Speciation of Arsenic in Medium Containing Bacterial Strains of Lysinibacillus boronitolerans and Bacillus cereus: Mechanism of Arsenic Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling, and Bacterial Isolation
2.2. Determination of Bacterial Resistance and Molecular Identification
2.3. Arsenic Accumulation and Metabolic Transformation Assays
2.4. Microscopic Analyses (AFM and SEM–EDS)
2.5. Exopolysaccharide (EPS) Production
2.6. Chemical Speciation of as in the Presence of Bacteria Using LC-ICP-MS Coupling
3. Results and Discussion
3.1. Evaluation of Exopolysaccharide (EPS) Production by Isolated Bactéria
3.2. Arsenic Oxidation by Bacterial Isolates
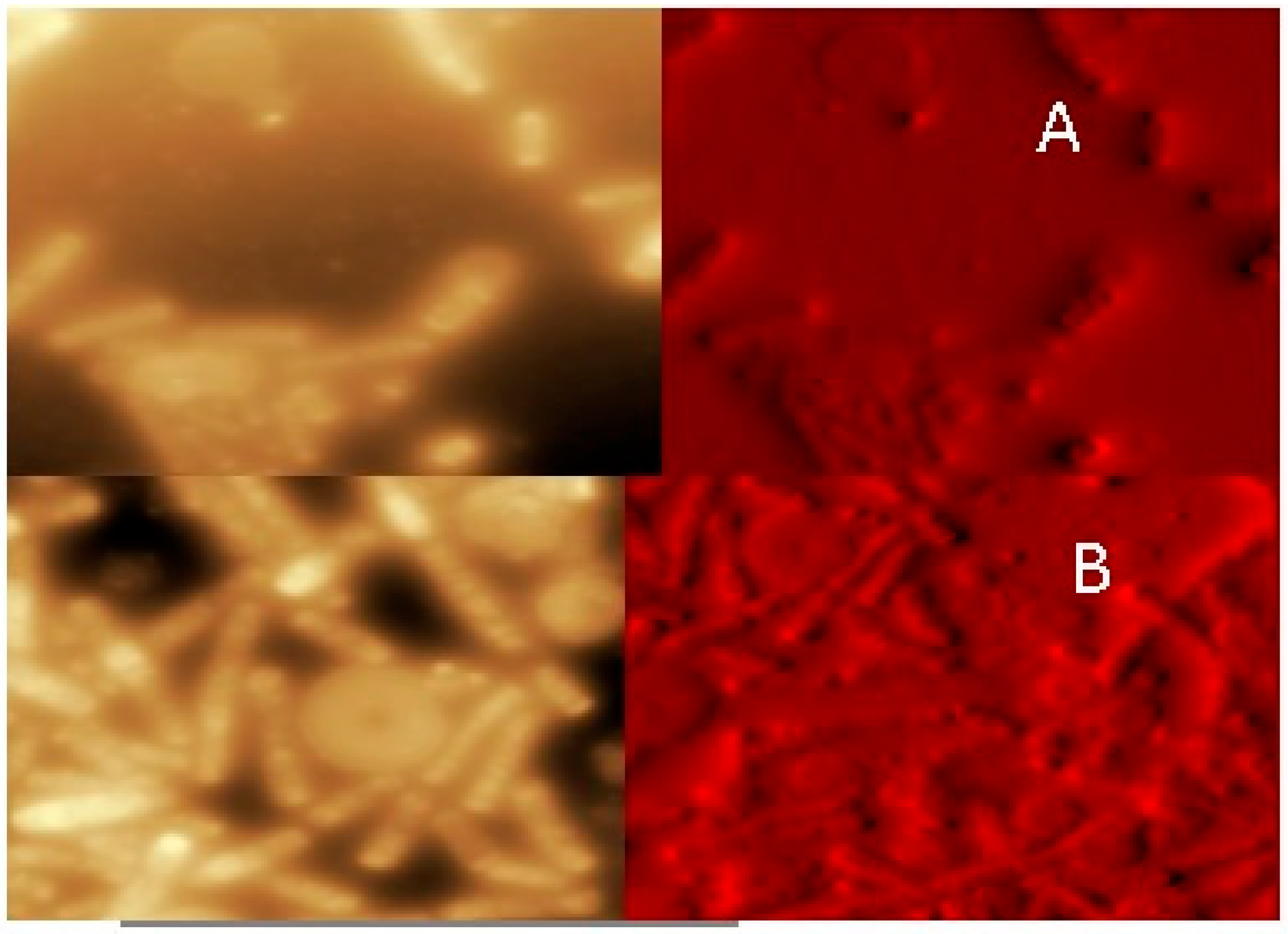
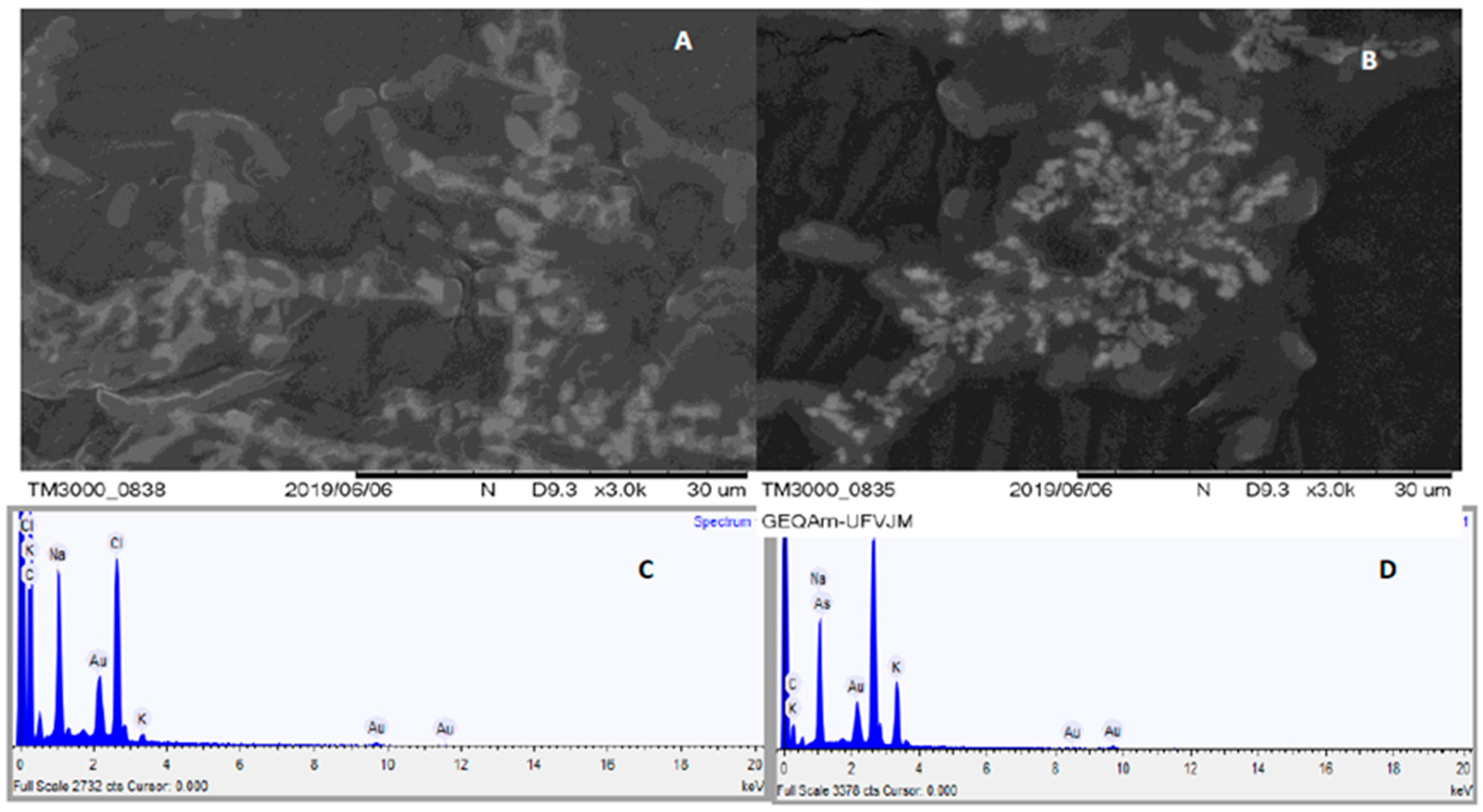
3.3. Images Obtained by AFM and Percentage of As in Bacteria by SEM–EDS
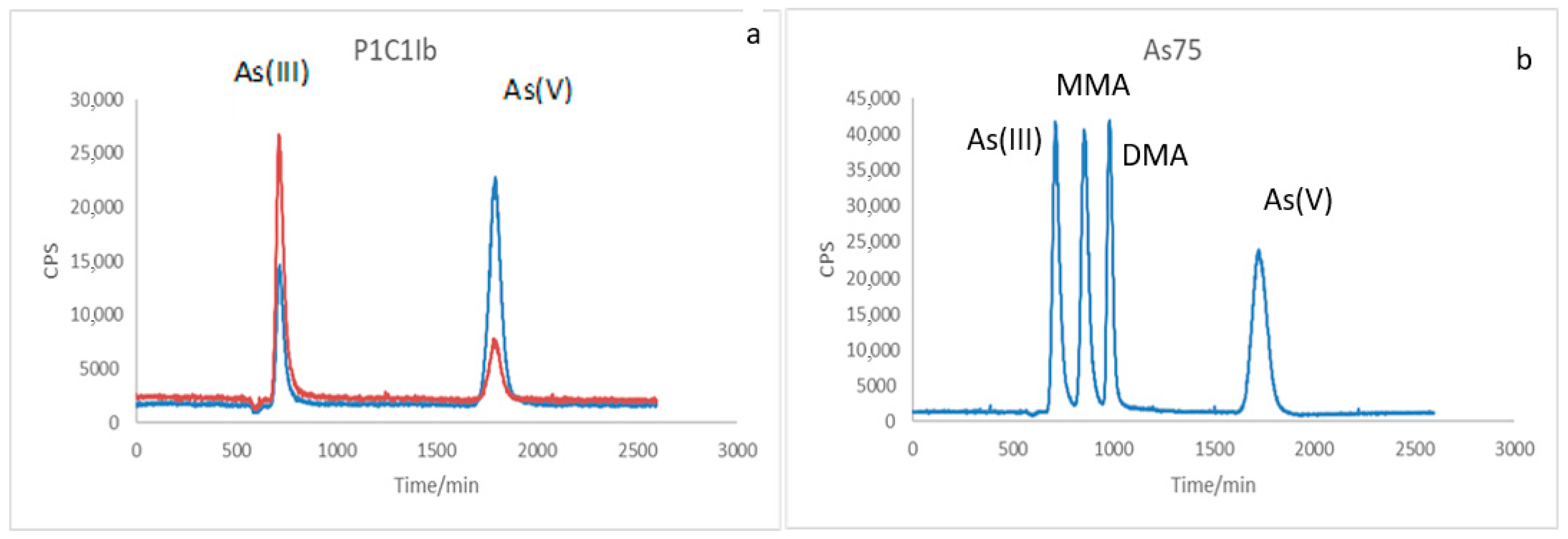
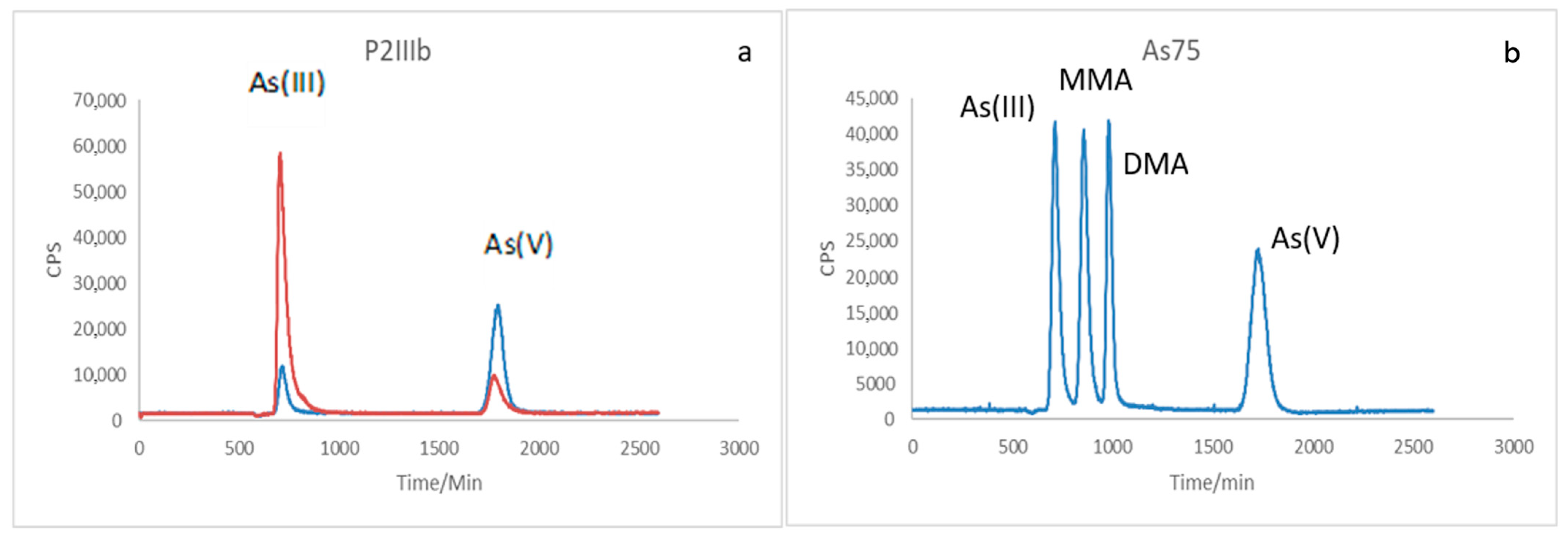
3.4. Chemical Speciation of as in the Medium Containing the Studied Bacteria Using LC-ICP-MS Coupling
3.5. Detoxification Mechanism Through Arsenic Volatilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Priority List of Hazardous Substances; ATSDR: Atlanta, Georgia, 2011. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 25 February 2017).
- Assis, I.R. Adsorption and Arsenic Availability in Soils with Different Mineralogical Compositions. Ph.D. Thesis, Federal University of Viçosa, Viçosa, Brasil, 2010. [Google Scholar]
- Salehizadeh, H.; Shojaosadati, A.S. A polysaccharide produced from Bacillus firmus removes metal ions from an aqueous solution. Biotechnol. Lett. 2003, 37, 4231–4235. [Google Scholar]
- Bidone, E.D.; Castilhos, Z.C.; Cesar, R.G.; Santos, M.C.B.; Ferreira, M.M.; Silva, R.S.V. Hydrogeochemistry of arsenic pollution in watersheds influenced by gold mining activities in Paracatu (Minas Gerais State, Brazil). Environ. Sci. Pollut. Res. 2016, 23, 8546–8555. [Google Scholar] [CrossRef]
- Budinhoff, C.R.; Hollibaugh, J.T. Arsenite-dependent photoautotrophy by an Ectothiorhodospira-dominated consortium. ISME J. 2008, 2, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Bowell, R.J.; Alpers, C.N.; Jamieson, H.E.; Nordstrom, D.K.; Majzlan, J. Environmental geochemistry of arsenic: An overview. Rev. Mineral. Geochem. 2014, 79, 1–16. [Google Scholar] [CrossRef]
- Kalantzi, I.; Mylona, K.; Sofoulaki, K.; Tsapakis, M.; Pergantis, S.A. Arsenic speciation in fish from Greek coastal areas. J. Environ. Sci. 2017, 56, 300–312. [Google Scholar] [CrossRef]
- Lima, C.A.; Castilhos, Z.C. Human health risk assessment by environmental exposure in Paracatu. In One Century of the Discovery of Arsenicosis in Latin America (1914–2014); Litter, M.I., Nicolli, H.B., Meichtry, M., Eds.; Taylor & Francis Group: London, UK, 2014; pp. 652–653. [Google Scholar]
- Craw, D.; Bowell, R.J. The characterization of mine waste. Rev. Mineral. Geochem. 2014, 79, 473–506. [Google Scholar] [CrossRef]
- El-Sheekh, L.M.; El-Shouny, W.A.; Osman, M.F.H.; El-Gammal, W.E. Growth and heavy metals removal affinity of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluent. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- Franco, M.W.; Ferreira, F.A.G.; Vasconcelos, I.F.; Batista, L.B.; Pujoni, G.F.; Magalhães, S.M.S.; Barbosa, F.; Barbosa, A.R. Arsenic biotransformation by cyanobacteria from mining areas: Evidence from culture experiments. Environ. Sci. Pollut. Res. 2015, 22, 500–505. [Google Scholar] [CrossRef]
- Faria, M.C.d.S.; Hott, R.C.; Santos, M.J.; Santos, M.S.; Andrade, T.G.; Bomfeti, C.A.; Rocha, B.A.; Barbosa, F., Jr.; Rodrigues, J.L. Arsenic in mining areas: Environmental contamination routes. Int. J. Environ. Res. Public Health 2023, 20, 4291. [Google Scholar] [CrossRef]
- Da Silva, H.T.; Magalhães, T.S.; Pires, S.A.; Santos, A.P.R.; Rodrigues, J.L.; Faria, M.C.d.S. Artisanal gem mining in Brazil: Evaluation of oxidative stress and genotoxicity biomarkers. Int. J. Environ. Res. Public Health 2024, 21, 871. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.R.; Silva, L.Z.; Freire, B.M.; Faria, M.C.d.S.; Batista, B.L.; Rocha, B.A.; Barbosa, F., Jr.; Rodrigues, J.L. Artisanal gem mining in Brazil: A source of genotoxicity and exposure to toxic elements. Int. J. Environ. Res. Public Health 2023, 20, 2510. [Google Scholar] [CrossRef]
- Conselho Nacional do Meio Ambiente (CONAMA). Resolução nº 430, de 13 de maio de 2011: Dispõe Sobre Condições e Padrões de Lançamento de Efluentes; Ministério do Meio Ambiente: Brasília, Brazil, 2011.
- Grabber, J.H.; Ralph, J.; Hatfield, R.D. Ferulate cross-link limits the enzymatic degradation of synthetically lignified primary walls of maize. J. Agric. Food Chem. 1998, 46, 2609–2614. [Google Scholar] [CrossRef]
- Melak, D.; Ferreccio, C.; Kalman, D.; Parra, R.; Acevedo, J.; Perez, L.; Steinmaus, C. Arsenic methylation and bladder and lung cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol. 2014, 274, 225–231. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). National primary drinking water regulations: Arsenic, compliance clarifications, and new source contaminant monitoring; final rule. Fed. Regist. 2013, 66, 6975–7066. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- World Health Organization. Exposure to Arsenic: A Significant Public Health Concern; WHO: Geneva, Switzerland, 2010; Available online: https://iris.who.int/server/api/core/bitstreams/ac5cc702-7983-4b6a-8094-869f4fe95593/content (accessed on 20 November 2016).
- Santos, M.S.; Mourão, A.O.; Santos, T.S.X.; Rodriguez, M.V.R.; Faria, M.C.d.S.; Franco, E.S.; Aguilar, N.A.; Rodrigues, J.L. Remove emerging contaminants (endocrine disruptors) using a photocatalyst and detection by high-performance liquid chromatography. Int. J. Environ. Res. Public Health 2025, 22, 334. [Google Scholar] [CrossRef]
- Cullen, W.R. Chemical mechanism of arsenic biomethylation. Chem. Res. Toxicol. 2014, 27, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.; Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Fréchet, J.M. Molecular-weight-dependent mobilities in regioregular poly(3-hexyl-thiophene) diodes. Appl. Phys. Lett. 2005, 86, 122110. [Google Scholar] [CrossRef]
- Hunt, K.M.; Srivastava, R.K.; Elmets, C.A.; Athar, M. The mechanistic basis of arsenicosis: Pathogenesis of skin cancer. Cancer Lett. 2014, 354, 211–219. [Google Scholar] [CrossRef]
- Mateos, L.M.; Ordóñez, E.; Letek, M.; Gil, J.A. Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int. Microbiol. 2006, 9, 207–215. [Google Scholar]
- Valls, M.; De Lorenzo, V. Exploring the genetic and biochemical capabilities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef]
- Yaminsky, I.V.; Akhmetova, A.I. The role of scanning microscopy in bacterial and bioremediation investigations. In Reduction of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–312. [Google Scholar]
- Sharma, A.K.; Tjell, J.C.; Sloth, J.J.; Holm, P.E. Review of arsenic contamination, exposure through water and food, and low-cost mitigation options for rural areas. Appl. Geochem. 2014, 41, 11–33. [Google Scholar] [CrossRef]
- Singh, S.; Fett, W. Stimulation of exopolysaccharide production by fluorescent pseudomonads in sucrose media due to dehydration and increased osmolarity. FEMS Microbiol. Lett. 1995, 130, 301–306. [Google Scholar] [CrossRef]
- Sanz, E.; Muñoz-Olivas, R.; Cámara, C. Evaluation of a focused sonication probe for arsenic speciation in environmental and biological samples. J. Chromatogr. A 2005, 1097, 1–8. [Google Scholar] [CrossRef]
- Jain, S.; Saluja, B.; Gupta, A.; Marla, S.S.; Goel, R. Validation of arsenic resistance in Bacillus cereus strain AG27 by comparative protein modeling of arsC gene product. Protein J. 2011, 30, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.C.; Batelochi, M.; Akiti, Y.; Maxwell, S.; Borges, A.L. Geology and characterization of Morro do Ouro mineral resources, Paracatu, Minas Gerais. In San Francisco Basin: Geology and Resources; Pinto, C.P., Martins-Neto, M.A., Eds.; CPRM: Belo Horizonte, Brazil, 2001. [Google Scholar]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Mende, D.R. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Butcher, B.G.; Rawlings, D.E. The divergent chromosomal ars operon of Acidithiobacillus ferrooxidans is regulated by an atypical ArsR protein. Microbiology 2002, 148, 3983–3992. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.M.; Sly, L.I.; Schnagl, R.D.; Macy, J.M. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: Phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 2000, 66, 92–97. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kawahata, H.; Gupta, L.P.; Kita, N.; Morishita, Y.; Ono, Y.; Komai, T. Arsenic resistance and removal by marine and non-marine bacteria. J. Biotechnol. 2007, 127, 434–442. [Google Scholar] [CrossRef]
- Kulp, T.R.; Hoeft, S.E.; Asao, M.; Madigan, M.T.; Hollibaugh, J.T.; Fisher, J.C.; Oremland, R.S. Arsenic(III) promotes anoxygenic photosynthesis in biofilms from Mono Lake, California hot springs. Science 2008, 321, 967–970. [Google Scholar] [CrossRef]
- Lin, D.Y.; Huang, Y.S.; Jeng, J.C.; Kuo, H.Y.; Chang, C.C.; Chao, T.T.; Hung, C.C. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell 2006, 24, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Rathod, J.; Jean, J.-S.; Jiang, W.-T.; Huang, I.-H.; Liu, B.H.; Lee, Y.-C. Micro-colonization of arsenic-resistant Staphylococcus sp. As-3 on arsenopyrite (FeAsS) drives arsenic mobilization under anoxic subsurface mimicking conditions. Sci. Total Environ. 2019, 669, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Catelani, T.; Expert, B.; Bellucci, F.; Lee, S.S.; Fenter, P.; Newville, M.; Costagliola, P. Arsenic uptake in bacterial calcite. Geochim. Cosmochim. Acta 2018, 222, 642–654. [Google Scholar] [CrossRef]
| LC Conditions | |
| Column | Hamilton PRP-X100 (5 μm, 150 mm, 4.6 mm) |
| Mobile phase | 10 mM HPO−24/H2PO 4, pH 8.5, 2% (v/v) MeOH |
| Mobile phase flow rate | 1 mL min−1 |
| Oven temperature (column) | 25 °C |
| Equilibrium | 1 min |
| Run | 9.0 min |
| Wash | 1 min |
| Mode | Isocratic |
| Injection volume | 100 μL |
| Measurement | Peak area |
| ICP-MS experimental conditions | |
| Radiofrequency power | 1200 W |
| Scan mode | Peak hopping |
| Gas flow rates | Plasma 15 L min−1; auxiliary 1.2 L min−1 |
| Nebulizer gas flow | 0.86–0.98 L min−1 |
| Internal standards (1) | Rh103 (10.0 mg/L) for total As determination |
| Internal standards (2) | Ga69 (0.5 mgL−1 for As speciation [ICP-MS’s peristaltic pump is set at 10 rpm (0.5 mL min−1)] for As speciation |
| Interface | Platinum cones |
| Sampler | 1.1 mm |
| Skimmer | 0.9 mm |
| Resolution | 0.7 amu |
| Isotope75As | |
| Bacterial Strain | As III | As V | As Volatilized |
|---|---|---|---|
| P1C1Ib | 27% | 59% | 14% |
| P1C1Ib | 76% | 9% | 15% |
| P2IIIb | 70% | 16% | 14% |
| P2IIIb | 24% | 61% | 15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, N.C.; Van Der Maas, A.S.; Santos, M.S.; Hott, R.d.C.; Faria, M.C.d.S.; Batista, B.L.; Bomfeti, C.A.; Mesquita, J.P.d.; Rodrigues, J.L. Speciation of Arsenic in Medium Containing Bacterial Strains of Lysinibacillus boronitolerans and Bacillus cereus: Mechanism of Arsenic Removal. Int. J. Environ. Res. Public Health 2025, 22, 1675. https://doi.org/10.3390/ijerph22111675
Aguilar NC, Van Der Maas AS, Santos MS, Hott RdC, Faria MCdS, Batista BL, Bomfeti CA, Mesquita JPd, Rodrigues JL. Speciation of Arsenic in Medium Containing Bacterial Strains of Lysinibacillus boronitolerans and Bacillus cereus: Mechanism of Arsenic Removal. International Journal of Environmental Research and Public Health. 2025; 22(11):1675. https://doi.org/10.3390/ijerph22111675
Chicago/Turabian StyleAguilar, Naidilene Chaves, Adriele Santos Van Der Maas, Mayra Soares Santos, Rodrigo de Carvalho Hott, Márcia Cristina da Silva Faria, Bruno Lemos Batista, Cleide Aparecida Bomfeti, João Paulo de Mesquita, and Jairo Lisboa Rodrigues. 2025. "Speciation of Arsenic in Medium Containing Bacterial Strains of Lysinibacillus boronitolerans and Bacillus cereus: Mechanism of Arsenic Removal" International Journal of Environmental Research and Public Health 22, no. 11: 1675. https://doi.org/10.3390/ijerph22111675
APA StyleAguilar, N. C., Van Der Maas, A. S., Santos, M. S., Hott, R. d. C., Faria, M. C. d. S., Batista, B. L., Bomfeti, C. A., Mesquita, J. P. d., & Rodrigues, J. L. (2025). Speciation of Arsenic in Medium Containing Bacterial Strains of Lysinibacillus boronitolerans and Bacillus cereus: Mechanism of Arsenic Removal. International Journal of Environmental Research and Public Health, 22(11), 1675. https://doi.org/10.3390/ijerph22111675








