Instability in the Penta-C and Penta-D Loci in Microsatellite-Unstable Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Sample Collection, and DNA Isolation
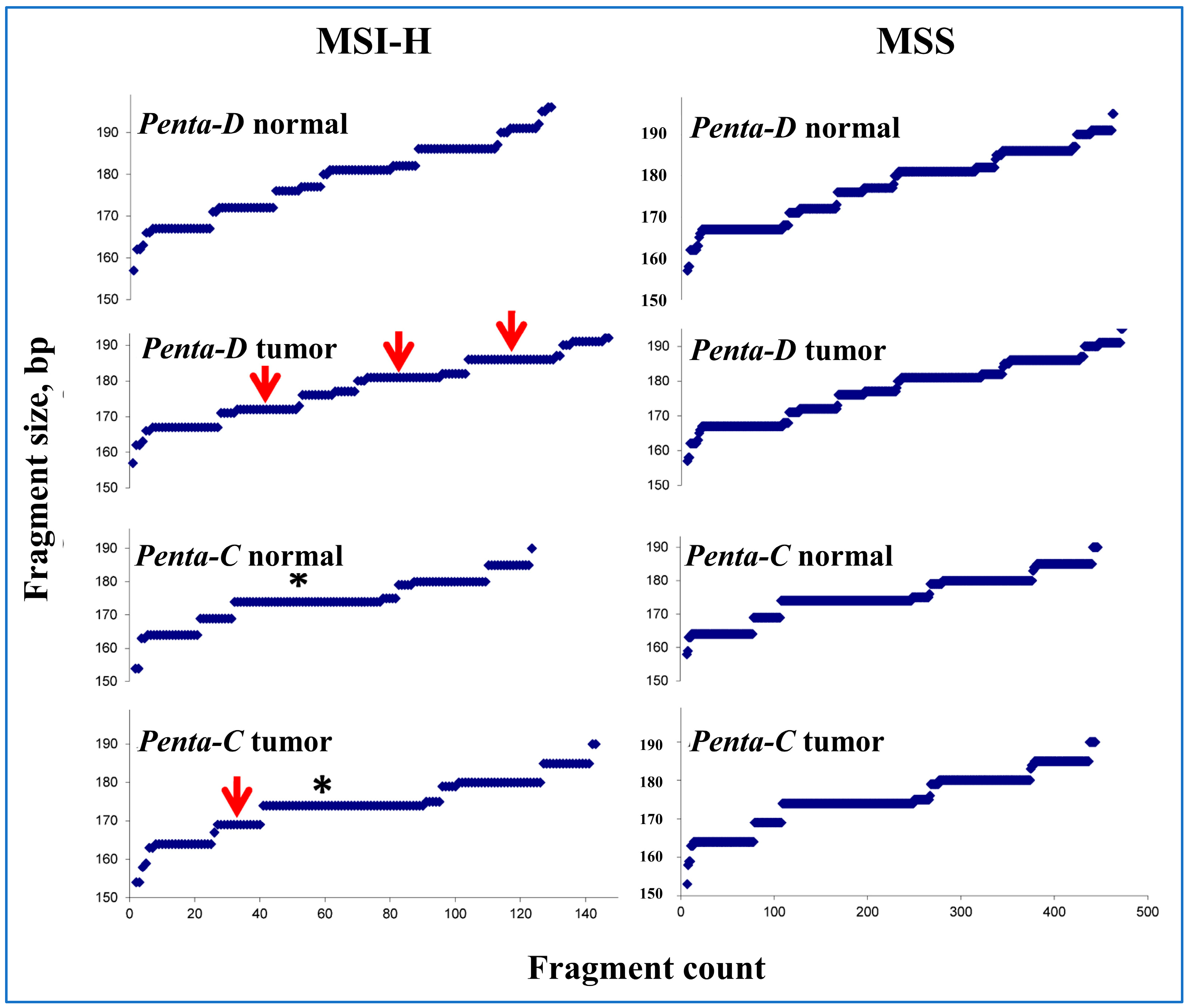
2.2. Microsatellite Instability Assay
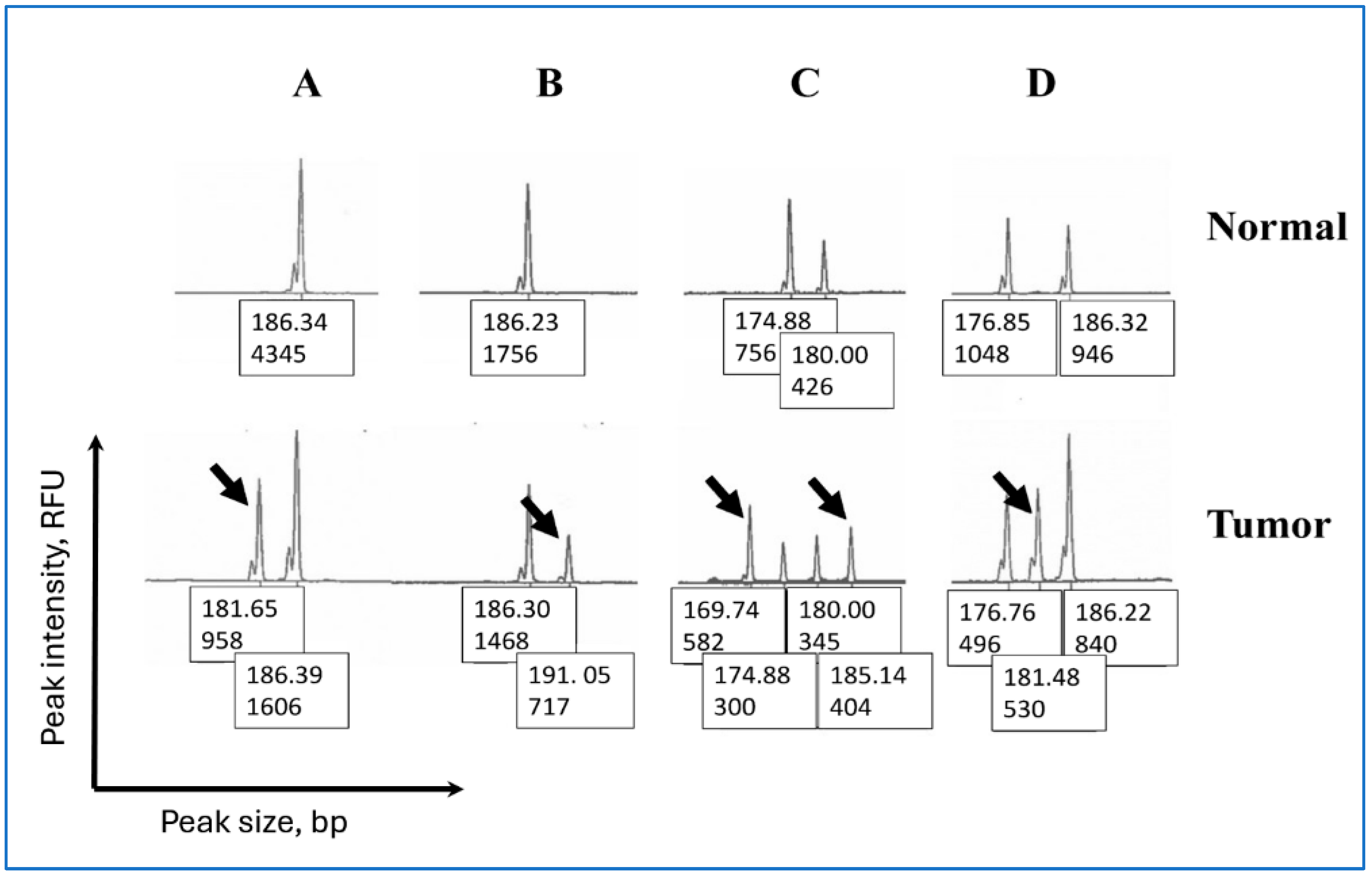
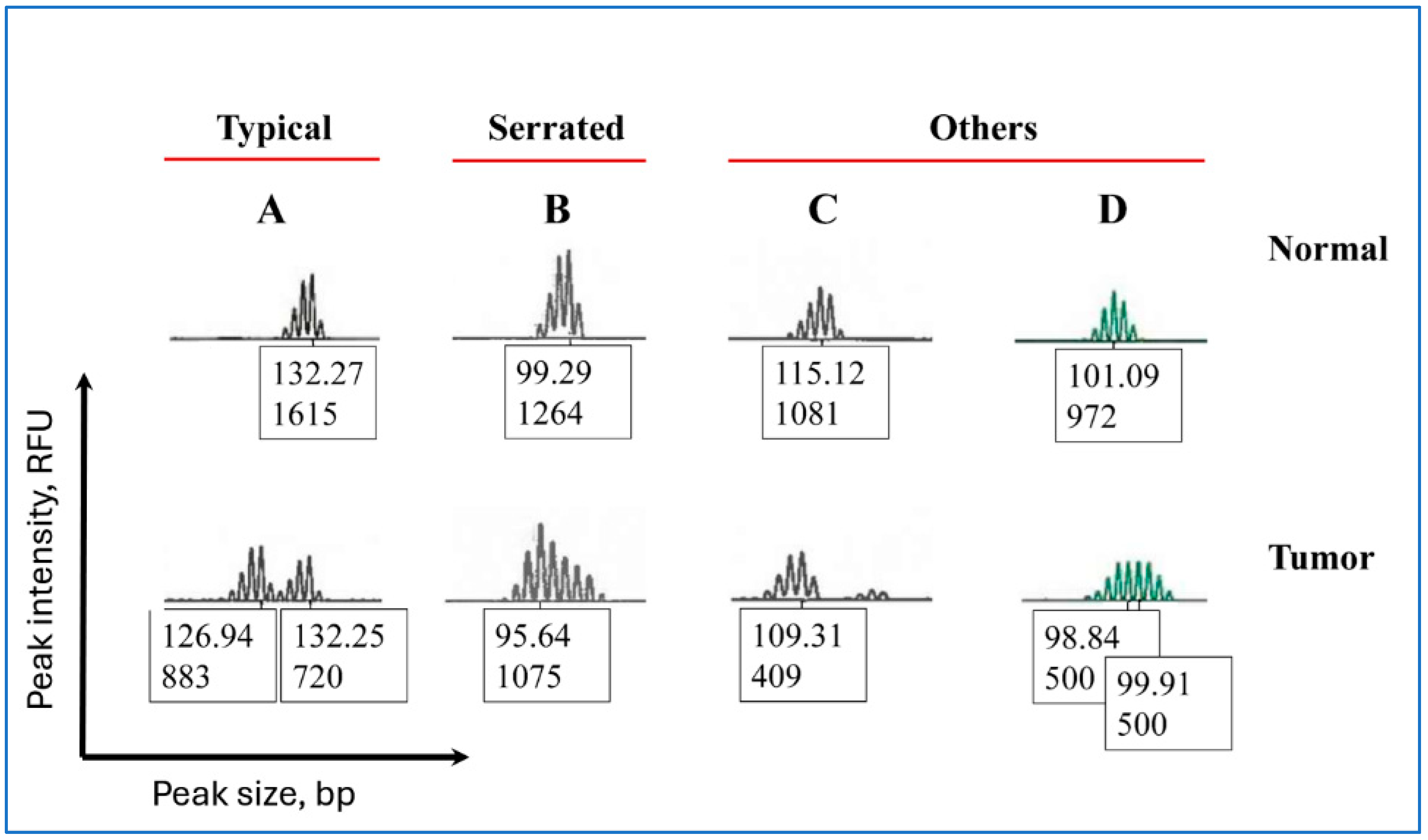
2.3. Classification of Peak Patterns in Mono- and Pentanucleotide Repeat Markers
2.4. MLH1 Hypermethylation Assay
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics of Patients
3.2. Both Penta-C and Penta-D Did Not Match in 8.2% of the MSI-H and 0.4% of the MSS Samples
3.3. Peak Patterns in Unstable Penta-C and Penta-D Are Similar
3.4. Penta-C Is More Stable than Penta-D
3.5. Clinical Characteristics in Patients with and Without Matching Penta-C and Penta-D
3.6. Peak Patterns in Unstable BAT-25 Are Correlated with IHC Staining and MLH1 Hypermethylation
3.7. Sample Mix-Ups Due to Matching in Penta-C and Penta-D Size by Random Chance Are Negligible
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellegren, H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004, 5, 435–445. [Google Scholar] [CrossRef] [PubMed]
- De’ Angelis, G.L.; Bottarelli, L.; Azzoni, C.; Angelis, N.D.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [PubMed]
- Shah, S.N.; Hile, S.E.; Eckert, K.A. Defective mismatch repair, microsatellite mutation bias, and variability in clinical cancer phenotypes. Cancer Res. 2010, 70, 431–435. [Google Scholar] [CrossRef]
- Javadian, P.; Nezhat, F. Endometrial Carcinoma and its Precursors. Adv. Exp. Med. Biol. 2020, 1242, 59–72. [Google Scholar]
- Obel, J.C.; Friberg, G.; Fleming, G.F. Chemotherapy in endometrial cancer. Clin. Adv. Hematol. Oncol. 2006, 4, 459–468. [Google Scholar]
- Muller, C.; Matthews, L.; Kupfer, S.S.; Weiss, J.M. Effective Identification of Lynch Syndrome in Gastroenterology Practice. Curr. Treat. Options Gastroenterol. 2019, 17, 666–680. [Google Scholar] [CrossRef]
- Hussein, Y.R.; Soslow, R.A. Molecular insights into the classification of high-grade endometrial carcinoma. Pathology 2018, 50, 151–161. [Google Scholar] [CrossRef]
- Iijima, M.; Banno, K.; Okawa, R.; Yanokura, M.; Iida, M.; Takeda, T.; Kunitomi-Irie, H.; Adachi, M.; Nakamura, K.; Umene, K.; et al. Genome-wide analysis of gynecologic cancer: The Cancer Genome Atlas in ovarian and endometrial cancer. Oncol. Lett. 2017, 13, 1063–1070. [Google Scholar] [CrossRef]
- Ogunmuyiwa, J.; Williams, V. Emerging Advances in Endometrial Cancer: Integration of Molecular Classification into Staging for Enhanced Prognostic Accuracy and Implications for Racial Disparities. Cancers 2024, 16, 1172. [Google Scholar] [CrossRef]
- Teng, Q.; Yuan, Z.; Mu, Y.; Ma, X.; Wang, S.; Sun, C.; Chin, L.; Huang, Z.; Zhu, C.; Yin, A.; et al. Molecular subtyping of endometrial cancer via a simplified one-step NGS classifier, ARID1A and ZFHX4 mutations help further subclassify CNL/MSI-H patients. Diagn. Pathol. 2025, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Guo, Q.; Xu, J.; Wu, X.; Cai, C.; Jiao, Y.; Ming, W.; Wen, H.; Wang, X. Prediction of molecular subtypes for endometrial cancer based on hierarchical foundation model. Bioinformatics 2025, 41, btaf059. [Google Scholar] [CrossRef]
- Casak, S.J.; Marcus, L.; Fashoyin-Aje, L.; Mushti, S.L.; Cheng, J.; Shen, Y.-L.; Pierce, W.F.; Her, L.; Goldberg, K.B.; Theoret, M.R.; et al. FDA Approval Summary: Pembrolizumab for the First-line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin. Cancer Res. 2021, 27, 4680–4684. [Google Scholar] [CrossRef]
- Green, A.K.; Feinberg, J.; Makker, V. A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Levy, J.; Rouyer, M.; Demange, J.; Husson, M.; Bonnet, C.; Salleron, J.; Leroux, A.; Merlin, J.-L.; Harlé, A. Evaluation of 3 molecular-based assays for microsatellite instability detection in formalin-fixed tissues of patients with endometrial and colorectal cancers. Sci. Rep. 2020, 10, 16386. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M. Genetics and genomics of core short tandem repeat loci used in human identity testing. J. Forensic Sci. 2006, 51, 253–265. [Google Scholar] [CrossRef]
- Bacher, J.W.; Flanagan, L.A.; Smalley, R.L.; Nassif, N.A.; Burgart, L.J.; Halberg, R.B.; Megid, W.M.; Thibodeau, S.N. Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis. Markers 2004, 20, 237–250. [Google Scholar] [CrossRef]
- Murphy, K.M.; Zhang, S.; Geiger, T.; Hafez, M.J.; Bacher, J.; Berg, K.D.; Eshleman, J.R. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J. Mol. Diagn. 2006, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Pearlman, R.; de la Chapelle, A.; Pritchard, C.C.; Zhao, W.; Jones, D.; Yilmaz, A.; Chen, W.; Frankel, W.L.; Suarez, A.A.; et al. Double somatic mismatch repair gene pathogenic variants as common as Lynch syndrome among endometrial cancer patients. Gynecol. Oncol. 2021, 160, 161–168. [Google Scholar] [CrossRef]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Bacher, J.W.; Clipson, L.; Steffen, L.S.; Halberg, R.B. Microsatellite Instability and Its Significance to Hereditary and Sporadic Cancer; IntechOpen: London, UK, 2016; pp. 187–227. [Google Scholar]
- Aska, E.M.; Zagidullin, B.; Pitkänen, E.; Kauppi, L. Single-Cell Mononucleotide Microsatellite Analysis Reveals Differential Insertion-Deletion Dynamics in Mouse T Cells. Front. Genet. 2022, 13, 913163. [Google Scholar] [CrossRef]
- Viguera, E.; Canceill, D.; Ehrlich, S. Replication slippage involves DNA polymerase pausing and dissociation. EMBO J. 2001, 20, 2587–2595. [Google Scholar] [CrossRef]
- Hunt, J.L. Identifying cross contaminants and specimen mix-ups in surgical pathology. Adv. Anat. Pathol. 2008, 15, 211–217. [Google Scholar] [CrossRef]
- Weyers, W. Confusion-specimen mix-up in dermatopathology and measures to prevent and detect it. Dermatol. Pract. Concept. 2014, 4, 27–42. [Google Scholar] [CrossRef]
- Gast, A.; Shachar, D.; Kotik, A.; Kugel, C.; Bublil, N. Identification of comingled tissue within a formalin-fixed paraffin-embedded sample using forensic genetics—A tool for prevention of misdiagnosis. Harefuah 2019, 158, 778–782. [Google Scholar] [PubMed]
- Baloğlu, H.; Yığıt, N. A new remedy in pathology practice: Molecular solution to sample mix-up. Turk Patoloji Derg. 2011, 27, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, P.J.; Buttin, B.M.; Herzog, T.J.; Rader, J.S.; Gibb, R.K.; Swisher, E.; Look, K.; Walls, K.C.; Fan, M.-Y.; Mutch, D.G. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc. Natl. Acad. Sci. USA 2003, 100, 5908–5913. [Google Scholar] [CrossRef]
- Buttin, B.M.; Powell, M.A.; Mutch, D.G.; Rader, J.S.; Herzog, T.J.; Gibb, R.K.; Huettner, P.; Edmonston, T.B.; Goodfellow, P.J. Increased risk for hereditary nonpolyposis colorectal cancer-associated synchronous and metachronous malignancies in patients with microsatellite instability-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin. Cancer Res. 2004, 10, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.; Baert, T.; Schmutzler, R.; du Bois, A.; Schneider, S.; Rhiem, K.; Schömig-Markiefka, B.; Siemanowski, J.; Heikaus, S.; Traut, A.; et al. Modern day screening for Lynch syndrome in endometrial cancer: The KEM experience. Arch. Gynecol. Obstet. 2021, 304, 975–984. [Google Scholar] [CrossRef]
- Peterson, L.M.; Kipp, B.R.; Halling, K.C.; Kerr, S.E.; Smith, D.I.; Distad, T.J.; Clayton, A.C.; Medeiros, F. Molecular characterization of endometrial cancer: A correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. Int. J. Gynecol. Pathol. 2012, 31, 195–205. [Google Scholar] [CrossRef]
| Parameter | N | % | |
|---|---|---|---|
| Age younger than 50 years | 43 | 13.3 | |
| MSI status | |||
| MSI-H | 73 | 22.5 | |
| MSI-low | 12 | 3.7 | |
| MSS | 239 | 73.8 | |
| MLH1 promoter hypermethylation | |||
| Present | 67 | 20.7 | |
| Absent | 17 | 5.2 | |
| Immunohistochemistry | |||
| Absent MLH1/PMS2 | 71 | 21.9 | |
| Absent MSH2/MSH6 | 8 | 2.5 | |
| Absent PMS2 only | 2 | 0.6 | |
| Absent MSH6 only | 7 | 2.2 | |
| IHC intact | 233 | 71.9 | |
| Others | 3 | 0.9 | |
| Race | |||
| White | 313 | 96.6 | |
| Black | 7 | 2.2 | |
| Asian | 3 | 0.9 | |
| Unknown | 1 | 0.3 | |
| Penta-C | Penta-D | Both Penta-C and Penta-D | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Match | Does Not Match | Match | Does Not Match | Match | Do Not Match | Only Penta-C Does Not Match | Only Penta-D Does Not Match | |||||||||||
| N | TC | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| MSI-H | 73 | 79.7 | 61 | 83.6 | 12 | 16.4 | 54 | 74 | 19 | 26 | 48 | 65.8 | 6 | 8.2 | 6 | 8.2 | 13 | 17.8 |
| MSI-low | 12 | 73.5 | 8 | 66.7 | 4 | 33.3 | 9 | 75 | 3 | 25 | 5 | 41.7 | 0 | 0.0 | 4 | 33.3 | 3 | 25.0 |
| MSS | 239 | 76.7 | 234 | 97.9 | 5 | 2.1 | 233 | 97.5 | 6 | 2.5 | 229 | 95.8 | 1 | 0.4 | 4 | 1.7 | 5 | 2.1 |
| Penta-C | Penta-D | ||||||
|---|---|---|---|---|---|---|---|
| Peak Patterns | TC | N | % | TC | N | % | |
| Normal tissue has a single peak | |||||||
| 5 bp deletion | 68.3 | 3 | 14.3 | 85 | 3 | 10.7 | |
| 5 bp duplication | 90 | 1 | 4.8 | 80 | 2 | 7.1 | |
| 10 bp deletion | Not available | 1 | 4.8 | 80 | 1 | 3.6 | |
| Indeterminate | Not available | 0 | 0.0 | 70 | 1 | 3.6 | |
| Normal tissue has two or more peaks | |||||||
| 5 bp deletion in the shorter fragment | 76 | 6 | 28.6 | 82 | 5 | 17.9 | |
| 5 bp duplication in the longer fragment | 81.7 | 3 | 14.3 | 90.8 | 4 | 14.3 | |
| 1 bp duplication in the shorter fragment | Not available | 0 | 0 | 90 | 1 | 3.6 | |
| 5 bp deletion in the longer fragment | 90 | 2 | 9.5 | 81 | 5 | 17.9 | |
| Indeterminate | 73.8 | 5 | 23.8 | 75 | 6 | 21.4 | |
| Total deletions | 74.6 | 12 | 57.1 | 82.1 | 14 | 50 | |
| Total duplications | 83.8 | 4 | 19.1 | 87.6 | 7 | 25 | |
| Total “Indeterminate” | 82.5 | 5 | 23.8 | 74.3 | 7 | 25 | |
| Total | 77.5 | 21 | 100 | 81.5 | 28 | 100 | |
| Penta-C | Penta-D | Both Penta-C and Penta-D | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matches | Does Not Match | Matches | Does Not Match | Match | Do Not Match | Only Penta-C Does Not Match | Only Penta-D Does Not Match | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| TC% | 77 | 78 | 77 | 82 | 77 | 82 | 75 | 81 | |||||||||
| UNS | 0.8 | 2.8 | 0.7 | 2.9 | 0.7 | 4.1 | 2.1 | 2.5 | |||||||||
| HYP | |||||||||||||||||
| Present | 55 | 82.1 | 12 | 17.9 | 49 | 73.1 | 18 | 26.9 | 41 | 61.2 | 4 | 6 | 8 | 11.9 | 14 | 20.9 | |
| Absent | 15 | 88.2 | 2 | 11.8 | 12 | 70.6 | 5 | 29.4 | 12 | 70.6 | 2 | 11.8 | 0 | 3 | 17.6 | ||
| AAD | |||||||||||||||||
| <50 yr | 41 | 95.3 | 2 | 4.7 | 41 | 95.3 | 2 | 4.7 | 40 | 93 | 1 | 2.3 | 1 | 2.3 | 1 | 2.3 | |
| ≥50 yr | 261 | 93.2 | 19 | 6.8 | 254 | 90.7 | 26 | 9.3 | 241 | 86.1 | 6 | 2.1 | 13 | 4.6 | 20 | 7.1 | |
| Absent IHC | |||||||||||||||||
| MLH1/PMS2 | 59 | 83.1 | 12 | 16.9 | 53 | 74.6 | 18 | 25.4 | 45 | 63.4 | 4 | 5.6 | 8 | 11.3 | 14 | 19.7 | |
| MSH2/MSH6 | 7 | 87.5 | 1 | 12.5 | 6 | 75 | 2 | 25 | 6 | 75 | 1 | 12.5 | 0 | 0 | 1 | 12.5 | |
| PMS2 only | 2 | 100 | 0 | 0 | 0 | 0 | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 100 | |
| MSH6 only | 6 | 85.7 | 1 | 14.3 | 7 | 100 | 0 | 0 | 6 | 85.7 | 0 | 0 | 1 | 14.3 | 0 | 0 | |
| Others | 2 | 100 | 0 | 0 | 2 | 100 | 0 | 0 | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IHC intact | 226 | 97 | 7 | 3 | 227 | 97.4 | 6 | 2.6 | 222 | 95.3 | 2 | 0.9 | 5 | 2.1 | 4 | 1.7 | |
| Age at Diagnosis | MLH1/PMS2 Staining | MSH2/MSH6 Staining | MLH1 Hypermethylation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <50 yr | >50 yr | Present | Absent | Present | Absent | Present | Absent | |||||||||||
| Pattern | TC | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| NR21 | ||||||||||||||||||
| Serrated | 81.9 | 3 | 21.4 | 11 | 78.6 | 2 | 12.5 | 14 | 87.5 | 15 | 88.2 | 2 | 11.8 | 11 | 78.6 | 3 | 21.4 | |
| Typical | 76.3 | 0 | 0 | 12 | 100 | 2 | 18.2 | 9 | 81.8 | 9 | 75 | 3 | 25 | 8 | 66.7 | 4 | 33.3 | |
| Other | 80.75 | 2 | 10 | 18 | 90 | 1 | 5 | 19 | 95 | 20 | 100 | 0 | 0 | 17 | 85 | 3 | 15 | |
| NR24 | ||||||||||||||||||
| Serrated | 79.9 | 2 | 8 | 23 | 92 | 4 | 16 | 21 | 84 | 22 | 91.7 | 2 | 8 | 17 | 68 | 8 | 32 | |
| Typical | 82.1 | 2 | 15.4 | 11 | 84.6 | 5 | 38.5 | 8 | 61.5 | 10 | 76.9 | 3 | 23.1 | 7 | 53.8 | 6 | 46.2 | |
| Other | 81.6 | 2 | 12.5 | 14 | 87.5 | 1 | 5.88 | 16 | 94.1 | 16 | 94.1 | 1 | 5.88 | 14 | 87.5 | 2 | 12.5 | |
| BAT25 | ||||||||||||||||||
| Serrated | 75.3 | 0 | 0 | 17 | 100 | 2 | 10 | 18 | 90 | 19 | 100 | 0 | 0 | 16 | 94.1 | 1 | 5.9 | |
| Typical | 82.3 | 2 | 18.2 | 9 | 81.8 | 4 | 44.4 | 5 | 55.6 | 6 | 60 | 4 | 40 | 3 | 27.3 | 8 | 72.7 | |
| Other | 84.04 | 3 | 11.5 | 23 | 88.5 | 5 | 17.2 | 24 | 82.8 | 25 | 89.3 | 3 | 10.7 | 21 | 80.8 | 5 | 19.2 | |
| BAT26 | ||||||||||||||||||
| Serrated | 70.6 | 2 | 14.3 | 12 | 85.7 | 4 | 23.5 | 13 | 76.5 | 15 | 100 | 0 | 0 | 11 | 78.6 | 3 | 21.4 | |
| Typical | 82.3 | 1 | 4.5 | 21 | 95.5 | 2 | 9.52 | 19 | 90.5 | 19 | 86.4 | 3 | 13.6 | 15 | 68.2 | 7 | 31.8 | |
| Other | 82.4 | 5 | 16.7 | 25 | 83.3 | 7 | 21.2 | 26 | 78.8 | 27 | 87.1 | 4 | 12.9 | 24 | 80 | 6 | 20 | |
| MONO27 | ||||||||||||||||||
| Serrated | 78.8 | 3 | 15 | 17 | 85 | 7 | 30.4 | 16 | 69.6 | 18 | 85.7 | 3 | 14.3 | 13 | 65 | 7 | 35 | |
| Typical | 71.3 | 1 | 5.6 | 17 | 94.4 | 2 | 11.8 | 15 | 88.2 | 15 | 83.3 | 3 | 16.7 | 13 | 72.2 | 5 | 27.8 | |
| Other | 84.8 | 1 | 9.1 | 10 | 90.9 | 0 | 0 | 10 | 100 | 11 | 100 | 0 | 0 | 7 | 63.6 | 4 | 36.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Frankel, W.L.; Zhao, W.; Suarez, A.A.; Chen, W.; Coleman, J.F.; McElroy, J.P.; Pearlman, R.; Goodfellow, P.J.; Hampel, H. Instability in the Penta-C and Penta-D Loci in Microsatellite-Unstable Endometrial Cancer. Int. J. Environ. Res. Public Health 2025, 22, 1674. https://doi.org/10.3390/ijerph22111674
Yilmaz A, Frankel WL, Zhao W, Suarez AA, Chen W, Coleman JF, McElroy JP, Pearlman R, Goodfellow PJ, Hampel H. Instability in the Penta-C and Penta-D Loci in Microsatellite-Unstable Endometrial Cancer. International Journal of Environmental Research and Public Health. 2025; 22(11):1674. https://doi.org/10.3390/ijerph22111674
Chicago/Turabian StyleYilmaz, Ahmet, Wendy L. Frankel, Weiqiang Zhao, Adrian A. Suarez, Wei Chen, Joshua F. Coleman, Joseph P. McElroy, Rachel Pearlman, Paul J. Goodfellow, and Heather Hampel. 2025. "Instability in the Penta-C and Penta-D Loci in Microsatellite-Unstable Endometrial Cancer" International Journal of Environmental Research and Public Health 22, no. 11: 1674. https://doi.org/10.3390/ijerph22111674
APA StyleYilmaz, A., Frankel, W. L., Zhao, W., Suarez, A. A., Chen, W., Coleman, J. F., McElroy, J. P., Pearlman, R., Goodfellow, P. J., & Hampel, H. (2025). Instability in the Penta-C and Penta-D Loci in Microsatellite-Unstable Endometrial Cancer. International Journal of Environmental Research and Public Health, 22(11), 1674. https://doi.org/10.3390/ijerph22111674






