Lunasin-like Peptide in Legume and Cereal Seeds: A Review
Abstract
1. Introduction
2. Lunasin in Soybean
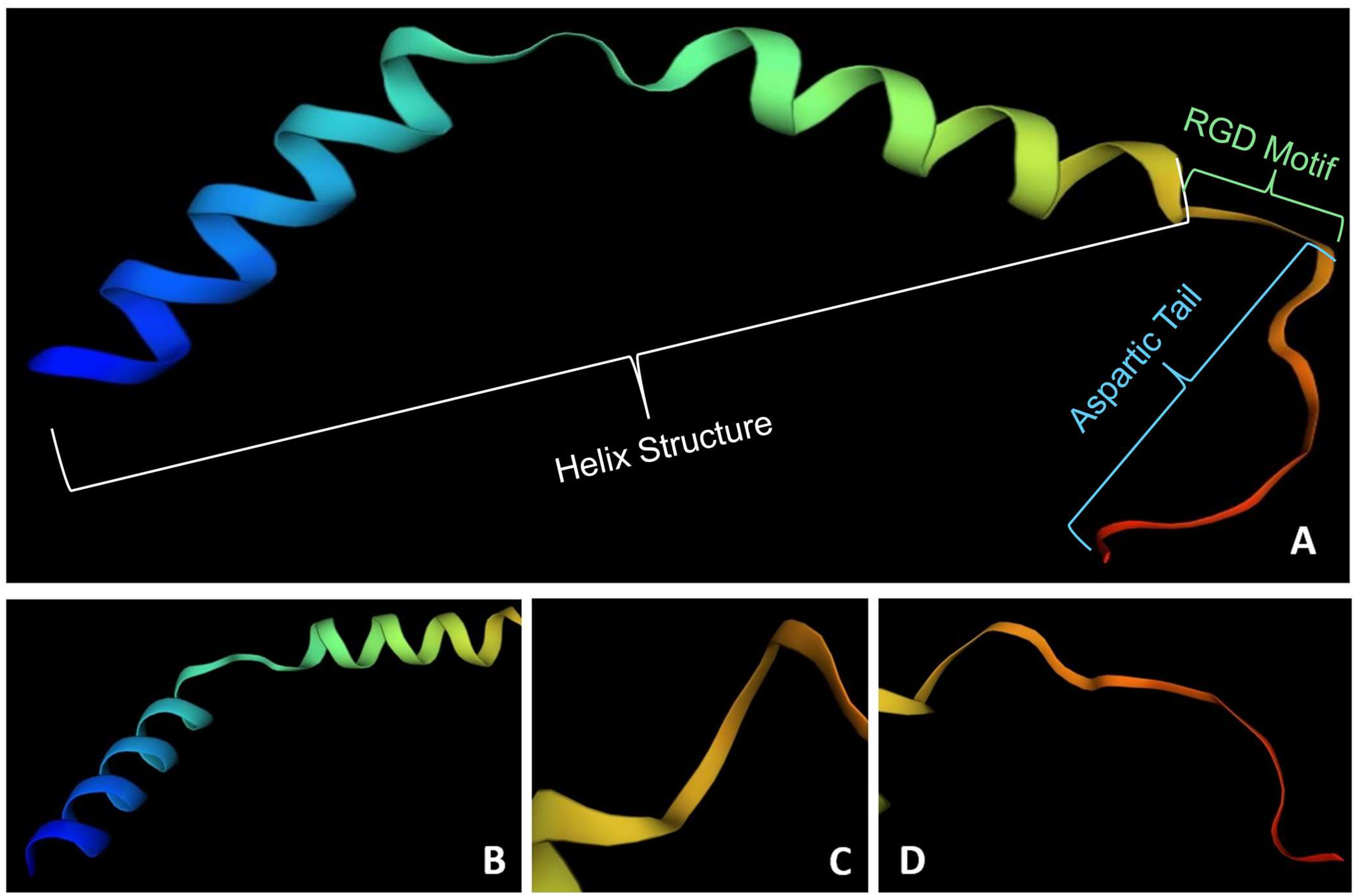
3. Lunasin-like Peptide from Other Sources
| Source and Reference | Type of Bioactivity Study | Identification Techniques | Purification | Amino Acid Sequence | Activity or Analytical Test Results |
|---|---|---|---|---|---|
| Barley [10] | Studies in vitro in cell lines. | Western blot Matrix-Assisted Laser Desorption Ionization (MALDI) | Ion-exchange column chromatography | QLQGVNLTPCEK WQHQQDSC*R qLQGVNLTPC*EK QLQGVNLTPC*EK SKWQHQQDSCR SKWQHQQDSC*R KQLQGVNLTPC*EK q = Pyroglutamic acid C* = Amidomethyl-cysteine | Barley lunasin suppressed colony formation in ras (oncogene)-transfected mouse fibroblast cells induced with IPTG. They also inhibited histone acetylation in NIH-3T3 mouse fibroblasts and MCF-7 human breast cells in the presence of the histone deacetylase inhibitor sodium butyrate. |
| Wheat [11] | Studies in vitro with enzymes. | Western blot Liquid chromatography–electrospray ionization with mass spectrometry (LC-ESI-MS) | HPLC C18 | KQLQGVNLTPCEKH | Lunasin obtained from wheat seeds dose-dependently inhibited histone acetylation (H3 and H4). |
| Rye [54] | Studies in vitro for digestion and in vivo in rats. | Western blot | HPLC C18 | No data | Rye lunasin inhibited the activity of histone acetyltransferase (HAT). |
| Triticale | No data | Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) | HPLC | No data | Identification of the presence of lunasin peptide in triticale, wheat, and rye. |
| Wheat | |||||
| Rye [53] | |||||
| Oats [52] | Studies in vitro in cells and with enzymes. | Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) | HPLC | No data | Lunasin present in oats was found to have an antioxidant effect and a cell proliferation test in HEK 293 showed decrement of 20% in proliferation. |
| Black Belladonna [56] | Studies in vitro with enzymes and for digestion. | Western blot | Ion-exchange column chromatography | No data | Lunasin, isolated from SNL, inhibited core histone H3 and H4 acetylation, HAT activities, and Rb protein phosphorylation. |
| Black Belladonna [57] | Studies in vitro for scavenging effects. | Western blot | HPLC C18 | No data | Lunasin from SNL protects DNA by chelating Fe2+, reducing its oxidation. |
| Amaranth [12] | Studies in vitro in cell lines. | Western blot Matrix-assisted laser Desorption Ionization coupled to an ion detector (MALDI-TOF) LC-MS/MS peptide de novo identification | Immunoprecipitation | HIMQK WQHQQDCR WQHQQDCRK QLQGVNLTPCEK | Trypsin-digested glutelin extracts showed induction of apoptosis against HeLa cells. Predicted other bioactive peptides in amaranth globulins and glutelins were mainly antihypertensive. |
| Amaranth [58] | Studies in vitro in cell lines. | SDS-PAGE | No data | No data | Amaranthus protein isolate was tested for its antiproliferative and antimutagenic effects in four cell lines (MC3T3E1 osteoblastic mouse calvaria-derived cells, UMR106 rat osteosarcoma-derived cells, and the Caco-2 and TC7 human colon-tumor lines), confirming these effects. |
| Amaranth [15] | Studies in vitro in cell lines. | Western blot PCR | Elution method | MTKFTILLISLLFCIAHTCSASKWQH-QQDSCRKQLQGFKMTATPPCEKHIT-RAFRRAPIQQRGISTRRGDDDDDDD-DDNHILSTRRDDEERTMRGRINYIRR-NEGKDPTPTLILREDE | The lunasin-like peptide is reported to internalize into the nucleus of NIH-3T3 cells in less time than soybean lunasin, inhibiting histone acetylation and the transformation of these cells into cancerous foci. |
| Amaranth [59] | Studies in vitro in cell lines. | SDS-PAGE MALDI-TOF | CAPYYLERWYRRKLF, EGDAZPGE, and GTFNE for unprocessed RPWWWHPGGGGGGGGLGAGT, HGSEPFGPR, RPRYPWRYT, and RDGPFPWPWYSH for extruded | Amaranth protein hydrolysates were tested in human and mouse macrophages that inhibited lipopolysaccharide-induced inflammation through inhibition of the NF-κB signaling pathway in THP-1 and RAW 264.7 cells; furthermore, extrusion enhanced this effect. | |
| Amaranth [60] | Studies in vitro in cell lines. | SDS-PAGE | No data | No data | The anticancer effect of three amaranth protein hydrolysates was studied, proving their antioxidant and antiproliferative activity in MCF-7, A549, and HEK 293 cell lines. A549 and HEK 293 cell lines, with the tryptic hydrolyzate, were the ones with the highest anticancer activity. |
| Amaranth [7] | Studies in vitro in cell lines. | Western blot Tandem mass spectrometry | Elution method | No data | Changes in the proteomic profile of NIH-3T3 cells were analyzed in the presence of 3-methylcholanthrene, which was inhibited in the presence of lunasin-like. In addition, an NF-κB factor-related pathway of action produced by the amaranth lunasin-like peptide was revealed. |
| Beans Chickpea Grass pea Lentils Pea [55] | Studies in vitro in cell lines and in silico for sequences. | Western blot Immunoidentification HPLC coupled to NanoESI-MS/MS analysis Nano-LC–ESI–MS–MS | HPLC | Absence of lunasin before fermentation is reported; however, different bands of immunoreactive polypeptides were found. The number and intensity of lunasin-like polypeptides increased during sourdough fermentation. An inhibitory effect on the proliferation of human adenocarcinoma Caco-2 cells by sourdough extracts is reported. |
3.1. Lunasin-like Peptide in Barley
3.2. Lunasin-like Peptide in Wheat
3.3. Lunasin-like Peptide in Rye
3.4. Lunasin-like Peptide in Amaranth
3.5. Lunasin-like Peptide in Triticale
3.6. Lunasin-like Peptide in Oat
3.7. Lunasin-like Peptide in Solanum
3.8. Lunasin-like Peptide in Legumes
3.9. Lunasin-like Peptide in Maize
4. Controversy on Lunasin-like Peptide in Different Plant Sources
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef]
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Deokar, G.S.; Nirmal, N.P.; Kshirsagar, S.J. Plant Seeds: A Potential Bioresource for Isolation of Nutraceutical and Bioactive Compounds. In Bioactive Extraction and Application in Food and Nutraceutical Industries. Methods and Protocols in Food and Nutraceutical Industries; Humana: New York, NY, USA, 2024; pp. 333–372. [Google Scholar] [CrossRef]
- Xavier, J.R.; Sanjay, B.S.; Gupta, D.; Mehta, S.; Chauhan, O.P. Bioactive compounds of foods: Phytochemicals and peptides. Food Humanit. 2024, 3, 100354. [Google Scholar] [CrossRef]
- Bano, N.; Izhar, S.K.; Gupta, A.; Zaheer, M.R. Prospects of Plant Derived Bioactive Compounds as Nanopar-ticles for Biotechnological Applications. Recent Pat. Biotechnol. 2024, 18, 113–127. [Google Scholar] [CrossRef]
- Unal, G.; Akalın, A.S. Antioxidant and angiotensin-converting enzyme inhibitory activity of yoghurt fortified with sodium calcium caseinate or whey protein concentrate. Dairy Sci. Technol. 2012, 92, 627–639. [Google Scholar] [CrossRef]
- Mazorra-Carrillo, J.L.; De León-Rodríguez, A.; Huerta-Ocampo, J.A.; Velarde-Salcedo, A.J.; de Mejía, E.G.; de la Rosa, A.P.B. Proteomic analysis of chemically transformed NIH-3T3 cells reveals novel mechanisms of action of amaranth lunasin-like peptide. Food Res. Int. 2022, 157, 111374. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Hsieh, C.; Martínez-Villaluenga, C.; O de Lumen, B.; Hernández-Ledesma, B. Updating the research on the chemopreventive and therapeutic role of the peptide lunasin. J. Sci. Food Agric. 2018, 98, 2070–2079. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lam, Y.; de Lumen, B.O. Barley Lunasin Suppresses ras-Induced Colony Formation and Inhibits Core Histone Acetylation in Mammalian Cells. J. Agric. Food Chem. 2002, 50, 5903–5908. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; Park, J.H.; Lee, J.B.; Kweon, D.-H.; Chung, G.Y.; Seo, E.W.; de Lumen, B.O. The cancer preventive peptide lunasin from wheat inhibits core histone acetylation. Cancer Lett. 2007, 255, 42–48. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; de la Rosa, A.P.B.; León-Galván, M.F.; de Lumen, B.O.; de León-Rodríguez, A.; de Mejía, E.G. Bioactive Peptides in Amaranth (Amaranthus hypochondriacus) Seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wu, B.; Li, M.; Yuan, M.; Qiao, L.; Zhao, J.; Zheng, X.; Li, X.; Wang, Y.; Wang, Y.; et al. Functional exploration of lunasin peptide in transgenic maize (Zea mays L.) and its role in controlling mitophagy in MDA-MB-231 cells. Food Biosci. 2024, 58, 103726. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud, “Cancer”. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 April 2023).
- Maldonado-Cervantes, E.; Jeong, H.J.; León-Galván, F.; Barrera-Pacheco, A.; De León-Rodríguez, A.; de Mejia, E.G.; de Lumen, B.O.; de la Rosa, A.P.B. Amaranth lunasin-like peptide internalizes into the cell nucleus and inhibits chemical carcinogen-induced transformation of NIH-3T3 cells. Peptides 2010, 31, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, A.; Gómez-Favela, M.A.; Reyes-Moreno, C.; Milán-Carrillo, J.; de Mejía, E.G. Identification of Bioactive Peptide Sequences from Amaranth (Amaranthus hypochondriacus) Seed Proteins and Their Potential Role in the Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2015, 14, 139–158. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, J.H.; Lam, Y.; de Lumen, B.O. Characterization of Lunasin Isolated from Soybean. J. Agric. Food Chem. 2003, 51, 7901–7906. [Google Scholar] [CrossRef]
- Odani, S.; Koide, T.; Ono, T. Amino acid sequence of a soybean (Glycine max) seed polypeptide having a poly(L-aspartic acid) structure. J. Biol. Chem. 1987, 262, 10502–10505. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Lunasin, a novel seed peptide for cancer prevention. Peptides 2009, 30, 426–430. [Google Scholar] [CrossRef]
- Galvez, A.F.; Chen, N.; Macasieb, J.; O De Lumen, B. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001, 61, 7473–7478. Available online: http://aacrjournals.org/cancerres/article-pdf/61/20/7473/2488898/ch2001007473.pdf (accessed on 27 March 2023).
- Fernández-Tomé, S.; Xu, F.; Han, Y.; Hernández-Ledesma, B.; Xiao, H. Inhibitory Effects of Peptide Lunasin in Colorectal Cancer HCT-116 Cells and Their Tumorsphere-Derived Subpopulation. Int. J. Mol. Sci. 2020, 21, 537. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Haddad, E.; Sabaté, J. The Presence of Lunasin, a Soy-Derived Bioactive Peptide in Plasma: A Randomized Clinical Trial. Curr. Dev. Nutr. 2020, 4, 400. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Hernández-Ledesma, B.; Jeong, H.J.; Park, J.H.; de Lumen, B.O. Complementary Roles in Cancer Prevention: Protease Inhibitor Makes the Cancer Preventive Peptide Lunasin Bioavailable. PLoS ONE 2010, 5, e8890. [Google Scholar] [CrossRef]
- Wan, X.; Liu, H.; Sun, Y.; Zhang, J.; Chen, X.; Chen, N. Lunasin: A promising polypeptide for the prevention and treatment of cancer. Oncol. Lett. 2017, 13, 3997–4001. [Google Scholar] [CrossRef]
- Dia, V.P.; de Mejia, E.G. Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to α5β1 integrin and suppresses FAK/ERK/NF-κB signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef]
- Galvez, A.F.; de Lumen, B.O. A soybean cDNA encoding a chromatin-binding peptide inhibits mitosis of mammalian cells. Nat. Biotechnol. 1999, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Fido, R.; Shewry, P.; Archer, D.B.; Alcocer, M.J. The expression and processing of two recombinant 2S albumins from soybean (Glycine max) in the yeast Pichia pastoris. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2004, 1698, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.F. The forgotten 2S albumin proteins: Importance, structure, and biotechnological application in agriculture and human health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, S.M.; Bothe, J.R.; Neri, C.; Walsh, P.L.; Zhang, J.; Pierson, E.; Mao, Y.; Gindy, M.; Leone, A.; Templeton, A.C. New and Evolving Techniques for the Characterization of Peptide Therapeutics. J. Pharm. Sci. 2016, 105, 2989–3006. [Google Scholar] [CrossRef]
- Mitra, M.S.; DeMarco, S.; Holub, B.; Thiruneelakantapillai, L.; Thackaberry, E.A. Development of peptide therapeutics: A nonclinical safety assessment perspective. Regul. Toxicol. Pharmacol. 2020, 117, 104766. [Google Scholar] [CrossRef]
- Wu, L. Regulatory Considerations for Peptide Therapeutics. RSC Drug Discov. Ser. 2019, 72, 1–30. [Google Scholar] [CrossRef]
- de Souza, S.M.A.; Hernández-Ledesma, B.; de Souza, T.L.F. Lunasin as a Promising Plant-Derived Peptide for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 9548. [Google Scholar] [CrossRef]
- Jones, G.; Srivastava, A. Understanding Lunasin’s biology and potential as a cancer therapeutic by utilizing Drosophila genetics. Exp. Biol. Med. 2014, 239, 519–528. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.; de Lumen, B.O. Relationship between lunasin’s sequence and its inhibitory activity of histones H3 and H4 acetylation. Mol. Nutr. Food Res. 2011, 55, 989–998. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Barnett, B.; Cai, J.; McConnell, E. “Sequence 1 from Patent US 9066906-Protein-NCBI”, Lunasin-Containing Complex and Purification of Lunasin from Plants. Available online: https://www.ncbi.nlm.nih.gov/protein/AMF28072.1 (accessed on 20 September 2025).
- DeLano, W.L. PyMOL. Available online: http://www.pymol.org (accessed on 20 September 2025).
- Chang, H.-C.; Lewis, D.; Tung, C.-Y.; Han, L.; Henriquez, S.M.P.; Voiles, L.; Lupov, I.P.; Pelloso, D.; Sinn, A.L.; Pollok, K.E.; et al. Soypeptide lunasin in cytokine immunotherapy for lymphoma. Cancer Immunol. Immunother. 2014, 63, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Indiano-Romacho, P.; Fernández-Tomé, S.; Amigo, L.; Hernández-Ledesma, B. Multifunctionality of lunasin and peptides released during its simulated gastrointestinal digestion. Food Res. Int. 2019, 125, 108513. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.; Galvez, A.; de Lumen, B.O. Lunasin™ Suppresses E1A-Mediated Transformation of Mammalian Cells but Does Not Inhibit Growth of Immortalized and Established Cancer Cell Lines. Nutr. Cancer 2009, 47, 88–94. [Google Scholar] [CrossRef]
- Lumen, B.O. Lunasin: A Cancer-Preventive Soy Peptide. Nutr. Rev. 2005, 63, 16–21. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Vásconez, M.; de Lumen, B.O.; Nelson, R. Lunasin Concentration in Different Soybean Genotypes, Commercial Soy Protein, and Isoflavone Products. J. Agric. Food Chem. 2004, 52, 5882–5887. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, H.J.; de Lumen, B.O. Contents and Bioactivities of Lunasin, Bowman−Birk Inhibitor, and Isoflavones in Soybean Seed. J. Agric. Food Chem. 2005, 53, 7686–7690. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Castañeda-Reyes, E.D.; Mojica, L.; Dia, V.; Wang, H.; Wang, T.; Johnson, L.A. Potential Health Benefits Associated with Lunasin Concentration in Dietary Supplements and Lunasin-Enriched Soy Extract. Nutrients 2021, 13, 1618. [Google Scholar] [CrossRef]
- Kusumah, J.; Preciado, J.A.; Yuan, J.; de Mejia, E.G. Lunasin, soluble protein concentration and profile in Glycine soja compared to Glycine max, bioaccessibility and peptides bioactivity. Food Biosci. 2025, 68, 106370. [Google Scholar] [CrossRef]
- Gullapalli, K.; Karthika, A.; Nagappan, K.; Shivanna, N.; Hernández-Ledesma, B. Quantification of bioactive peptide lunasin from soybean, wheat, and their commercial products by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Food Meas. Charact. 2023, 17, 4927–4937. [Google Scholar] [CrossRef]
- Wang, W.; Dia, V.P.; Vasconez, M.; de Mejia, E.G.; Nelson, R.L. Analysis of Soybean Protein-Derived Peptides and the Effect of Cultivar, Environmental Conditions, and Processing on Lunasin Concentration in Soybean and Soy Products. J. AOAC Int. 2008, 91, 936–946. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Amaya-Farfán, J.; Berhow, M.A.; Mandarino, J.M.G.; de Mejia, E.G.; Kil Chang, Y. A high-protein soybean cultivar contains lower isoflavones and saponins but higher minerals and bioactive peptides than a low-protein cultivar. Food Chem. 2010, 120, 15–21. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Berhow, M.A.; Mandarino, J.M.G.; Kil Chang, Y.; de Mejia, E.G. Effect of time and temperature on bioactive compounds in germinated Brazilian soybean cultivar BRS 258. Food Res. Int. 2010, 43, 1856–1865. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Optimization of germination time and temperature to maximize the content of bioactive compounds and the antioxidant activity of purple corn (Zea mays L.) by response surface methodology. LWT 2017, 76, 236–244. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Cruz-Huerta, E.; Amigo, L.; Recio, I.; Hernández-Ledesma, B. In Vitro Gastrointestinal Digestion and Transepithelial Transport of Bioactive Peptide Lunasin: Study of Released and Bioavailable Peptides. 2017. Available online: https://digital.csic.es/handle/10261/151644 (accessed on 20 September 2025).
- Nakurte, I.; Kirhnere, I.; Namniece, J.; Saleniece, K.; Krigere, L.; Mekss, P.; Vicupe, Z.; Bleidere, M.; Legzdina, L.; Muceniece, R. Detection of the lunasin peptide in oats (Avena sativa L). J. Cereal Sci. 2013, 57, 319–324. [Google Scholar] [CrossRef]
- Nakurte, I.; Klavins, K.; Kirhnere, I.; Namniece, J.; Adlere, L.; Matvejevs, J.; Kronberga, A.; Kokare, A.; Strazdina, V.; Legzdina, L.; et al. Discovery of lunasin peptide in triticale (X Triticosecale Wittmack). J. Cereal Sci. 2012, 56, 510–514. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, J.R.; Jeong, J.B.; Park, J.H.; Cheong, Y.-K.; de Lumen, B.O. The Cancer Preventive Seed Peptide Lunasin From Rye Is Bioavailable and Bioactive. Nutr. Cancer 2009, 61, 680–686. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Hernández-Ledesma, B.; Fernández-Tomé, S.; Curiel, J.A.; Pinto, D.; Marzani, B.; Coda, R.; Gobbetti, M. Italian legumes: Effect of sourdough fermentation on lunasin-like polypeptides. Microb. Cell Factories 2015, 14, 1–20. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jeong, H.J.; Park, J.H.; Lee, S.H.; Lee, J.R.; Lee, H.K.; Chung, G.Y.; Choi, J.D.; de Lumen, B.O. Cancer-Preventive Peptide Lunasin from Solanum nigrum L. Inhibits Acetylation of Core Histones H3 and H4 and Phosphorylation of Retinoblastoma Protein (Rb). J. Agric. Food Chem. 2007, 55, 10707–10713. [Google Scholar] [CrossRef]
- Jeong, J.B.; De Lumen, B.O.; Jeong, H.J. Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Lett. 2010, 293, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Barrio, D.A.; Añón, M.C. Potential antitumor properties of a protein isolate obtained from the seeds of Amaranthus mantegazzianus. Eur. J. Nutr. 2010, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Ramkisson, S.; Dwarka, D.; Venter, S.; Mellem, J.J. In vitro anticancer and antioxidant potential of Amaranthus cruentus protein and its hydrolysates. Food Sci. Technol. 2020, 40, 634–639. [Google Scholar] [CrossRef]
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef]
- Andong University Industrial Cooperation Center. Isolation, Purification and Availability of Lunasin Peptide as a Prevention and Curing of Skin Aging and Allergy, Anticancer and Highly Functional Foods from Soybean and Barley. Patent KR1020060024079A, 15 March 2006. [Google Scholar]
- Jeong, H.J.; Jeong, J.B.; Hsieh, C.C.; Hernández-Ledesma, B.; De Lumen, B.O. Lunasin Is Prevalent in Barley and Is Bio-available and Bioactive in In Vivo and In Vitro Studies. Nutr. Cancer 2010, 62, 1113–1119. [Google Scholar] [CrossRef]
- Ma, D.; Wang, C.; Feng, J.; Xu, B. Wheat grain phenolics: A review on composition, bioactivity, and influencing factors. J. Sci. Food Agric. 2021, 101, 6167–6185. [Google Scholar] [CrossRef]
- Dinelli, G.; Bregola, V.; Bosi, S.; Fiori, J.; Gotti, R.; Simonetti, E.; Trozzi, C.; Leoncini, E.; Prata, C.; Massaccesi, L.; et al. Lunasin in wheat: A chemical and molecular study on its presence or absence. Food Chem. 2014, 151, 520–525. [Google Scholar] [CrossRef]
- Fan, X.; Qin, P.; Hao, Y.; Guo, H.; Blecker, C.; Everaert, N.; Ren, G. Overexpression of Soybean-Derived Lunasin in Wheat and Assessment of Its Anti-Proliferative Activity in Colorectal Cancer HT-29 Cells. Int. J. Mol. Sci. 2020, 21, 9594. [Google Scholar] [CrossRef]
- Kaur, P.; Sandhu, K.S.; Purewal, S.S.; Kaur, M.; Singh, S.K. Rye: A wonder crop with industrially important macromolecules and health benefits. Food Res. Int. 2021, 150, 110769. [Google Scholar] [CrossRef]
- Kigel, J. Development and Ecophysiology of Amaranths. Amaranth Biol. Chem. Technol. 2018, 39–73. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2012, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Caselato-Sousa, V.M.; Amaya-Farfán, J. State of Knowledge on Amaranth Grain: A Comprehensive Review. J. Food Sci. 2012, 77, R93–R104. [Google Scholar] [CrossRef] [PubMed]
- Paccapelo, H.; Ferreira, V.; Picca, A.; Ferrari, E.; Domínguez, R.; Grassi, E.; Ferreira, A.; Di Santo, H.; Castillo, E. TRITICALE (x Triticosecale Wittmack): RENDIMIENTO Y SUS COMPONENTES EN UN AMBIENTE SEMIÁRIDO DE LA ARGENTINA. Chil. J. Agric. Anim. Sci. 2017, 33, 1. [Google Scholar] [CrossRef]
- Galbas, M.E.; Porzucek, F.; Selwet, M.; Nowak, A.; Slomski, R. Lunasin—A bioactive peptide from triticale (X Triticosecale Wittmack) seeds, inhibits proliferation of cancer HeLa and SK-OV-3 cells. BioTechnologia 2018, 98, 219–224. [Google Scholar] [CrossRef]
- Sadiq Butt, M.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Chen, X.; Dai, X.; Liu, Y.; Yang, Y.; Yuan, L.; He, X.; Gong, G. Solanum nigrum Linn.: An Insight into Current Research on Traditional Uses, Phytochemistry, and Pharmacology. Front. Pharmacol. 2022, 13, 918071. [Google Scholar] [CrossRef]
- OMS; FAO. Cereales, Legumbres, Leguminosas y Productos Proteínicos Vegetales; CODEX ALIMENTARIUS. (Online Book); OMS: Rome, Italy, 2007; pp. 16–19. Available online: https://www.fao.org/4/a1392s/a1392s00.pdf (accessed on 18 June 2024).
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive Peptides and Hydrolysates from Pulses and Their Potential Use as Functional Ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Lu, H.; Tian, Z.; Zhao, H.; Wei, D.; Wang, S.; Huang, Z. Integration of transcriptome and metabolome analyses reveals key lodging-resistance-related genes and metabolic pathways in maize. Front. Genet. 2022, 13, 1001195. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Viewpoint: Agri-nutrition research: Revisiting the contribution of maize and wheat to human nutrition and health. Food Policy 2021, 100. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, A.A.; Krishnan, H.B. Immunological Investigation for the Presence of Lunasin, a Chemopreventive Soybean Peptide, in the Seeds of Diverse Plants. J. Agric. Food Chem. 2016, 64, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Lovegrove, A.; Shewry, P.R. Lunasin in cereal seeds: What is the origin? J. Cereal Sci. 2013, 57, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Alzaydi, A.; Barbhuiya, R.I.; Routray, W.; Elsayed, A.; Singh, A. Bioactive peptides: Synthesis, applications, and associated challenges. Food Bioeng. 2023, 2, 273–290. [Google Scholar] [CrossRef]
- Cavazos, A.; de Mejia, E.G. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef]
- Cermeño, M.; Connolly, A.; O’KEeffe, M.B.; Flynn, C.; Alashi, A.M.; Aluko, R.E.; FitzGerald, R.J. Identification of bioactive peptides from brewers’ spent grain and contribution of Leu/Ile to bioactive potency. J. Funct. Foods 2019, 60, 103455. [Google Scholar] [CrossRef]
- Gammoh, S.; Alu’datt, M.H.; Alhamad, M.N.; Tranchant, C.C.; Rababah, T.; Al-U’datt, D.; Hussein, N.; Alrosan, M.; Tan, T.-C.; Kubow, S.; et al. Functional and Bioactive Properties of Wheat Protein Fractions: Impact of Digestive Enzymes on Antioxidant, α-Amylase, and Angiotensin-Converting Enzyme Inhibition Potential. Molecules 2023, 28, 6012. [Google Scholar] [CrossRef]
- Gong, X.; An, Q.; Le, L.; Geng, F.; Jiang, L.; Yan, J.; Xiang, D.; Peng, L.; Zou, L.; Zhao, G.; et al. Prospects of cereal protein-derived bioactive peptides: Sources, bioactivities diversity, and production. Crit. Rev. Food Sci. Nutr. 2022, 62, 2855–2871. [Google Scholar] [CrossRef]
- Indrati, R. Bioactive Peptides from Legumes and Their Bioavailability. Legumes Res. 2021, 2, 12–35. [Google Scholar] [CrossRef]
- Islas-Martínez, D.; Ávila-Vargas, Y.N.; Rodríguez-Serrano, G.M.; González-Olivares, L.G.; Pérez-Flores, J.G.; Contreras-López, E.; Olloqui, E.J.; Pérez-Escalante, E. Multi-Bioactive Potential of a Rye Protein Isolate Hydrolysate by Enzymatic Processes. Biol. Life Sci. Forum 2023, 26, 38. [Google Scholar] [CrossRef]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Meza-Márquez, O.G.; Jiménez-Martínez, C. Biological functions of peptides from legumes in gastrointestinal health. A review legume peptides with gastrointestinal protection. J. Food Biochem. 2022, 46, e14308. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Taniya, M.; Mv, R.; Ps, S.; Krishnan, G.; S, P. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Thakur, S.; Punia, A.; Satyakam; Acharya, V.; Kumar, B.; Prasad, A.; Yadav, S.K.; Kumar, R. Bringing bioactive peptides into drug discovery: Challenges and opportunities for medicinal plants. Ind. Crop. Prod. 2024, 222, 119855. [Google Scholar] [CrossRef]
- Tok, K.; Moulahoum, H.; Kocazorbaz, E.K.; Zihnioglu, F. Bioactive peptides with multiple activities extracted from Barley (Hordeum vulgare L.) grain protein hydrolysates: Biochemical analysis and computational identification. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Weng, Z.; Chen, Y.; Liang, T.; Lin, Y.; Cao, H.; Song, H.; Xiong, L.; Wang, F.; Shen, X.; Xiao, J. A review on processing methods and functions of wheat germ-derived bioactive peptides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5577–5593. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, Z.; Zhou, Z.; Jin, M.; Li, Q.; Zhou, X. Recent advances in bioactive peptides from cereal-derived Foodstuffs. Int. J. Food Sci. Nutr. 2022, 73, 875–888. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Cheng, W.-L.; Li, X.-D.; Wang, X.; Yang, F.-W.; Xiao, J.-S.; Li, Y.-X.; Zhao, G.-P. Extraction, bioactive function and application of wheat germ protein/peptides: A review. Curr. Res. Food Sci. 2023, 6, 100512. [Google Scholar] [CrossRef]
- Zhu, F. Amaranth proteins and peptides: Biological properties and food uses. Food Res. Int. 2023, 164, 112405. [Google Scholar] [CrossRef]
- Zhuang, M.; Li, J.; Wang, A.; Li, G.; Ke, S.; Wang, X.; Ning, M.; Sheng, Z.; Wang, B.; Zhou, Z. Structurally manipulated antioxidant peptides derived from wheat bran: Preparation and identification. Food Chem. 2024, 442, 138465. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Yongsawatdigul, J. Production and purification of antioxidant peptides from a mungbean meal hydrolysate by Virgibacillus sp. SK37 proteinase. Food Chem. 2013, 141, 992–999. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Proteins Extracted from Defatted Wheat Germ: Nutritional and Structural Properties. Cereal Chem. 2006, 83, 69–75. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Q. Histone deacetylase inhibitors and cell death. Cell. Mol. Life Sci. 2014, 71, 3885–3901. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov. Today 2019, 24, 685–702. [Google Scholar] [CrossRef]
- Kaufman-Szymczyk, A.; Kaczmarek, W.; Fabianowska-Majewska, K.; Lubecka-Gajewska, K. Lunasin and Its Epigenetic Impact in Cancer Chemoprevention. Int. J. Mol. Sci. 2023, 24, 9187. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Pan, T.-M. Recombinant expression of bioactive peptide lunasin in Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 88, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; James, K.A.; McPherson, M.J. Recombinant production of the therapeutic peptide lunasin. Microb. Cell Factories 2012, 11, 28. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-López, J.O.; Castañeda-Reyes, E.D.; Dávila-Ortiz, G. Lunasin-like Peptide in Legume and Cereal Seeds: A Review. Int. J. Environ. Res. Public Health 2025, 22, 1505. https://doi.org/10.3390/ijerph22101505
Gutiérrez-López JO, Castañeda-Reyes ED, Dávila-Ortiz G. Lunasin-like Peptide in Legume and Cereal Seeds: A Review. International Journal of Environmental Research and Public Health. 2025; 22(10):1505. https://doi.org/10.3390/ijerph22101505
Chicago/Turabian StyleGutiérrez-López, Jorge Oswaldo, Erick Damián Castañeda-Reyes, and Gloria Dávila-Ortiz. 2025. "Lunasin-like Peptide in Legume and Cereal Seeds: A Review" International Journal of Environmental Research and Public Health 22, no. 10: 1505. https://doi.org/10.3390/ijerph22101505
APA StyleGutiérrez-López, J. O., Castañeda-Reyes, E. D., & Dávila-Ortiz, G. (2025). Lunasin-like Peptide in Legume and Cereal Seeds: A Review. International Journal of Environmental Research and Public Health, 22(10), 1505. https://doi.org/10.3390/ijerph22101505








