.; Zhang, Y. Risk Factors Associated with 31-Day Unplanned Readmission in 50,912 Discharged Patients after Stroke in China. BMC Neurol. 2018, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Lesaine, E.; Francis, F.; Domecq, S.; Miganeh-Hadi, S.; Sevin, F.; Sibon, I.; Rouanet, F.; Pradeau, C.; Coste, P.; Cetran, L.; et al. Social and Clinical Vulnerability in Stroke and STEMI Management during the COVID-19 Pandemic: A Registry-Based Study. BMJ Open 2024, 14, e073933. [Google Scholar] [CrossRef]
- Tong, X.; Carlson, S.A.; Kuklina, E.V.; Coronado, F.; Yang, Q.; Merritt, R.K. Social Vulnerability Index and All-Cause Mortality After Acute Ischemic Stroke, Medicare Cohort 2020–2023. JACC Adv. 2024, 3, 101258. [Google Scholar] [CrossRef]
- Carmo, M.E.D.; Guizardi, F.L. O Conceito de Vulnerabilidade e Seus Sentidos Para as Políticas Públicas de Saúde e Assistência Social. Cad. Saúde Pública 2018, 34, e00101417. [Google Scholar] [CrossRef]
- Dimenstein, M.; Cirilo Neto, M. Abordagens conceituais da vulnerabilidade no âmbito da saúde e assistência social. Rev. Pesqui. Práticas Psicossociais 2020, 15, 1–17. [Google Scholar]
- Vieira-Meyer, A.P.G.F.; Morais, A.P.P.; Campelo, I.L.B.; Guimarães, J.M.X. Violência e Vulnerabilidade No Território Do Agente Comunitário de Saúde: Implicações No Enfrentamento Da COVID-19. Ciênc. Saúde Coletiva 2021, 26, 657–668. [Google Scholar] [CrossRef]
- Souza, C.D.F.D.; Oliveira, D.J.D.; Silva, L.F.D.; Santos, C.D.D.; Pereira, M.C.; Paiva, J.P.S.D.; Leal, T.C.; Mariano, R.D.S.; Araújo, A.K.B.F.D.; Baggio, J.A.D.O. Tendência Da Mortalidade Por Doenças Cerebrovasculares No Brasil (1996–2015) e Associação Com Desenvolvimento Humano e Vulnerabilidade Social. Arq. Bras. Cardiol. 2021, 116, 89–99. [Google Scholar] [CrossRef]
- Mendes, E.V. O Cuidado das Condições Crônicas na Atenção Primária à Saúde: O Imperativo da Consolidação da Estratégia da Saúde da Família, 1st ed.; Organização Pan-Americana da Saúde: Brasília, Brazil, 2012; ISBN 978-85-7967-078-7. [Google Scholar]
- Polit, D.; Beck, C. Fundamentos de Pesquisa em Enfermagem: Avaliação de Evidências Para a Prática da Enfermagem; Artmed: Porto Alegre, Brazil, 2021; ISBN 978-85-8271-489-8. [Google Scholar]
- Malta, M.; Cardoso, L.O.; Bastos, F.I.; Magnanini, M.M.F.; Silva, C.M.F.P.D. Iniciativa STROBE: Subsídios Para a Comunicação de Estudos Observacionais. Rev. Saúde Pública 2010, 44, 559–565. [Google Scholar] [CrossRef]
- Barbosa, A.M.D.L.; Pereira, C.C.M.; Miranda, J.P.R.; Rodrigues, J.H.D.L.; De Carvalho, J.R.O.; Rodrigues, A.C.E. Perfil Epidemiológico Dos Pacientes Internados Por Acidente Vascular Cerebral No Nordeste Do Brasil. Acervo Saúde 2021, 13, e5155. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Caneda, M.A.G.D.; Fernandes, J.G.; Almeida, A.G.D.; Mugnol, F.E. Confiabilidade de Escalas de Comprometimento Neurológico Em Pacientes Com Acidente Vascular Cerebral. Arq. Neuro-Psiquiatr. 2006, 64, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Girotto, E.; Andrade, S.M.D.; Cabrera, M.A.S.; Matsuo, T. Adesão Ao Tratamento Farmacológico e Não Farmacológico e Fatores Associados Na Atenção Primária Da Hipertensão Arterial. Ciênc. Saúde Coletiva 2013, 18, 1763–1772. [Google Scholar] [CrossRef][Green Version]
- Anderle, P.; Rockenbach, S.P.; Goulart, B.N.G.D. Reabilitação Pós-AVC: Identificação de Sinais e Sintomas Fonoaudiológicos Por Enfermeiros e Médicos Da Atenção Primária à Saúde. CoDAS 2019, 31, e20180015. [Google Scholar] [CrossRef]
- Organização Pan-Americana da Saúde (OPAS). Cuidados Inovadores Para Condições Crônicas: Organização e Prestação de Atenção de Alta Qualidade Às Doenças Crônicas Não Transmissíveis Nas Américas; OPAS: Washington, DC, USA, 2015; ISBN 978-92-75-71738-7. [Google Scholar]
- Fabrizzio, G.C.; Erdmann, A.L.; Oliveira, L.M.D.; Lorenzini, E.; Jensen, R.; Santos, J.L.G.D. Prediction of COVID-19 Patients’ Admission to the Intensive Care Unit Based on the Precision Nursing Framework. J. Health Inform. 2024, 16. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Sathyanarayanan, S.; Tantri, B.R. Confusion Matrix-Based Performance Evaluation Metrics. Afr. J. Biomed. Res. 2024, 27, 4023–4031. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.G.; Lee, S.-I. Consistent Individualized Feature Attribution for Tree Ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- Chicco, D.; Jurman, G. Machine Learning Can Predict Survival of Patients with Heart Failure from Serum Creatinine and Ejection Fraction Alone. BMC Med. Inform. Decis. Mak. 2020, 20, 16. [Google Scholar] [CrossRef]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Man, S.; Bruckman, D.; Tang, A.S.; Uchino, K.; Schold, J.D. The Association of Socioeconomic Status and Discharge Destination with 30-Day Readmission after Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106146. [Google Scholar] [CrossRef] [PubMed]
- Organização Pan-Americana da Saúde (OPAS). La Situación de Los Cuidados a Largo Plazo en América Latina y el Caribe; OPAS: Washington, DC, USA, 2023; ISBN 978-92-75-32687-9. [Google Scholar]
- Gaspari, A.P.; Cruz, E.D.D.A.; Batista, J.; Alpendre, F.T.; Zétola, V.; Lange, M.C. Preditores de Internação Prolongada Em Unidade de Acidente Vascular Cerebral (AVC). Rev. Lat.-Am. Enferm. 2019, 27, e3197. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Almeida, P.F.D. Atenção Primária Integral e Sistemas Segmentados de Saúde Na América Do Sul. Cad. Saúde Pública 2017, 33, e00118816. [Google Scholar] [CrossRef]
- Florian Ángeles, J.M. Sistemas de Salud En Latinoamérica Durante El Periodo 2020 al 2023. Rev. Climatol. 2024, 24, 1374–1381. [Google Scholar] [CrossRef]
- Almeida, P.F.D.; Giovanella, L.; Schenkman, S.; Franco, C.M.; Duarte, P.O.; Houghton, N.; Báscolo, E.; Bousquat, A. Perspectivas Para Las Políticas Públicas de Atención Primaria En Salud En Suramérica. Ciênc. Saúde Coletiva 2024, 29, e03792024. [Google Scholar] [CrossRef]
- Nishimura, F.; Carrara, A.F.; Freitas, C.E.D. Effect of the Melhor Em Casa Program on Hospital Costs. Rev. Saúde Pública 2019, 53, 104. [Google Scholar] [CrossRef]
- Chaverri-Carvajal, A.; Matus-López, M. Cuidados de larga duración en Costa Rica: Enseñanzas para América Latina desde la evidencia internacional. Rev. Panam. Salud Pública 2021, 45, e146. [Google Scholar] [CrossRef]
- Sánchez, Y.C.A. Recomendaciones Para la Articulación de los Programas de Atención Domiciliaria en Paciente Crónico de la Red Pública y Privada En Bogotá: Una Reflexión Desde las Ciencias Contemporáneas. Master’s Thesis, Facultad de Medicina, Universidad El Bosque, Bogotá, Colombia, 2021. [Google Scholar]
| Socioeconomic Characteristics | n (%) | Health Background and Lifestyle | n (%) | Health Conditions and Functional Dependence | n (%) | |||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 140 (52.4) | Smoking (current or past) | Yes | 99 (37.1) | Previous medical assistance before stroke | Yes | 107 (40.1) |
| Female | 127 (47.6) | No | 168 (62.9) | No | 160 (59.9) | |||
| Age | (min/max) | (38/111) * | Alcohol use (current or past) | Yes | 69 (25.8) | Type of stroke | Ischemic stroke | 235 (88.0) |
| Mean (SD) | 70.5 (12.1) | No | 198 (74.2) | Hemorrhagic stroke | 20 (7.5) | |||
| Race/skin color | Non-white | 241 (90.3) | Hypertension | Yes | 201 (75.3) | Rehospitalized | Both | 12 (4.5) |
| White | 26 (9.7) | No | 66 (24.7) | Yes, stroke-related | 76 (28.5) | |||
| Marital status | With partner | 145 (54.3) | Diabetes Mellitus | Yes | 95 (35.6) | Yes, other causes | 49 (18.3) | |
| Without partner | 122 (45.7) | No | 172 (64.4) | Not rehospitalized | 142 (53.2) | |||
| Education (years of schooling) | Illiterate | 136 (50.9) | Previous stroke | Yes | 66 (24.7) | Rehospitalized within one year after stroke | Yes | 99 (37.1) |
| Up to 8 years | 102 (38.2) | No | 201 (75.3) | No | 168 (62.9) | |||
| 8 years or more | 29 (10.9) | Dyslipidemia | Yes | 74 (27.7) | Hospitalization location (first stroke) | Medical ward only | 147 (55.1) | |
| Income (minimum wage) | Less than one | 17 (6.4) | No | 193 (72.3) | Emergency room/ICU | 57 (21.4) | ||
| 1 to <3 | 245 (91.8) | Dementia | Yes | 46 (17.2) | Both (ward and emergency/ICU) | 46 (17.2) | ||
| 3 to <5 | 5 (1.9) | No | 221 (82.8) | Not hospitalized | 17 (6.4) | |||
| Family composition | Min/max | 1/9 * | Pneumonia | Yes | 47 (17.6) | Better home care | Yes | 143 (53.6) |
| Mean (SD) | 3.8 (1.6) | No | 220 (82.4) | No | 124 (46.6) | |||
| Place of residence | Urban area | 219 (82.0) | COVID-19 | Yes | 58 (21.7) | Continuous medication use | Yes | 249 (93.3) |
| Rural area | 48 (18.0) | No | 209 (78.3) | No | 18 (6.7) | |||
| Work activity | Yes | 41 (15.4) | Falls | Yes | 76 (28.5) | Functional dependence (Barthel Index) | Totally independent | 102 (38.2) |
| No | 226 (84.6) | No | 191 (71.5) | Slight dependence | 54 (20.2) | |||
| Caregiver | No caregiver | 156 (58.4) | Cardiac comorbidity | Yes | 33 (12.3) | Moderate dependence | 24 (9.0) | |
| Informal caregiver | 101 (37.8) | No | 234 (84.7) | |||||
| Formal caregiver | 10 (3.7) | Hospitalized complications | Yes | 63 (23.6) | Severe dependence | 23 (8.6) | ||
| No | 204 (76.4) | Total dependence | 64 (24.0) | |||||
| Modelo | Validação | Acurácia | AUC-ROC | Kappa de Cohen | RMSE | Falsos Positivos n (%) | Falsos Negativos n (%) |
|---|---|---|---|---|---|---|---|
| Regressão Logística (Ridge) | 80/20 | 75.9% | 80.3% | 0.48 | 0.48 | 7 (13.0%) | 6 (11.1%) |
| 70/30 | 66.7% | 78.4% | 0.29 | 0.48 | 14 (17.3%) | 13 (16.0%) | |
| 5-KFold | 70.0% ± 0.08 | 77.9% ± 0.084 | 0.36 ± 0.17 | 0.46 ± 0.01 | 15.75% | 14.27% | |
| Árvore de Decisão (CART) | 80/20 | 70.4% | 81.8% | 0.43 | 0.41 | 14 (25.9%) | 2 (3.7%) |
| 70/30 | 74.1% | 80.3% | 0.47 | 0.41 | 15 (18.5%) | 6 (7.4%) | |
| 5-KFold | 69.6% ± 0.04 | 72.4% ± 0.04 | 0.36 ± 0.06 | 0.46 ± 0.01 | 17.6% | 12.7% |
| Variable | Classical Logistic Regression | Ridge Logistic Regression with Penalization for Control of Collinearity | Importance | ||||
|---|---|---|---|---|---|---|---|
| Coef Classic | Odds Ratio | Coef Ridge | Odds Ratio | 95% Confidence Interval (Bootstrap with 1000 Resamples) | |||
| OR_2.5% | OR_97.5% | ||||||
| Complications during hospitalization | 0.4168 | 1.5171 | 0.0263 | 1.0267 | 1.0185 | 1.0463 | 18.17% |
| Fall | 0.4761 | 1.6098 | 0.0264 | 1.0268 | 1.0155 | 1.0444 | 16.48% |
| Skin Lesion | 0.4987 | 1.6466 | 0.0322 | 1.0327 | 1.0142 | 1.0428 | 16.21% |
| Type of Stroke | 0.4172 | 1.5177 | 0.0054 | 1.0054 | 1.0125 | 1.0403 | 14.92% |
| Caregiver Presence | 0.3849 | 1.4694 | 0.0287 | 1.0291 | 1.0133 | 1.0405 | 14.85% |
| Sleep difficulty | 0.2151 | 1.24 | 0.0292 | 1.0296 | 1.0011 | 1.0295 | 8.78% |
| Time since stroke in months | −0.2019 | 0.8172 | 0.0156 | 1.0157 | 0.9768 | 0.9976 | 7.55% |
| Better at Home | 0.1314 | 1.1404 | −0.0134 | 0.9867 | 0.9918 | 1.02 | 3.04% |
| CCCM Element | Finding from Decision Tree | Finding from Logistic Regression | Meaning in the Chronic Care Context | Level of Care/Professional Involved |
|---|---|---|---|---|
| Risk stratification and proactive care management | The variable “Complications during hospitalization” was the main decision node, indicating a higher risk of readmission. | The variable “Complications during hospitalization” showed the highest importance in predicting readmission risk. | Represents the need for early identification of complex cases and active follow-up by primary healthcare (PHC) after hospital discharge. | Primary and secondary care—multidisciplinary team (community health worker, physician, nurse, physiotherapist). |
| Supported self-care and patient engagement | The presence of a “Caregiver” strongly influenced the readmission outcome. | The presence of a “Caregiver” contributed to nearly 15% of the readmission outcome. | Reflects the role of family and community support in maintaining treatment adherence and preventing complications. | Primary care—nurse, community health worker. |
| Clinical decision support and multidisciplinary approach | Variables such as “Sleep difficulty” and “Fall” appear in the lower levels of the tree. | Fall, skin lesion, type of stroke, sleep difficulty, and time since stroke appear as predictors. | Demonstrates the need for an integrated clinical approach, considering functional and behavioral symptoms. | Primary care and rehabilitation (Family Health Support Centers)—physician, nurse, psychologist, physiotherapist. |
| Health information systems and continuous monitoring | The tree shows predictable readmission patterns based on simple clinical data. | The regression reveals predictable readmission variables based on simple clinical information. | Highlights the potential of health data use to guide interventions and continuous surveillance. | Health management and surveillance units—data analysts, program managers, coordination teams. |
| Integration across care levels and continuity of care | The model emphasizes the importance of communication between hospital and primary care after discharge. | Participation in the “Home Care Program” acted as a protective factor against readmission. | Indicates that lack of care coordination may contribute to avoidable readmissions. | Integrated Health Network (IHN)—care transition professionals, network managers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, E.S.; Moreira, T.M.M.; de Souza, A.C.C.; Santos, A.M.R.d.; da Silva, A.R.V.; Falcão, L.M.; Pereira, L.C.; da Penha, J.C.; da Silva Junior, M.B.; de Lima Fontes, F.L.; et al. Hospital Readmission in Stroke Survivors in Social Vulnerability: Predictive Modeling with Machine Learning from the Perspective of the Chronic Conditions Care Model. Int. J. Environ. Res. Public Health 2025, 22, 1705. https://doi.org/10.3390/ijerph22111705
da Silva ES, Moreira TMM, de Souza ACC, Santos AMRd, da Silva ARV, Falcão LM, Pereira LC, da Penha JC, da Silva Junior MB, de Lima Fontes FL, et al. Hospital Readmission in Stroke Survivors in Social Vulnerability: Predictive Modeling with Machine Learning from the Perspective of the Chronic Conditions Care Model. International Journal of Environmental Research and Public Health. 2025; 22(11):1705. https://doi.org/10.3390/ijerph22111705
Chicago/Turabian Styleda Silva, Erisonval Saraiva, Thereza Maria Magalhães Moreira, Ana Célia Caetano de Souza, Ana Maria Ribeiro dos Santos, Ana Roberta Vilarouca da Silva, Lariza Martins Falcão, Livia Carvalho Pereira, Jardeliny Corrêa da Penha, Manoel Borges da Silva Junior, Francisco Lucas de Lima Fontes, and et al. 2025. "Hospital Readmission in Stroke Survivors in Social Vulnerability: Predictive Modeling with Machine Learning from the Perspective of the Chronic Conditions Care Model" International Journal of Environmental Research and Public Health 22, no. 11: 1705. https://doi.org/10.3390/ijerph22111705
APA Styleda Silva, E. S., Moreira, T. M. M., de Souza, A. C. C., Santos, A. M. R. d., da Silva, A. R. V., Falcão, L. M., Pereira, L. C., da Penha, J. C., da Silva Junior, M. B., de Lima Fontes, F. L., Sayaverde, I. W. D., Gallardo, M. d. P. S., & Borges, J. W. P. (2025). Hospital Readmission in Stroke Survivors in Social Vulnerability: Predictive Modeling with Machine Learning from the Perspective of the Chronic Conditions Care Model. International Journal of Environmental Research and Public Health, 22(11), 1705. https://doi.org/10.3390/ijerph22111705







