Osteoporosis in the Elderly: A Cross-Sectional Study in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
Study Design, Sample, Population
3. Research Variables
3.1. Quantitative Ultrasound Examination of the Calcaneus
3.2. Analysis of Actual Nutrition
4. Data Analysis
5. Ethical Considerations
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OP | Osteoporosis |
| BMI | Body Mass Index |
| BMD | Bone Mineral Density |
| 25(OH)D | 25-hydroxyvitamin D |
| DXA | Dual-energy X-ray Absorptiometry |
| WHO | World Health Organization |
| FAO | Food and Agriculture Organization of the United Nations |
| FRAX | Fracture Risk Assessment Tool |
| CI | Confidence Interval |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| IBM SPSS | Statistical Package for the Social Sciences |
| SOS | Speed of Sound |
| BUA | Broadband Ultrasound Attenuation |
| MUFA | Monounsaturated Fatty Acids |
| RE | Retinol Equivalent |
| LCB | Local Committee on Bioethics |
References
- Kemmak, A.R.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran. 2020, 34, 154. [Google Scholar]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- SAbrams, A.; Atkinson, S.A. Calcium, magnesium, phosphorus and vitamin D fortification of complementary foods. J. Nutr. 2003, 133, 2994S–2999S. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, A.; Davoodi, S.H.; Vahid, F. Vitamin D status, including serum levels and sun exposure are associated or correlated with bone mass measurements diagnosis, and bone density of the spine. BMC Nutr. 2023, 9, 48. [Google Scholar] [CrossRef]
- Bouillon, R.; Norman, A.W.; Lips, P. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 1980–1981. [Google Scholar] [PubMed]
- Liu, J.; Curtis, E.M.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef]
- Panel, N.I.H.C. Osteoporosis prevention, diagnosis, and therapy. Jama 2001, 285, 785–795. [Google Scholar]
- Barrett-Connor, E.; Wade, S.W.; Downs, R.W.; Ganiats, T.; Hochberg, M.; Recker, R.R.; Stolshek, B.S. Self-reported calcium use in a cohort of postmenopausal women receiving osteoporosis therapy: Results from POSSIBLE USTM. Osteoporos. Int. 2015, 26, 2175–2184. [Google Scholar] [CrossRef]
- Political Declaration and Madrid International Plan of Action on Ageing, Second World Assembly on Ageing, Madrid, Spain, 8–12 April 2002. New York City (NY): United Nations. 2002. Available online: https://www.un.org/development/desa/ageing/madrid-plan-of-action-and-its-implementation.html (accessed on 25 October 2025).
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Madiyeva, M.; Rymbayeva, T.; Kaskabayeva, A.; Bersimbekova, G.; Kanapiyanova, G.; Prilutskaya, M.; Akhmetzhanova, D.; Alimbayeva, A.; Omarov, N. The Prevalence and Risk Factors of Low Bone Mineral Density in the Population of the Abay Region of Kazakhstan. Int. J. Environ. Res. Public Health 2024, 21, 681. [Google Scholar] [CrossRef]
- Lesnyak, O.; Bilezikian, J.P.; Zakroyeva, A. Report on the Audit on Burden of Osteoporosis in Eight Countries of the Eurasian Region: Armenia, Belarus, Georgia, Moldova, Kazakhstan, the Kyrgyz Republic, the Russian Federation, and Uzbekistan. Arch. Osteoporos. 2020, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Population of the Republic of Kazakhstan by Gender and Type of Locality at the Beginning of 2025. Bureau of National Statistics of Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Available online: https://stat.gov.kz/en/industries/social-statistics/demography/publications/330951/ (accessed on 25 October 2025).
- Slivkina, N.; Abduldayeva, A.; Tardjibayeva, S.; Doszhanova, G.; Kuanyshbayeva, G. The health of the population, according to prenosological diagnostics. Georgian Med. News 2020, 303, 188–193. [Google Scholar]
- Rodionova, S.S.; Khakimov, U.R. Risk factors of bone mineral density deficit and low-energy fractures in primary osteoporosis in men. NN Priorov J. Traumatol. Orthop. 2018, 25, 22–29. [Google Scholar] [CrossRef]
- Yohannan, J.M. Assessment of the Risk Factors and Knowledge on Osteoporosis Among Pre-Menopausal Women in a Selected Community of Mangalore; Rajiv Gandhi University of Health Sciences: Bengaluru, India, 2013. [Google Scholar]
- Antiushko, D.P. Evaluation of gerodietetic product’s for enteral nutrition protein value. J. Chem. Technol. 2020, 28, 161–167. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Doszhanova, G.; Abduldayeva, A.A. Hygienic assessment of nutrition status of the population of the gerontological group. Gig. I Sanit. 2017, 96, 1084–1087. [Google Scholar] [CrossRef]
- Norms of Physiological Requirements for Energy and Nutrients for Various Population Groups in the Republic of Kazakhstan. National Methodological Recommendations. Available online: https://online.zakon.kz/Document/?doc_id=38710734&pos=14;-55#pos=14;-55 (accessed on 25 October 2025).
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The declaration of Helsinki. Br. Med. J. Publ. Group 2007, 335, 624–625. [Google Scholar]
- Samouei, R.; Keyvanara, M. Identifying strategies for dealing with the aging population from the perspective of health system experts: A qualitative study. J. Educ. Health Promot. 2022, 11, 210. [Google Scholar]
- Doszhanova, G.; Abduldayeva, A.; Dosmambetova, K. Aging biomarkers for evaluating the life style quality of elderly and senile people. Iran. J. Public Health 2018, 47, 757. [Google Scholar]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting food-sourced vitamins for consumer diet and health needs: A perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ 2021, 9, e11940. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; A Shapses, S.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef]
- Groenendijk, I.; den Boeft, L.; van Loon, L.J.; de Groot, L.C. High Versus low Dietary Protein Intake and Bone Health in Older Adults: A Systematic Review and Meta-Analysis. Comput. Struct. Biotechnol. J. 2019, 17, 1101–1112. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Y.; Zhu, Z. Association between dietary protein intake and bone mineral density based on NHANES 2011–2018. Sci. Rep. 2025, 15, 8638. [Google Scholar] [CrossRef]
- MIsanejad; Sirola, J.; Mursu, J.; Kröger, H.; Tuppurainen, M.; Erkkilä, A. Association of Protein Intake with Bone Mineral Density and Bone Mineral Content among Elderly Women: The OSTPRE Fracture Prevention Study. J. Nutr. Health Aging 2017, 21, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Ran, X.; Cui, J.; Wei, F.; Yi, G.; Chen, W.; Luo, X.; Chen, Z. The Role of Zinc in Bone Mesenchymal Stem Cell Differentiation. Cell Reprogram. 2022, 24, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jia, L.; Gao, R. Association between dietary copper, iron, zinc, selenium intake and osteopenia or osteoporosis in elderly hypertensive patients: A retrospective cohort study. Front. Nutr. 2024, 11, 1419379. [Google Scholar] [CrossRef]
- Vergatti, A.; Abate, V.; Garofano, F.; Fiore, A.; De Filippo, G.; Strazzullo, P.; Rendina, D. Are Dietary Habits the Missing Link Between Hashimoto’s Thyroiditis and Osteoporosis? Nutrients 2025, 17, 2109. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Xie, H.; Wang, N.; He, H.; Yang, Z.; Wu, J.; Yang, T.; Wang, Y. The association between selenium and bone health: A meta-analysis. Bone Jt. Res. 2023, 12, 423–432. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, X.; Liu, S.; Li, Y.; Hu, L.; Zhang, J.; Hu, W.; Dong, S. Advances in selenium research for bone and joint-related diseases: From pathophysiological mechanisms to therapeutic implications of selenium-based biomaterials. Biomater. Transl. 2025, 2. [Google Scholar] [CrossRef]
- McLean, R.R.; Hannan, M.T. B vitamins, homocysteine, and bone disease: Epidemiology and pathophysiology. Curr. Osteoporos. Rep. 2007, 5, 112–119. [Google Scholar] [CrossRef]
- Dai, Z.; Koh, W.P. B-vitamins and bone health—A review of the current evidence. Nutrients 2015, 7, 3322–3346. [Google Scholar] [CrossRef]
- Kim, D.; Han, A.; Park, Y. Association of Dietary Total Antioxidant Capacity with Bone Mass and Osteoporosis Risk in Korean Women: Analysis of the Korea National Health and Nutrition Examination Survey 2008–2011. Nutrients 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.Y.; Alshaikhi, A.I.; Hazzazi, J.S.; Kurdi, J.R.; Ramadan, M.F. Recent insight on nutritional value, active phytochemicals, and health-enhancing characteristics of fig (Ficus craica). Food Saf. Heath 2024, 2, 179–195. [Google Scholar] [CrossRef]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Ahmed, H.H.; Basoudan, N. Protective effect of Citrus sinensis and Citrus aurantifolia against osteoporosis and their phytochemical constituents. J. Med. Plants Res. 2011, 5, 579–588. Available online: http://www.academicjournals.org/JMPR (accessed on 25 October 2025).
- Zhou, F.; Wang, T.; Li, L.; Yu, J.; Liu, Z.; Zhang, J.; Wang, G.; Li, J.; Shao, C.; Wang, P.; et al. Tea consumption and risk of bone health: An updated systematic review and meta-analysis. J. Bone Miner. Metab. 2024, 42, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Toroptsova, N.V.; Benevolenskaya, L.I.; Nikitinskaya, O.A. Osteoporosis: Clinical Guidelines. Available online: https://online.zakon.kz/Document/?doc_id=30469318&pos=4;-111#pos=4;-111 (accessed on 25 October 2025).
- Kanis, J.A.; Oden, A.; Johnell, O.; Jonsson, B.; de Laet, C.; Dawson, A. The burden of osteoporotic fractures: A method for setting intervention thresholds. Osteoporos. Int. 2001, 12, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Issayeva, S.; Lesnyak, O.; Zakroyeva, A.; Issayeva, B.; Dilmanova, D.; Johansson, H.; Liu, E.; Lorentzon, M.; Harvey, N.; McCloskey, E.; et al. Epidemiology of osteoporotic fracture in Kazakhstan and development of a country specific FRAX model. Arch. Osteoporos. 2020, 15, 30. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Y.; Liu, X.; Zhang, X.; Qian, X.; Zhang, H.; Zhang, G.; Wang, C. Association of obesity with bone mineral density and osteoporosis in adults: A systematic review and meta-analysis. Public Health 2020, 180, 22–28. [Google Scholar] [CrossRef]
- Cherukuri, L.; Kinninger, A.; Birudaraju, D.; Lakshmanan, S.; Li, D.; Flores, F.; Mao, S.S.; Budoff, M.J. Effect of body mass index on bone mineral density is age-specific. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1767–1773. [Google Scholar] [CrossRef]
- Jyoti, J.; Mahilange, A. A Study of Relationship of Bone Mineral Density with Age, Body Mass Index, Obesity and Serum Magnesium Level. J. Evol. Med. Dent. Sci. 2021, 10, 1563–1566. [Google Scholar]
- Bansal, A.; Bansal, S. Relationship of Body Mass Index and Bone Mineral Density in Adult Men. Int. J. Med. Dent. Sci. 2016, 5, 1033–1037. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, Y.; Jin, M.; Gu, Z.; Pei, Y.; Meng, P. Aged-Related Changes in Body Composition and Association between Body Composition with Bone Mass Density by Body Mass Index in Chinese Han Men over 50-year-old. PLoS ONE 2015, 10, e0130400. [Google Scholar]
- Reza, S.M.; Salamat, A.H.; Abedi, I.; Janghorbani, M. Relationship between Weight, Body Mass Index, and Bone Mineral Density in Men Referred for Dual-Energy X-Ray Absorptiometry Scan in Isfahan, Iran. J. Osteoporos. 2013, 2013, 205963. [Google Scholar]
- Oance, T.; Rodica, P.; Melania, B. Body weight, BMI, and stature have a protective effect on bone mineral density in women with postmenopausal vertebral osteoporosis, whereas greater age at menarche and years after menopause have a negative effect. Asian Biomed. 2017, 9, 81–86. [Google Scholar] [CrossRef]
- Adrian, K.; Sumardi, M. A REMS Scan-Based Report on Relation Between Body Mass Index and Osteoporosis in Urban Population of Medan at Royal Prima Hospital. Maj. Kedokt. Bdg. 2020, 52, 22–27. [Google Scholar] [CrossRef]
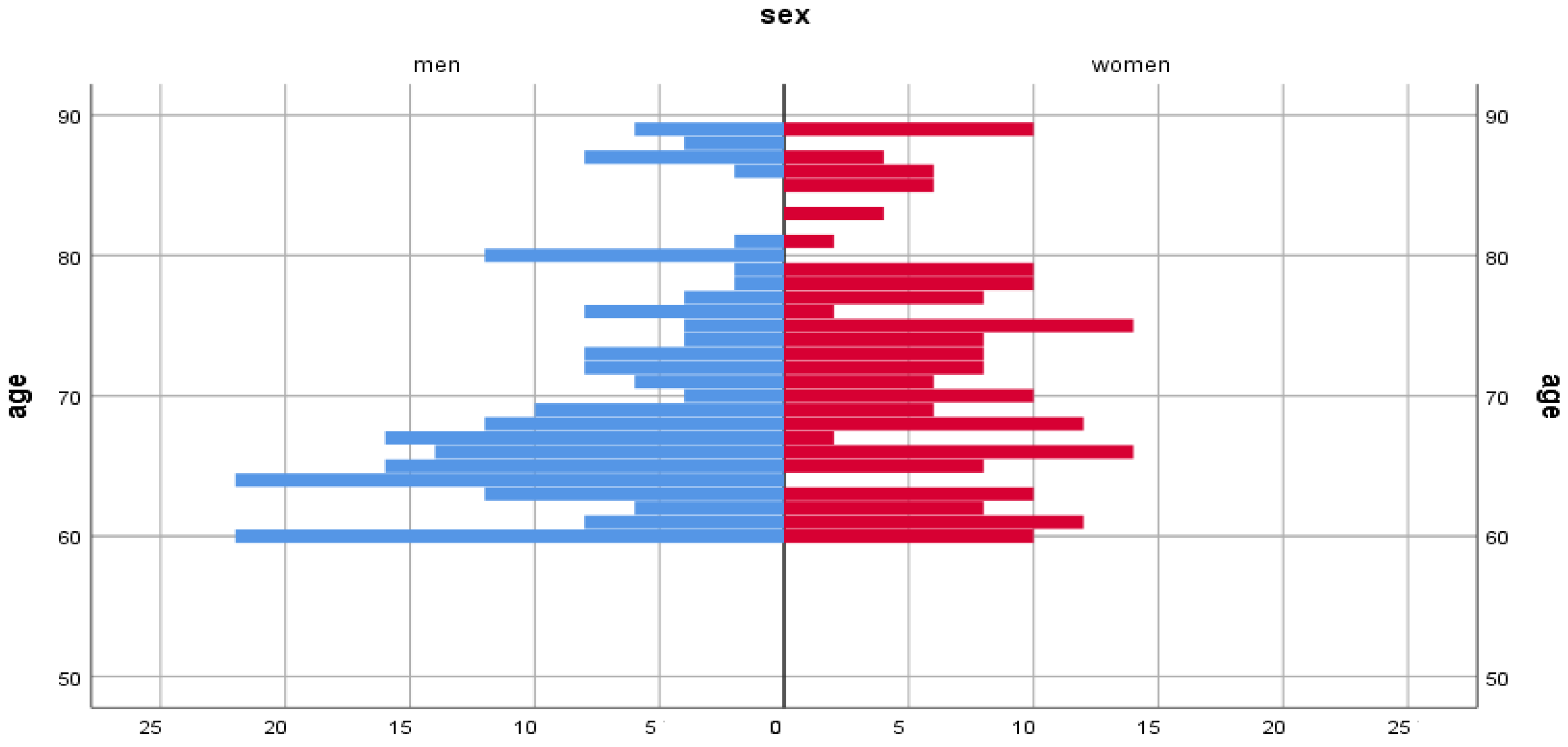
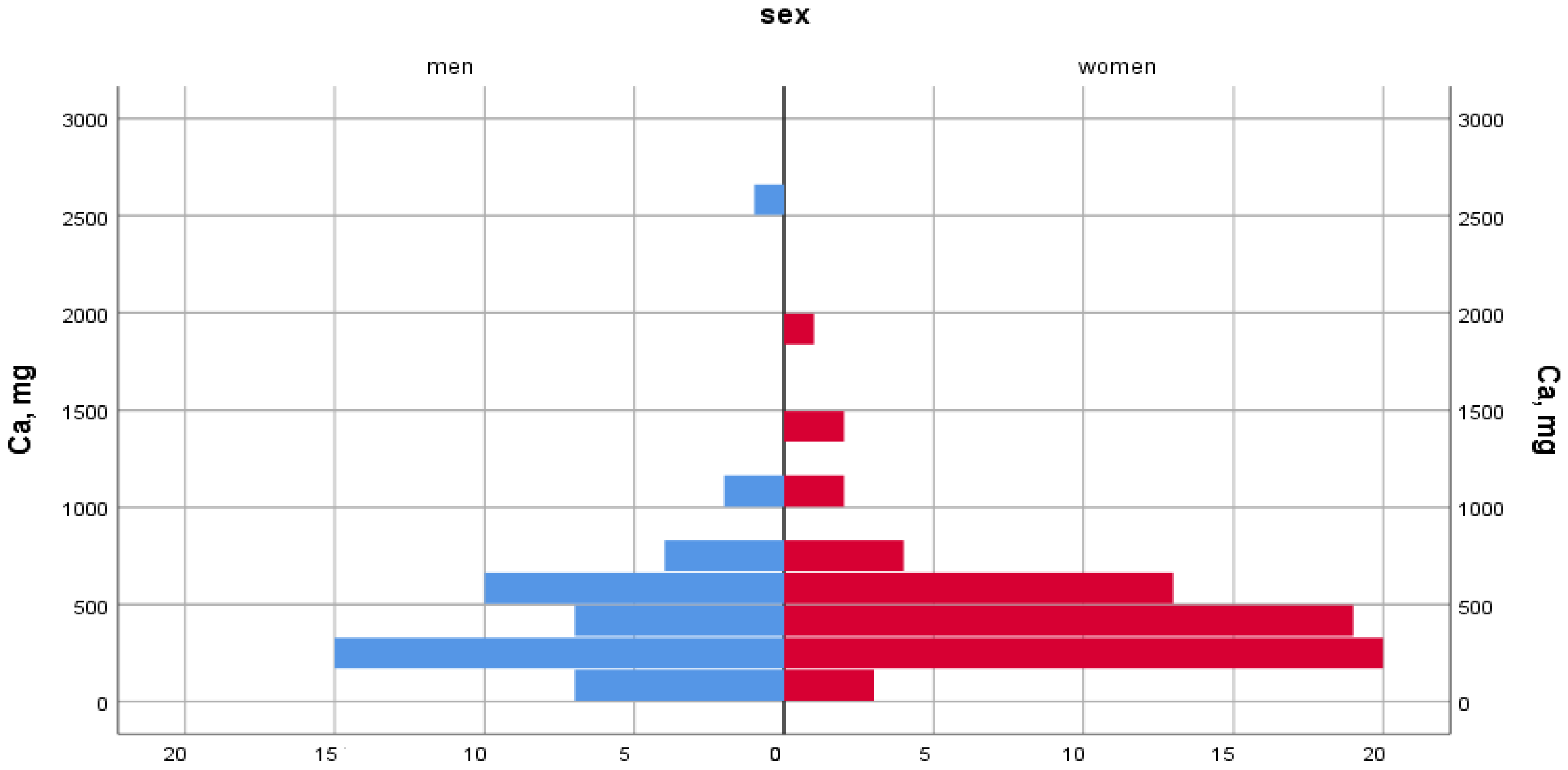
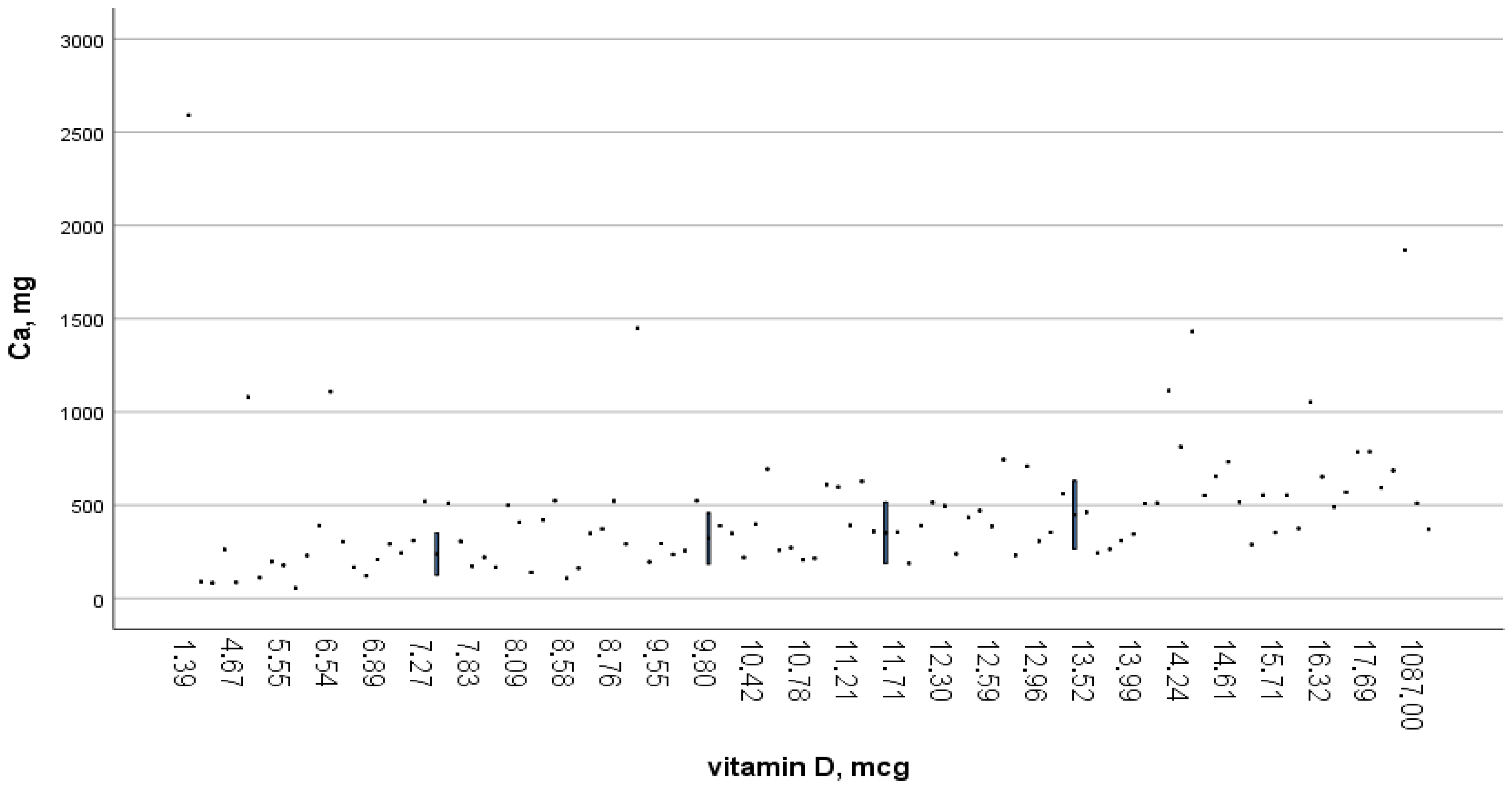
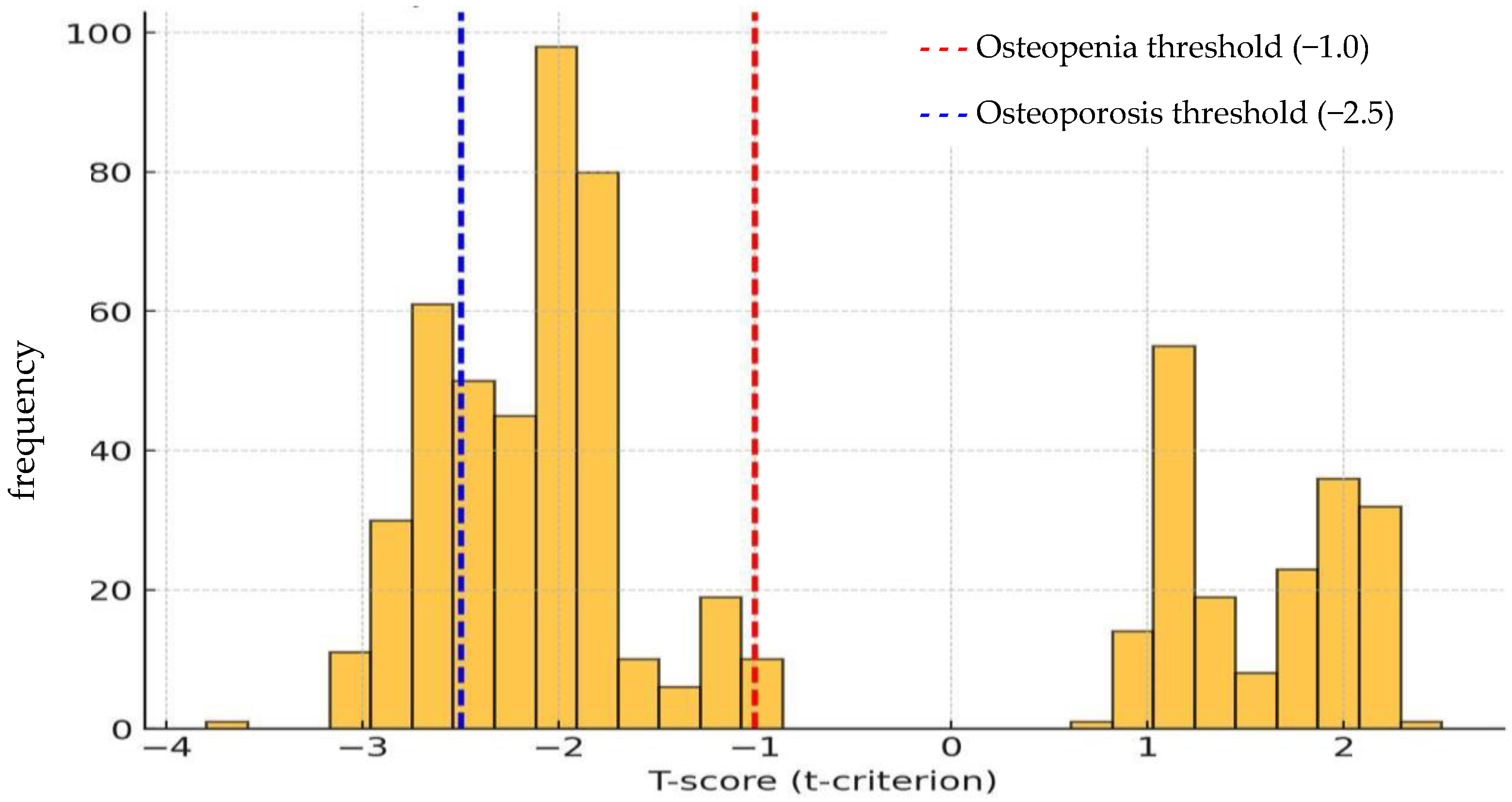
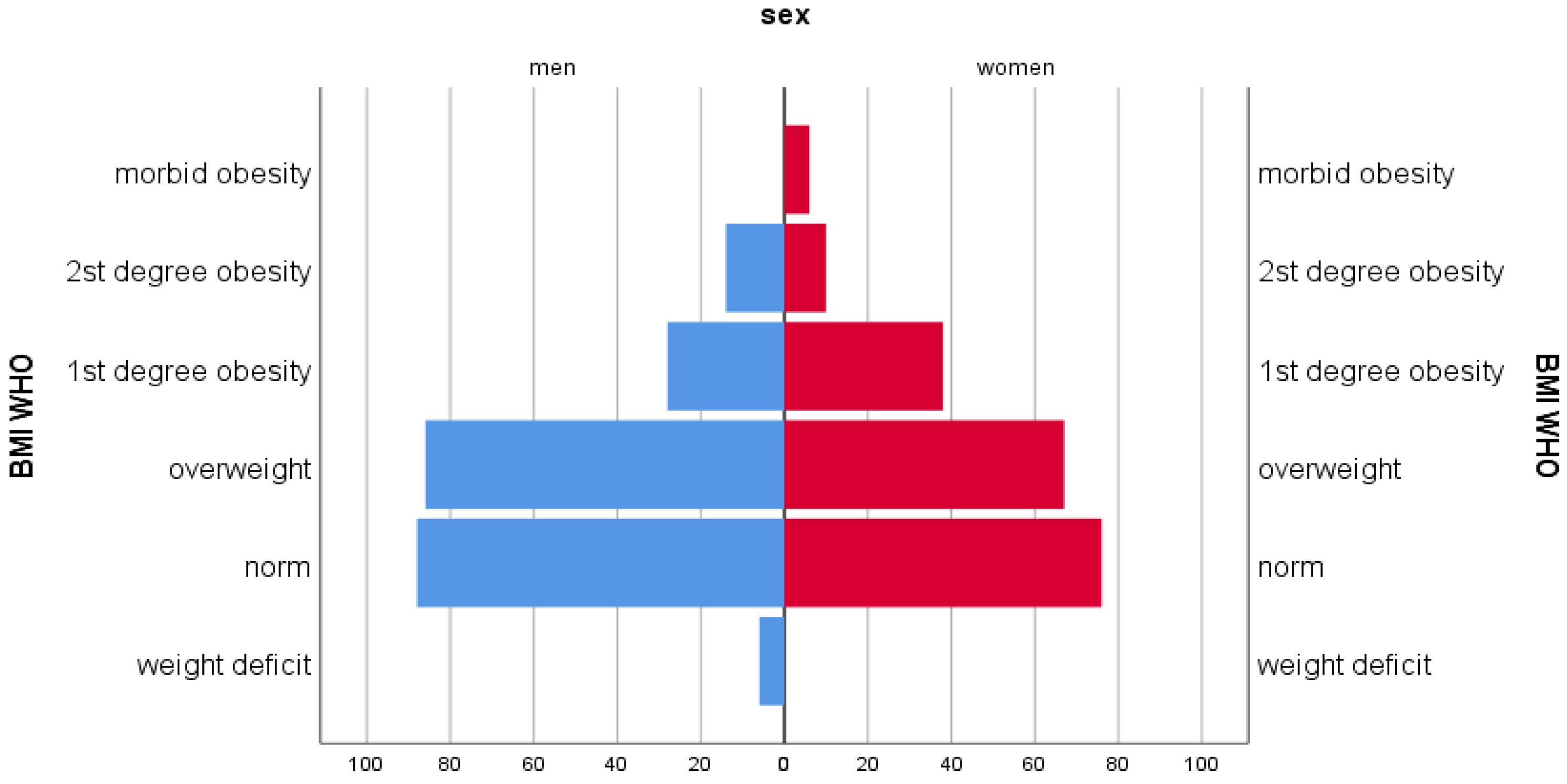

| Nutritional Factors | Women | Men |
|---|---|---|
| Calorie content, (kcal) | 1800.2 ± 242.3 | 1820.0 ± 322.2 |
| Protein, (g) | 70.2 ± 18.3 | 78.4 ± 32.5 |
| Animal protein, (g) | 37.8 ± 23.3 | 44.1 ± 29.3 |
| Fiber (cellulose), (g) | 5.7 ± 3.6 | 5.6 ± 3.4 |
| Monounsaturated fatty acids (MUFAs), (g) | 17.5 ± 9.5 | 19.8 ± 9.9 |
| Phytosterol, (mg) | 22.5 ± 37.0 | 23.9 ± 41.4 |
| Sodium, (mg) | 1191.0 ± 801.5 | 1541.3 ± 901.0 |
| Potassium, (mg) | 2681.1 ± 1252 | 2835.8 ± 1319.1 |
| Calcium, (mg) | 528.9 ± 308.2 | 549.6 ± 353.5 |
| Magnesium, (mg) | 249.6 ± 109.1 | 274.0 ± 111.4 |
| Phosphorus, (mg) | 918.2 ± 375 | 1019.0 ± 420.4 |
| Zinc, (mg) | 7.14 ± 4.06 | 8.51 ± 4.98 |
| Selenium, (mcg) | 35.5 ± 26.8 | 50.5 ± 44.5 |
| Iodine, (mcg) | 41.2 ± 32.6 | 44.1 ± 30.5 |
| Manganese, (mg) | 2.15 ± 1.18 | 2.55 ± 1.32 |
| RE (retinol equivalent), mcg | 682.8 ± 1536.2 | 739.2 ± 2068.9 |
| Vitamin D, (mcg) | 0.92 ± 1.71 | 1.15 ± 2.2 |
| Vitamin C, (mg) | 55.7 ± 53.9 | 52.5 ± 118.7 |
| Pearson’s Correlation | R | Value |
| Calcium, (mg) | 0.713 ** | Correlation is significant at 0.01 (two-way) |
| Protein, total, (g) | 0.538 ** | |
| Phytosterol, (mg) | 0.576 ** | |
| Zinc, (mg) | 0.575 ** | |
| Selenium, (mcg) | 0.630 ** | |
| Iodine, (mcg) | 0.609 ** | |
| Vitamin B6, (mg) | 0.630 ** | |
| ANOVA | F | Meaning |
| Animal proteins, total, (g) | 8.055 | 0.005 |
| Nitrogen, (g) | 7.254 | 0.008 |
| Vitamin C, (mg) | 6.904 | 0.010 |
| Fiber (cellulose), (g) | 6.358 | 0.013 |
| Monounsaturated fatty acids (MUFAs), (g) | 4.142 | 0.044 |
| Vitamin B6, (mg) | 3.735 | 0.056 |
| RE (retinol equivalent), (mcg) | 2.839 | 0.095 |
| Studied | All Surveyed Respondents Were Studied | % of Individuals with a Consumption Level of Less than 2/3 RDA * |
|---|---|---|
| Insufficient calcium intake | ||
| Whole group | 1961 (100%) | 1579 (80.5%) |
| Women | 1619 (100%) | 1305 (80.6%) |
| Men | 342 (100%) | 274 (80.1%) |
| Insufficient intake of vitamin D | ||
| The whole group | 1961 (100%) | 1923 (98.1%) |
| Women | 1619 (100%) | 1593 (98.4%) |
| Men | 342 (100%) | 330 (96.5%) |
| BMI, kg/m2 | <18.5 | 18.5–24.99 | 25–29.99 | 30–34.99 | 35+ | All | p | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| The entire group of respondents | ||||||||
| In general, n, % | of Norms | 2 (8.3) | 79 (16.1) | 147 (19.6) | 107 (23.9) | 69 (27.7) | 404 (20.6) | 0.001 |
| Osteopenia | 14 (58.3) | 299 (60.9) | 453 (60.4) | 264 (59.1) | 147 (59.0) | 1177 (60.0) | ||
| OP | 8 (33.3) | 113 (23.0) | 150 (20.0) | 76 (17.0) | 33 (13.3) | 380 (19.4) | ||
| Total | 24 (100.0) | 491 (100.0) | 750 (100.0) | 447 (100.0) | 249 (100.0) | 1961 (100.0) | ||
| Gender attribute | ||||||||
| Woman, n, % | of Norms | 2 (11.1) | 55 (14.4) | 112 (18.4) | 87 (22.9) | 61 (26.3) | 317 (19.6) | 0.001 |
| Osteopenia | 8 (44.4) | 236 (61.8) | 366 (60.2) | 226 (59.5) | 139 (59.9) | 975 (60.2) | ||
| OP | 8 (44.4) | 91 (23.8) | 130 (21.4) | 67 (17.6) | 32 (13.8) | 328 (20.2) | ||
| Total | 18 (100.0) | 382 (100.0) | 608 (100.0) | 380 (100.0) | 232 (100.0) | 1620 (100.0) | ||
| Men, n, % | of Norms | 0 | 24 (22.0) | 35 (24.6) | 20 (29.9) | 8 (47.1) | 87 (25.5) | 0.156 |
| Osteopenia | 6 (100.0) | 63 (57.8) | 87 (61.3) | 38 (56.7) | 8 (47.1) | 202 (59.2) | ||
| OP | 0 | 22 (20.2) | 20 (14.1) | 9.0 (13.4) | 1.0 (5.9) | 52 (15.2) | ||
| Total | 6 (100.0) | 109 (100.0) | 142 (100.0) | 67 (100.0) | 17 (100.0) | 341 (100.0) | ||
| Age, years | ||||||||
| 60–69 years, n, % | of Norms | 0 | 7.0 (10.0) | 18 (11.6) | 17 (16.5) | 17 (25.4) | 59 (14.8) | 0.06 |
| Osteopenia | 1.0 (33.3) | 39 (55.7) | 96 (61.9) | 56 (54.4) | 39 (58.2) | 231 (58.0) | ||
| OP | 2.0 (66.7) | 24 (34.3) | 41 (26.5) | 30 (29.1) | 11 (16.4) | 108 (27.1) | ||
| Total | 3 (100.0) | 70 (100.0) | 155 (100.0) | 103 (100.0) | 67 (100.0) | 398 (100.0) | ||
| 70–79 years, n, % | of norms | 0 | 3 (7.9) | 8 (9.5) | 4 (10.8) | 7.0 (24.1) | 22 (11.6) | 0.033 |
| Osteopenia | 0 | 14 (36.8) | 34 (40.5) | 23 (62.2) | 14 (48.3) | 85 (44.7) | ||
| OP | 2 (100.0) | 21 (55.3) | 42 (50.0) | 10 (27.0) | 8.0 (27.6) | 83 (43.7) | ||
| Total | 2 (100.0) | 38 (100.0) | 84 (100.0) | 37 (100.0) | 29 (100.0) | 190 (100) | ||
| 80–89 years, n, % | Osteopenia | 0 | 1.0 (14.3) | 1,0 (14.3) | 4.0 (50.0) | 0 | 6.0 (26.1) | 0.291 |
| OP | 0 | 6.0 (85.7) | 6.0 (85.7) | 4.0 (50.0) | 1.0 (100.0) | 17 (73.9) | ||
| Total | 0 | 7.0 (100.0) | 7.0 (100.0) | 8.0 (100.0) | 1.0 (100.0) | 23 (100.0) | ||
| Respondents | BMI | |
|---|---|---|
| Norm | 29.91 ± 3.92 | RR2 = 0.9997 |
| Osteopenia | 28.57 ± 1.69 | |
| Osteoporosis | 27.76 ± 5.79 | |
| Respondents | BMI | t | p | |
|---|---|---|---|---|
| Men | Women | |||
| Norm | 28.25 ± 3.51 | 30.36 ± 2.17 | 0.51 | p > 0.05 |
| Osteopenia | 26.76 ± 2.02 | 28.94 ± 1.88 | 0.79 | |
| Osteoporosis | 26.02 ± 4.17 | 28.03 ± 2.08 | 0.43 | |
| BMI | Osteoporosis | Violation of Bone Strength Properties (OP+ Osteopenia) | ||||||
|---|---|---|---|---|---|---|---|---|
| “+” n (%) | “−” n (%) | χ2 (df) | p | “+” n (%) | “−” n (%) | χ2 (df) | p | |
| <18.5 | 8 (33.3) | 16 (66.7) | 14.9 (4) | <0.005 | 22 (91.7) | 2 (8.3) | 19.5 (4) | <0.001 |
| 18.5–24.9 | 113 (23) | 378 (77) | 412 (83.9) | 79 (16.1) | ||||
| 25–29.9 | 150 (20) | 600 (80) | 603 (80.4) | 147 (19.6) | ||||
| 30–34.9 | 76 (17) | 371 (83) | 340 (76.1) | 107 (23.9) | ||||
| 35+ | 33 (13.3) | 216 (86.7) | 180 (72.3) | 69 (27.7) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduldayeva, A.; Doszhanova, G.; Iskakova, S.; Bukeyeva, Z.; Tarjibayeva, S.; Tolegenova, Y.; Kazbekova, A.; Kozhamkulov, O.; Baimagambetova, A.; Dosmyrzayeva, G. Osteoporosis in the Elderly: A Cross-Sectional Study in Kazakhstan. Int. J. Environ. Res. Public Health 2025, 22, 1694. https://doi.org/10.3390/ijerph22111694
Abduldayeva A, Doszhanova G, Iskakova S, Bukeyeva Z, Tarjibayeva S, Tolegenova Y, Kazbekova A, Kozhamkulov O, Baimagambetova A, Dosmyrzayeva G. Osteoporosis in the Elderly: A Cross-Sectional Study in Kazakhstan. International Journal of Environmental Research and Public Health. 2025; 22(11):1694. https://doi.org/10.3390/ijerph22111694
Chicago/Turabian StyleAbduldayeva, Aigul, Gulnur Doszhanova, Saule Iskakova, Zhanar Bukeyeva, Saule Tarjibayeva, Yerkezhan Tolegenova, Ainagul Kazbekova, Olzhas Kozhamkulov, Aigerm Baimagambetova, and Gulnaz Dosmyrzayeva. 2025. "Osteoporosis in the Elderly: A Cross-Sectional Study in Kazakhstan" International Journal of Environmental Research and Public Health 22, no. 11: 1694. https://doi.org/10.3390/ijerph22111694
APA StyleAbduldayeva, A., Doszhanova, G., Iskakova, S., Bukeyeva, Z., Tarjibayeva, S., Tolegenova, Y., Kazbekova, A., Kozhamkulov, O., Baimagambetova, A., & Dosmyrzayeva, G. (2025). Osteoporosis in the Elderly: A Cross-Sectional Study in Kazakhstan. International Journal of Environmental Research and Public Health, 22(11), 1694. https://doi.org/10.3390/ijerph22111694






