Clinical Outcomes of Severe Lassa Fever in West Africa: A Systematic Review and Meta-Analysis
Abstract
1. Background
2. Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Review Questions
2.4. Inclusion Criteria
2.5. Data Extraction
2.6. Methodological Quality and Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
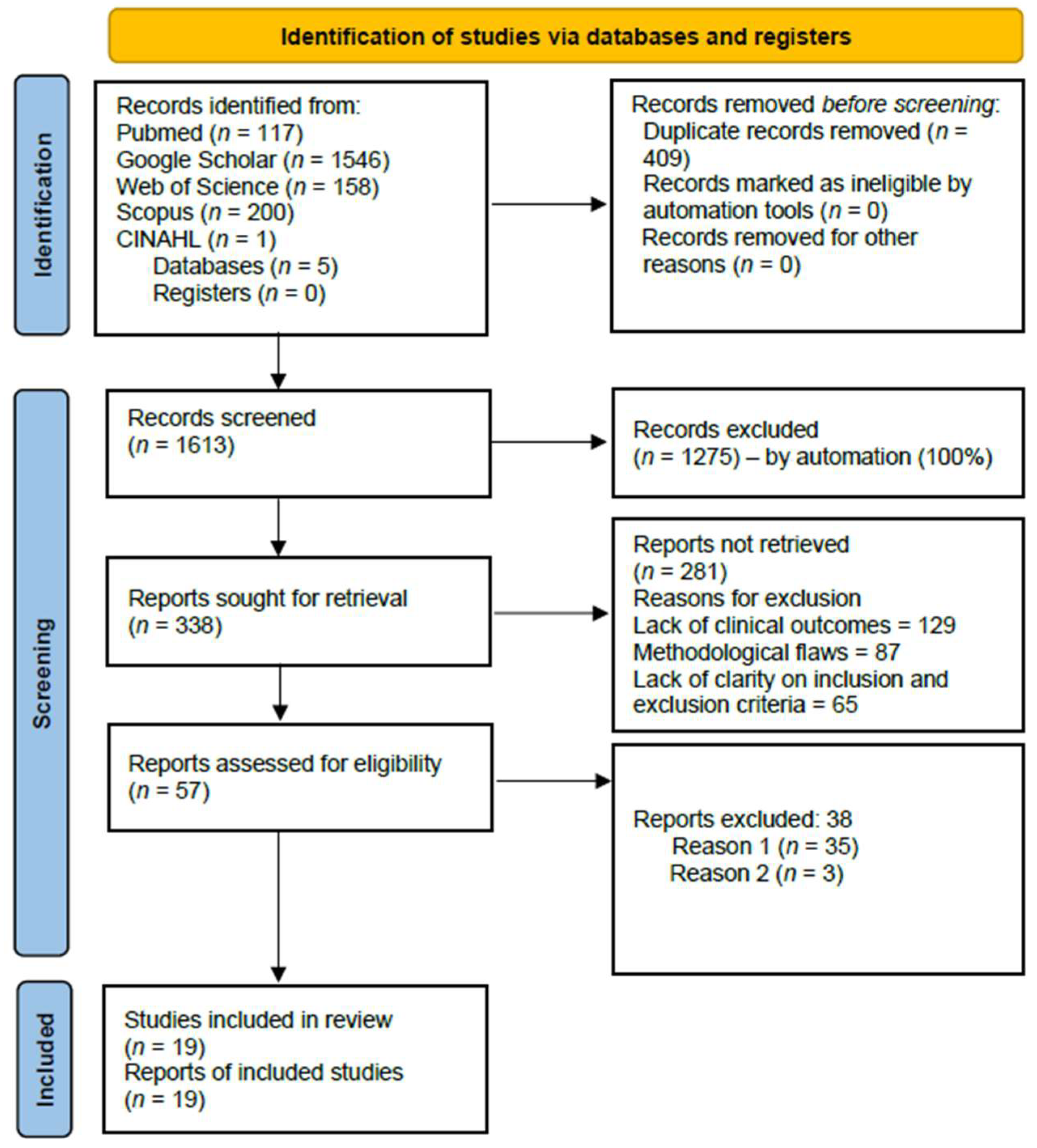
3.1. Selection of Studies
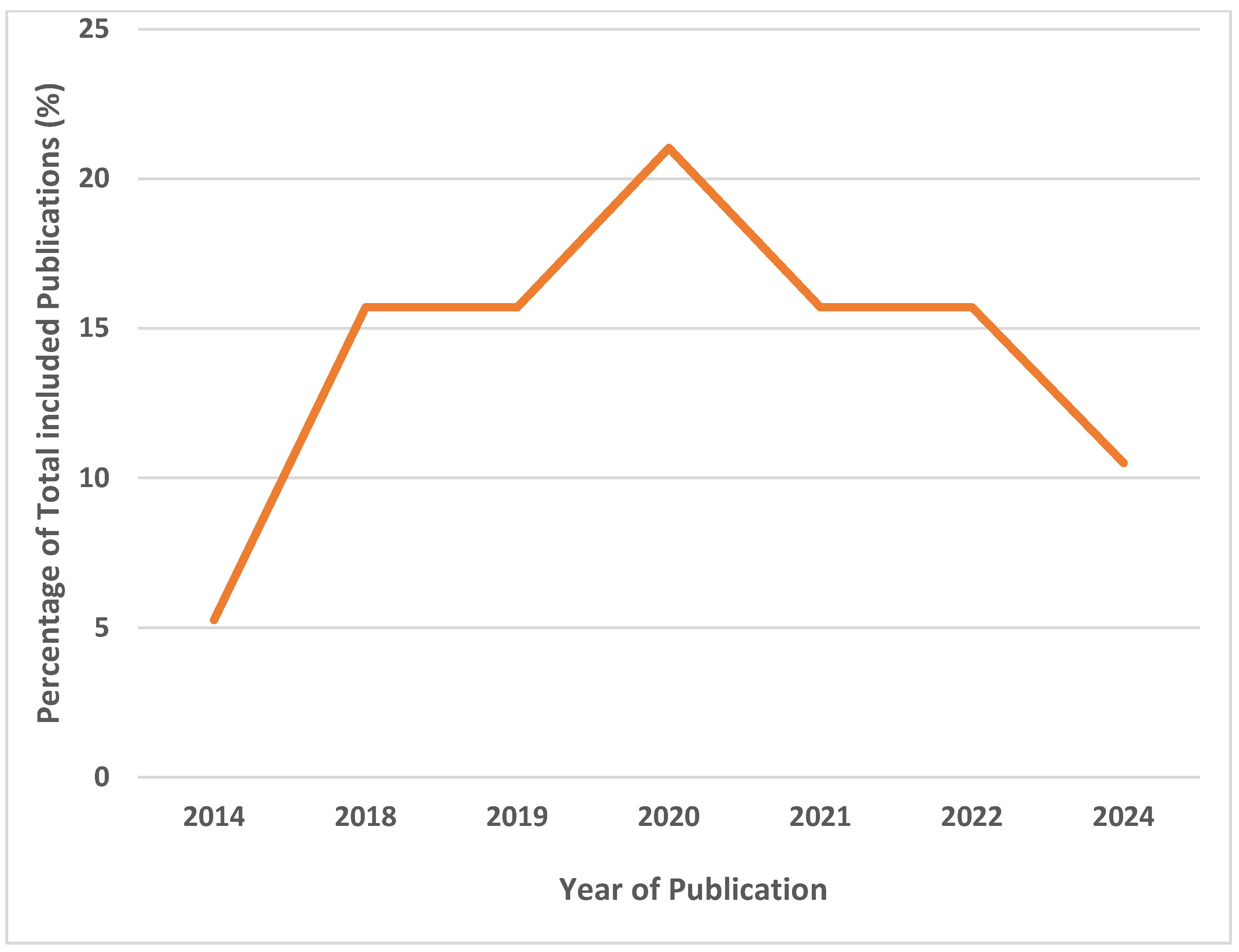
3.2. Characteristics of Included Studies
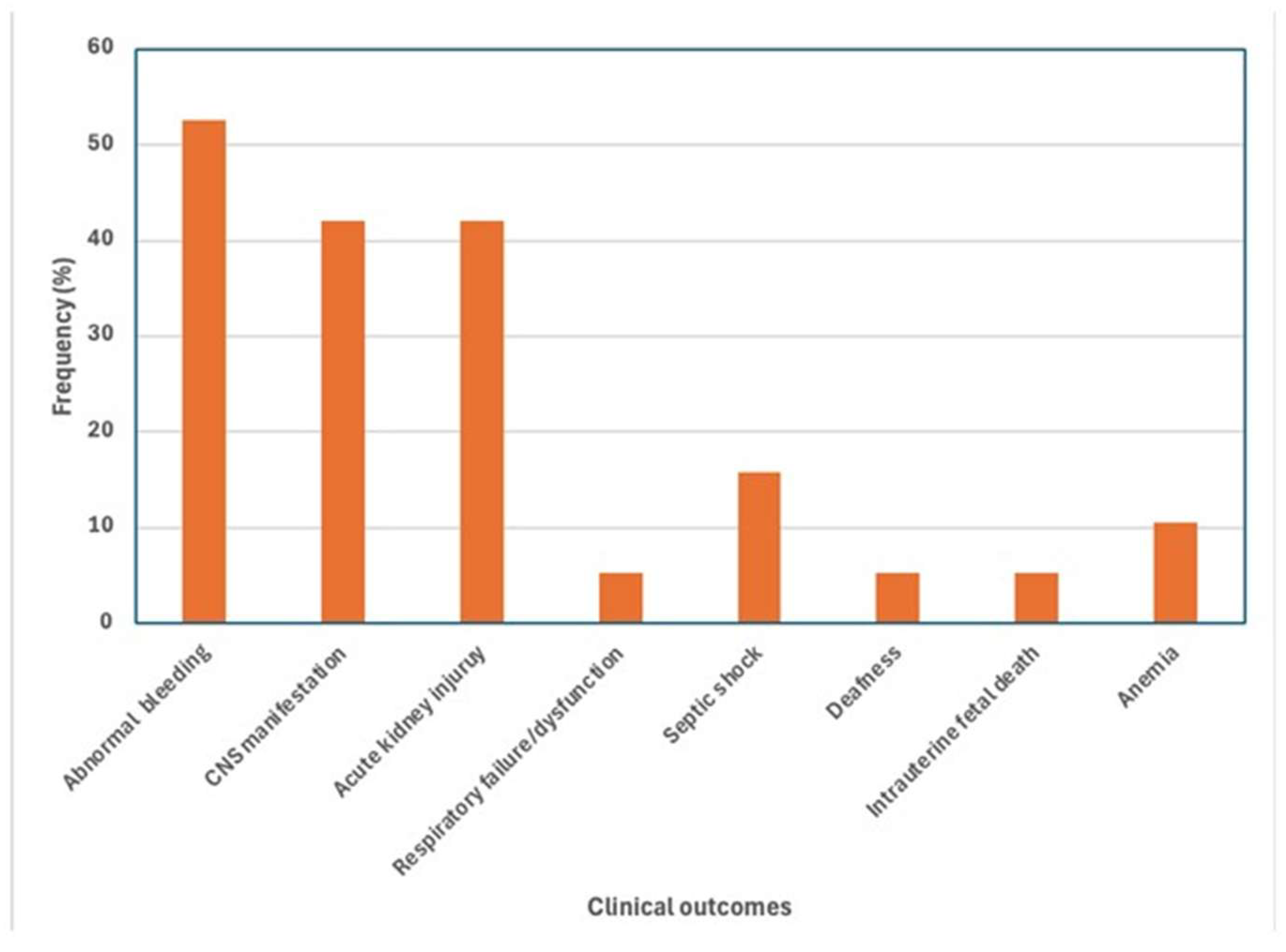
3.3. Primary Outcomes
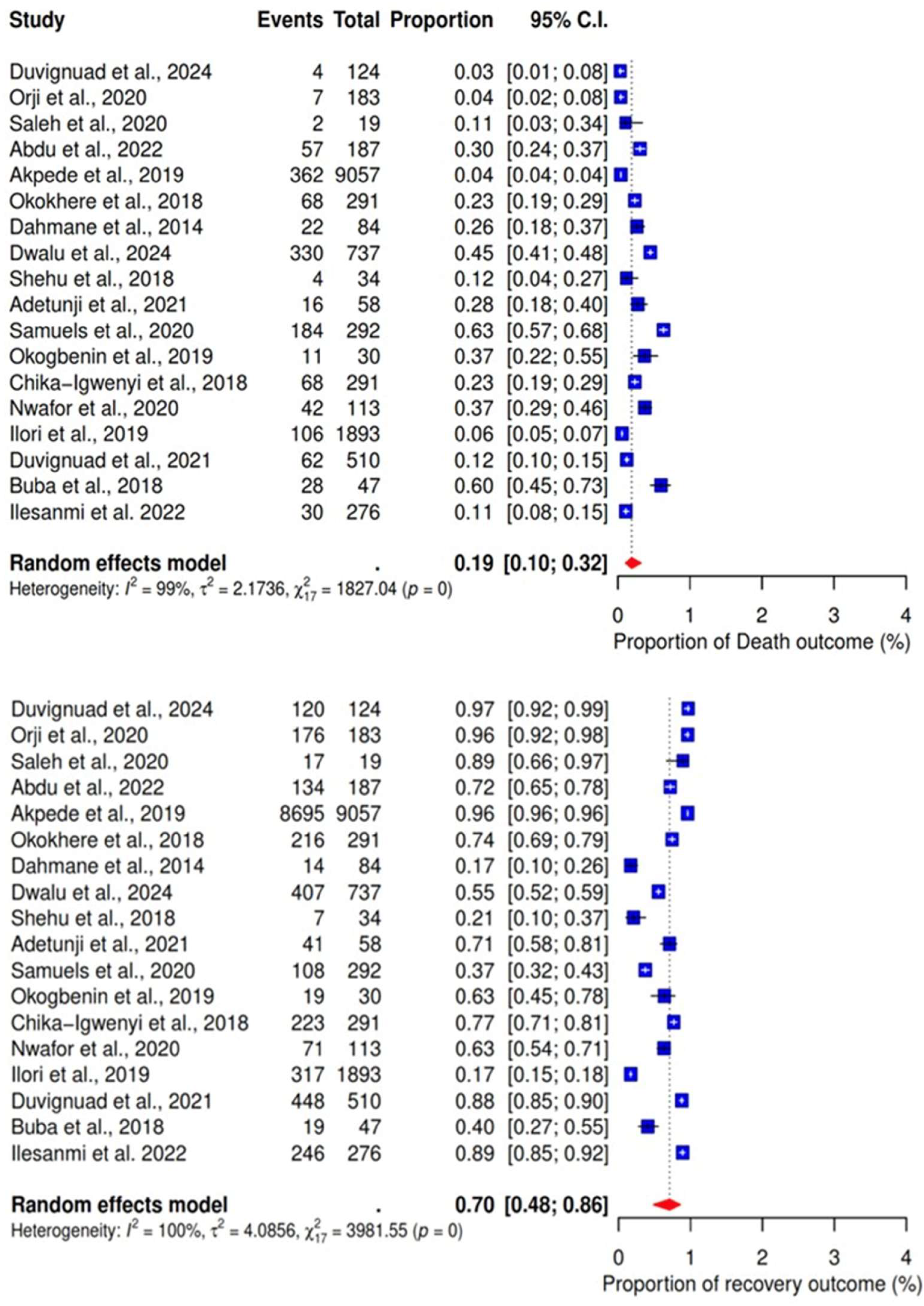
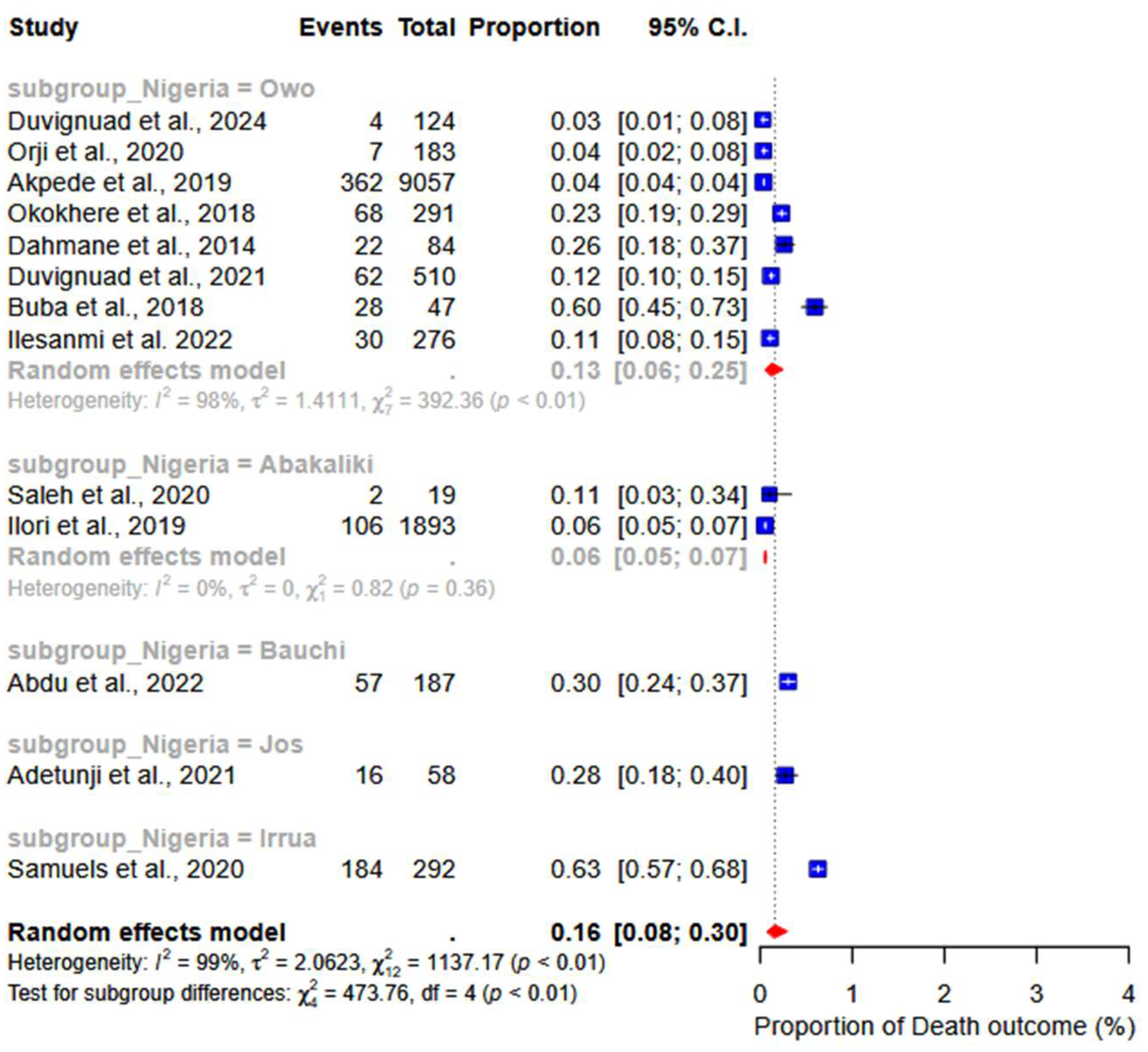
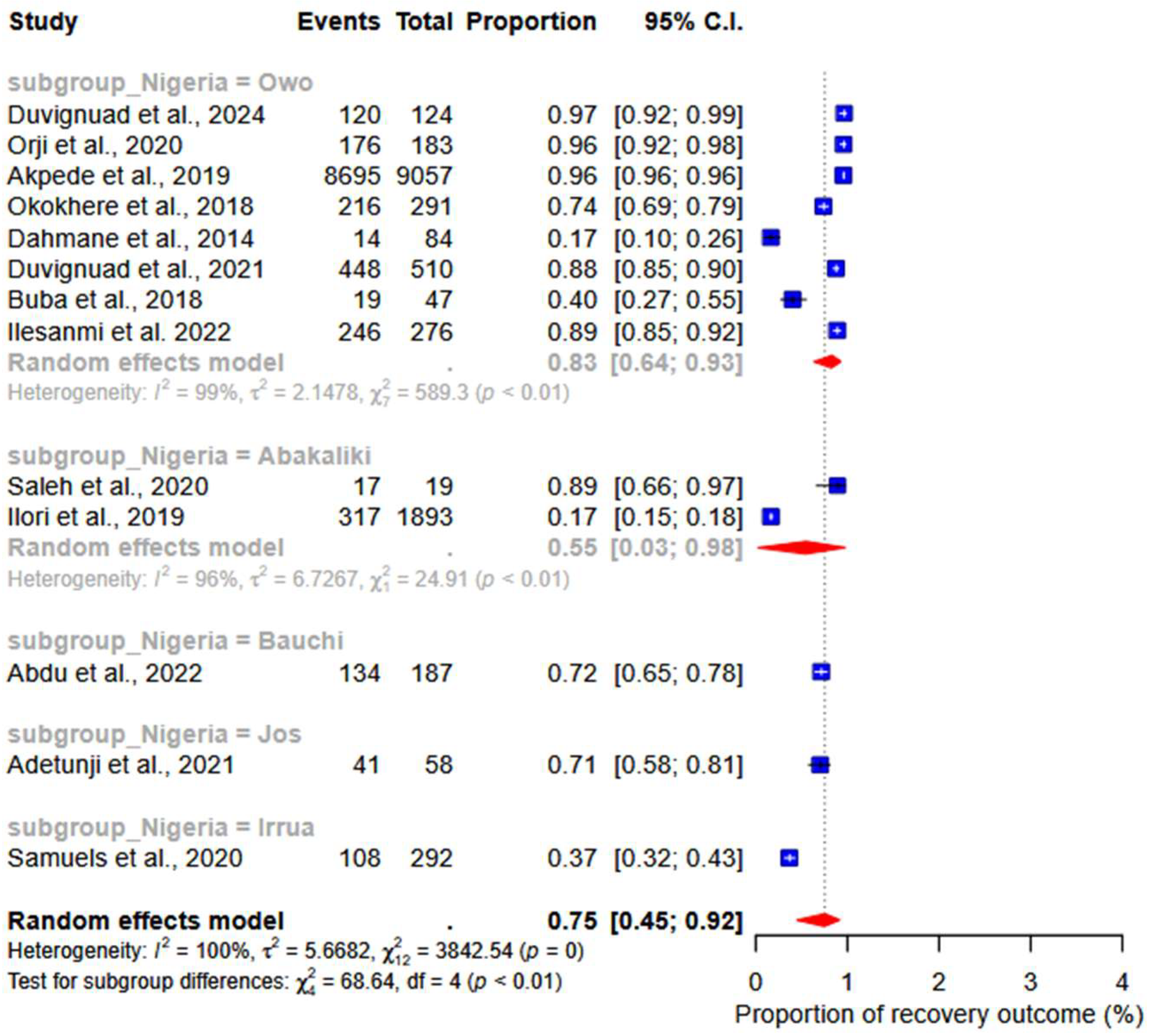
3.4. Meta-Analysis
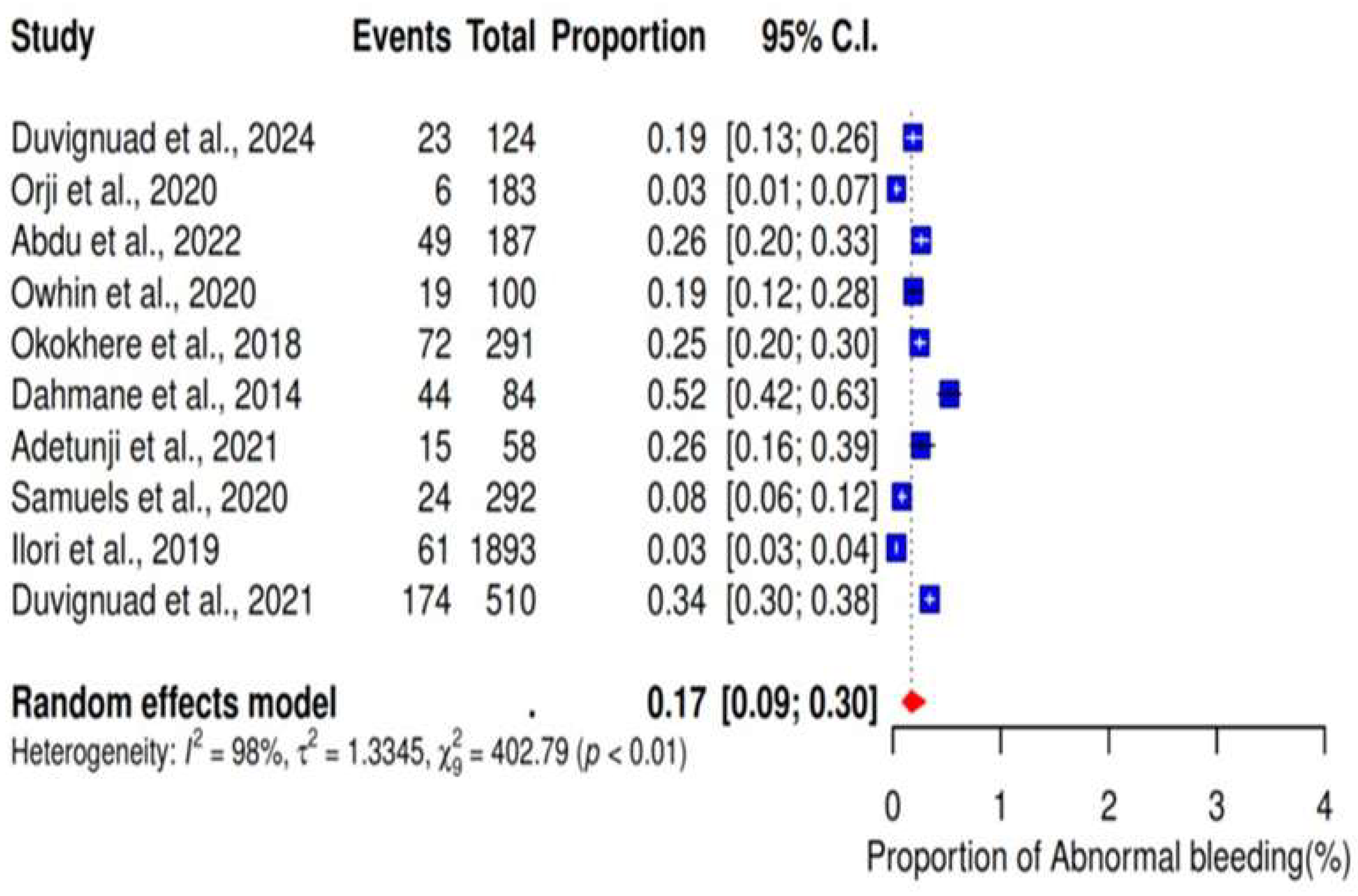
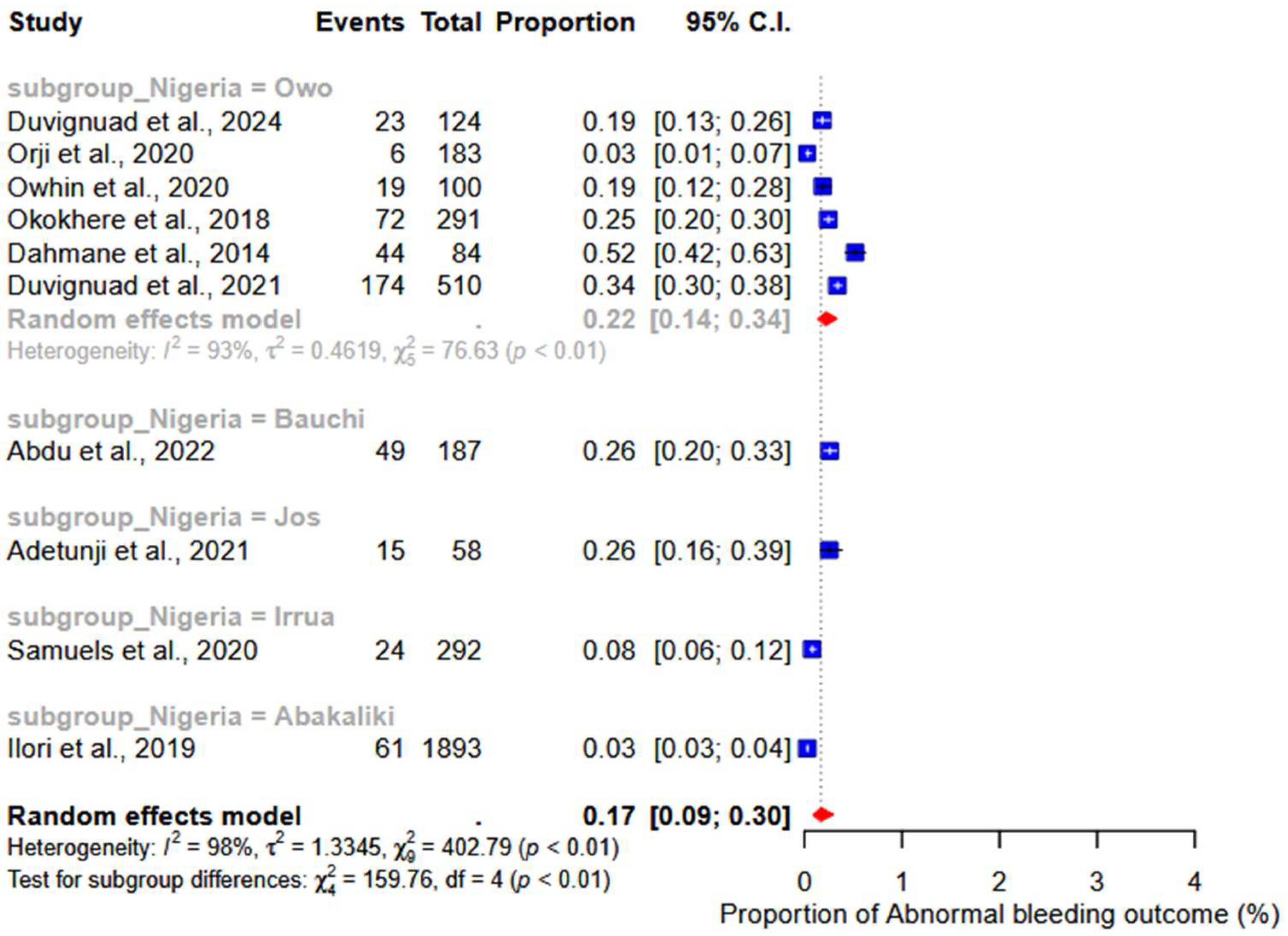
3.5. Abnormal Bleeding
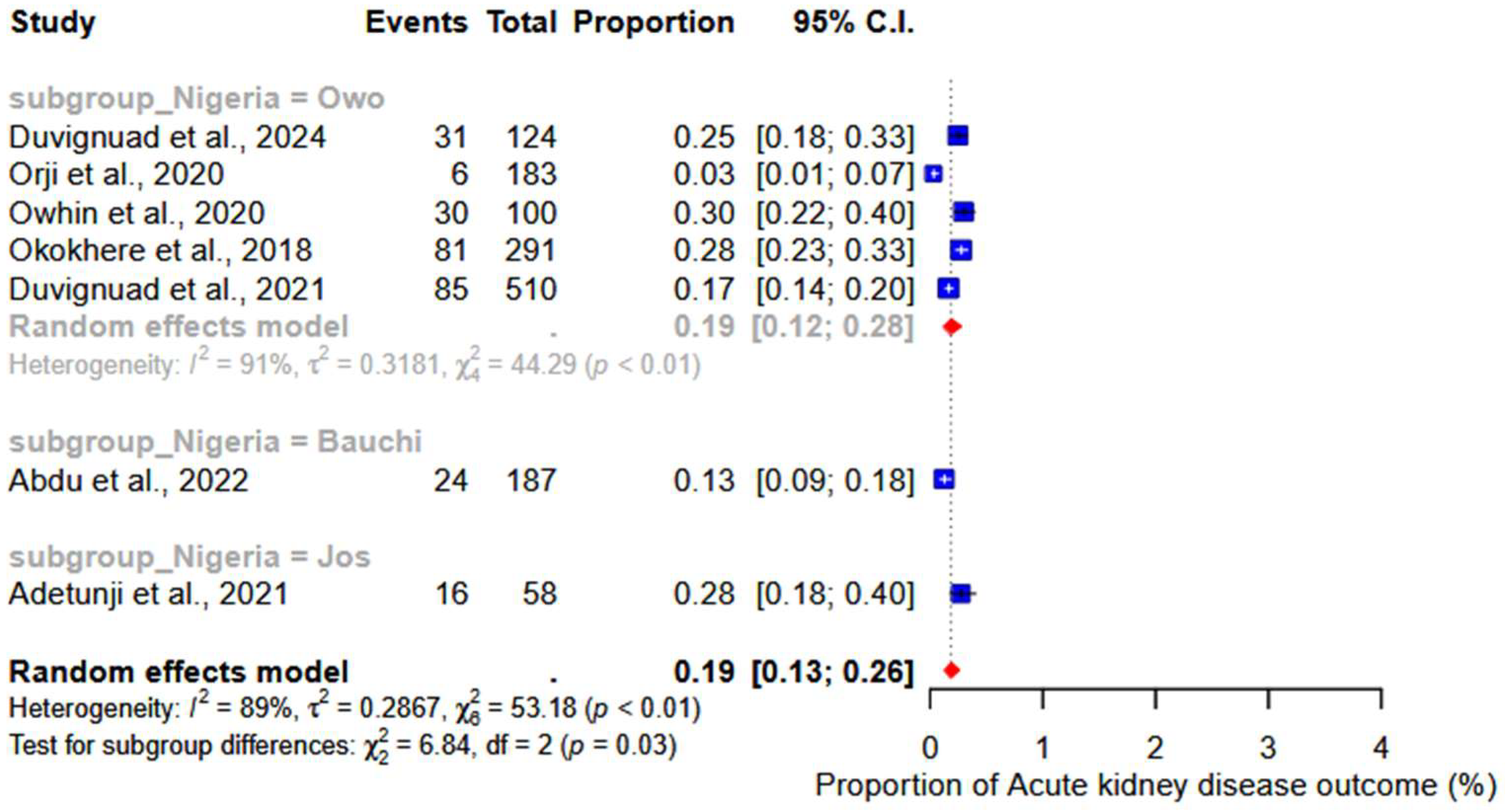
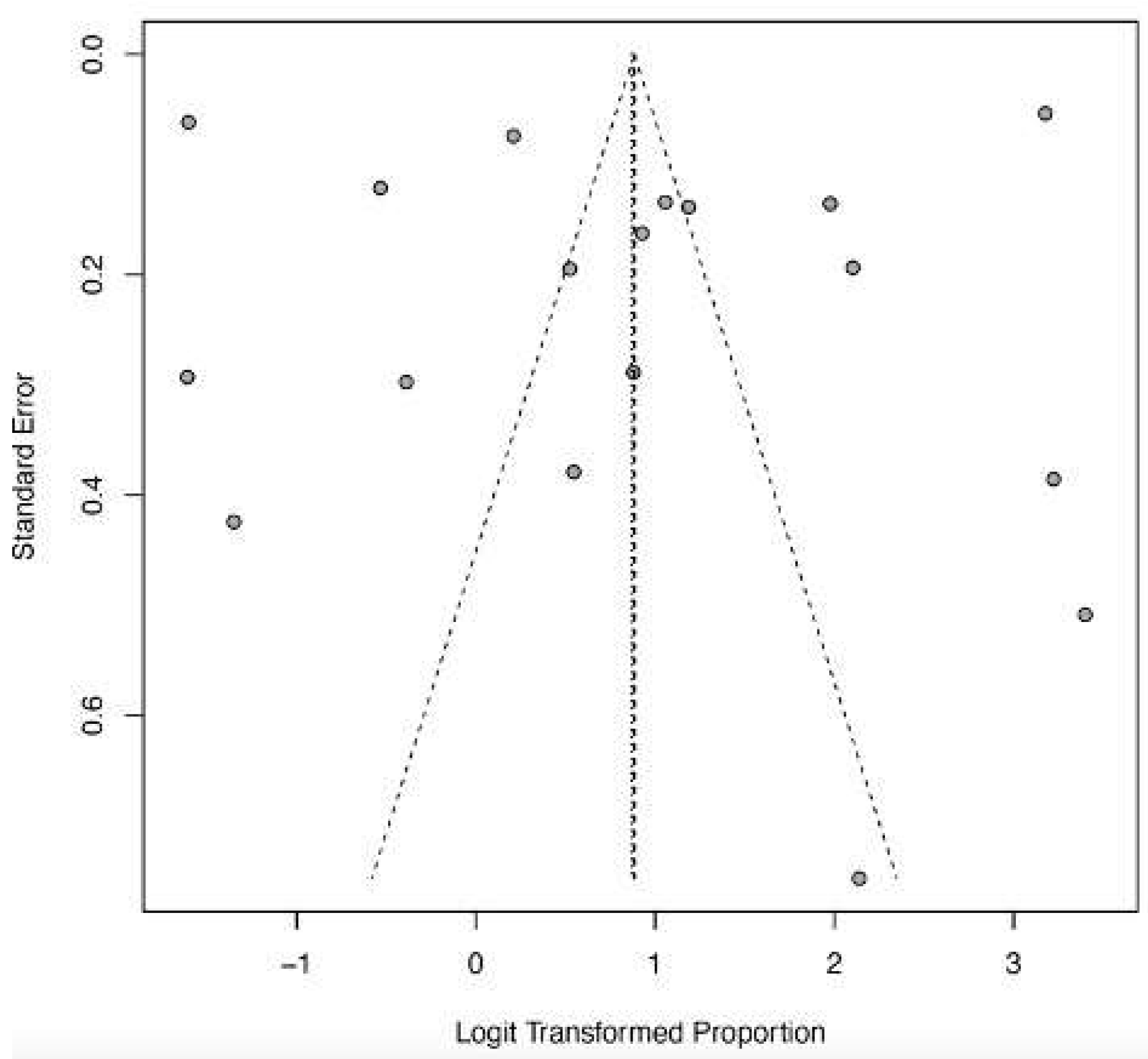
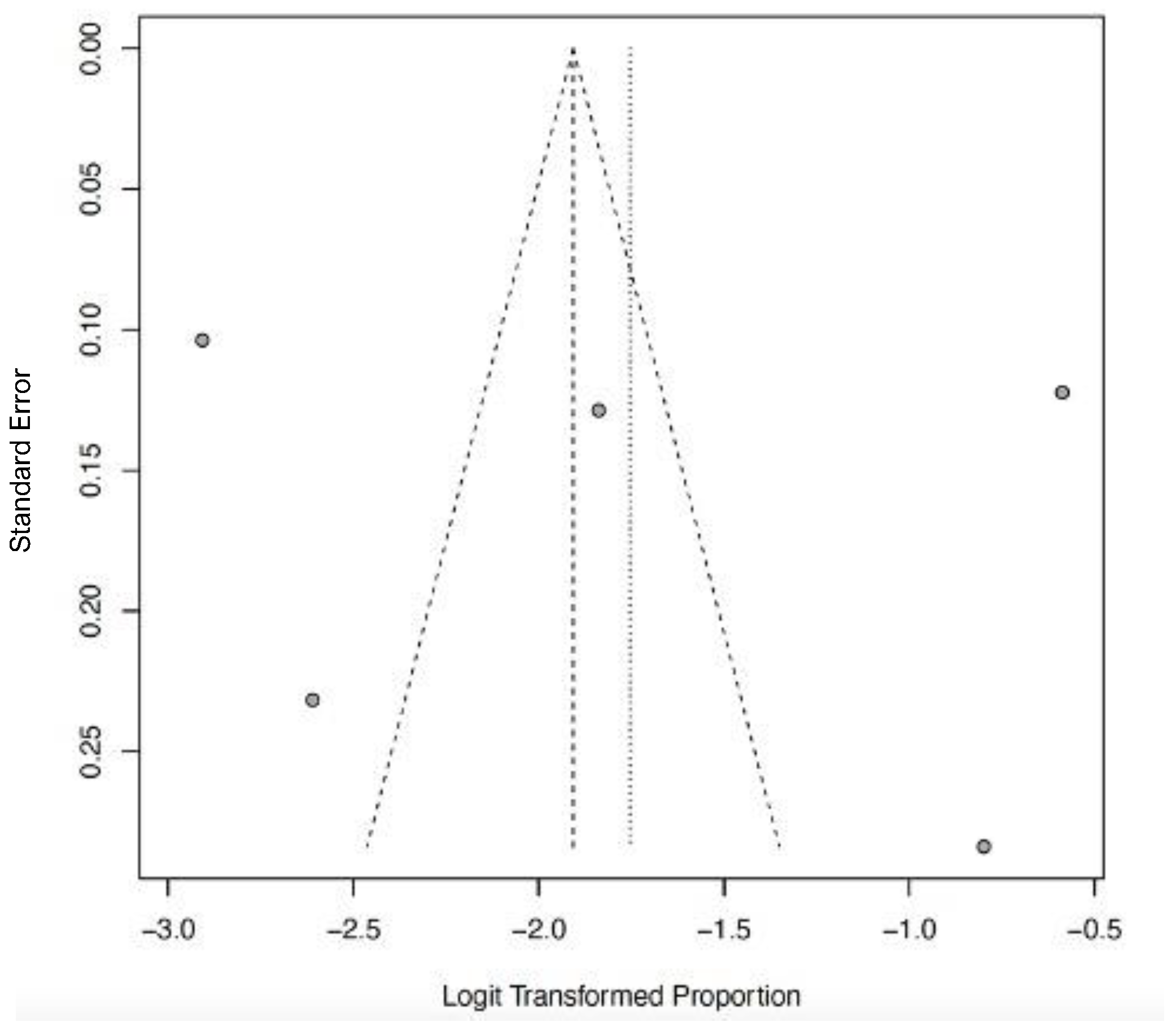
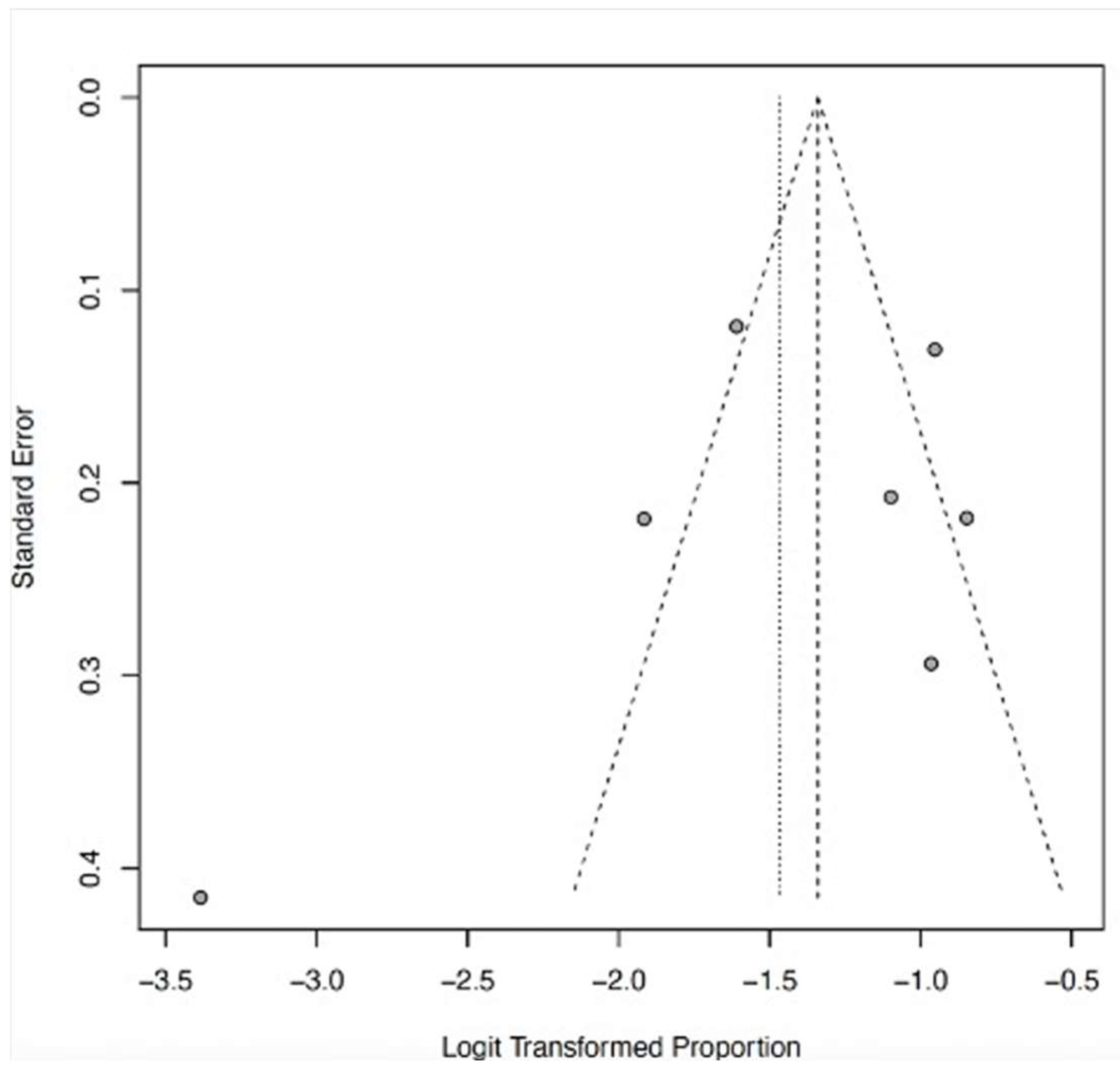
3.6. Acute Kidney Injury
3.7. CNS Dysfunction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization—Lassa Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/lassa-fever (accessed on 5 October 2024).
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulémou, K.; Sylla, O.; Kourouma, F.; Doré, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef]
- Adewuyi, G.M.; Fowotade, A.; Adewuyi, B.T. Lassa Fever: Another Infectious Menace. Afr. J. Clin. Exp. Microbiol. 2009, 10, 144–155. [Google Scholar] [CrossRef]
- Grace, J.-U.A.; Egoh, I.J.; Udensi, N. Epidemiological trends of Lassa fever in Nigeria from 2015–2021: A review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211058252. [Google Scholar] [CrossRef]
- Olayemi, A.; Cadar, D.; Magassouba, N.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New Hosts of The Lassa Virus. Sci. Rep. 2016, 6, 25280. [Google Scholar] [CrossRef]
- Olayemi, A.; Fichet-Calvet, E. Systematics, Ecology, and Host Switching: Attributes Affecting Emergence of the Lassa Virus in Rodents across Western Africa. Viruses 2020, 12, 312. [Google Scholar] [CrossRef]
- Lassa Fever Research and Development (R&D) Roadmap—DRAFT FOR PUBLIC COMMENT. Available online: https://www.who.int/publications/m/item/lassa-fever-research-and-development-roadmap-May (accessed on 6 October 2024).
- R&D Blueprint and Ebola/Marburg. Available online: https://www.who.int/teams/blueprint/lassa-fever (accessed on 6 October 2024).
- Moore, K.A.; Ostrowsky, J.T.; Mehr, A.J.; Johnson, R.A.; Ulrich, A.K.; Moua, N.M.; Fay, P.C.; Hart, P.J.; Golding, J.P.; Benassi, V.; et al. Lassa fever research priorities: Towards effective medical countermeasures by the end of the decade. Lancet Infect. Dis. 2024, 24, e696–e706. [Google Scholar] [CrossRef] [PubMed]
- Participants in Nigeria Vaccinated in First-Ever Phase 2 Lassa Fever Vaccine Clinical Trial | CEPI. Available online: https://cepi.net//participants-nigeria-vaccinated-first-ever-phase-2-lassa-fever-vaccine-clinical-trial (accessed on 6 October 2024).
- First-Ever Phase 2 Lassa Vaccine Clinical Trial Now Fully Active Across West Africa—IAVI. Available online: https://www.iavi.org/features/iavi-c105-lassa-vaccine-clinical-trial-fully-active/ (accessed on 5 October 2024).
- Cross, R.W.; Heinrich, M.L.; Fenton, K.A.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Woolsey, C.; Deer, D.J.; Dobias, N.S.; Rowland, M.M.; et al. A human monoclonal antibody combination rescues nonhuman primates from advanced disease caused by the major lineages of Lassa virus. Proc. Natl. Acad. Sci. USA 2023, 120, e2304876120. [Google Scholar] [CrossRef]
- Oestereich, L.; Rieger, T.; Lüdtke, A.; Ruibal, P.; Wurr, S.; Pallasch, E.; Bockholt, S.; Krasemann, S.; Muñoz-Fontela, C.; Günther, S. Efficacy of Favipiravir Alone and in Combination with Ribavirin in a Lethal, Immunocompetent Mouse Model of Lassa Fever. J. Infect. Dis. 2016, 213, 934–938. [Google Scholar] [CrossRef]
- Ehichioya, D.U.; Hass, M.; Olschläger, S.; Becker-Ziaja, B.; Onyebuchi Chukwu, C.O.; Coker, J.; Nasidi, A.; Ogugua, O.O.; Günther, S.; Omilabu, S.A.; et al. Lassa Fever, Nigeria, 2005–2008. Emerg. Infect. Dis. 2010, 16, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- SciELO—Brazil—The Joanna Briggs Institute Approach for Systematic Reviews. The Joanna Briggs Institute Approach for Systematic Reviews. Available online: https://www.scielo.br/j/rlae/a/3X4PW3B8fzcrpH6YvgZhCJH/?lang=en (accessed on 5 October 2024).
- Duvignaud, A.; Etafo, I.C.; Jaspard, M.; Salau, Q.; Serra, B.; Kareem, A.J.; Juchet, S.; Jegede, T.O.; Gabillard, D.; Abidoye, A.T.; et al. Presentation and Outcomes of Lassa Fever in Children in Nigeria: A Prospective Cohort Study (LASCOPE). J. Pediatr. Infect. Dis. Soc. 2024, 13, piae083. [Google Scholar] [CrossRef]
- Orji, M.L.; Ajayi, N.; Onyire, N.; Unigwe, U.; Ojide, C. Positivity rate, Predictors, and Outcome of Paediatric Lassa fever Disease (LFD) in a Lassa fever Endemic State, Southeast Nigeria. Niger. Med. J. 2022, 62, 133–138. [Google Scholar]
- Saleh, M.; Dan-Nwafor, C.; Ihekweazu, C.; Ipadeola, O.; Ukponu, W.; Abejegah, C.; Oyegeoke, A.; Adekanye, U.; Tuko, M.; Amao, L.; et al. Exposure Incidents and Outcome of Lassa Fever Virus (LASV) Infection among Healthcare Workers in Nigeria, 2019. J. Infect. Dis. Epidemiol. 2020, 6, 168. [Google Scholar]
- Abdu, A.; Ibrahim, M.M.; Muhammad, L.S.; Audi, Y.K.; Sabo, U.M.; Yusuf, J.B. Factors Affecting Outcome in Reverse Transcriptase-Polymerase Chain Reaction-Positive Lassa Fever Patients with Acute Kidney Injury: A Retrospective Analysis. Niger. J. Med. 2022, 31, 544–548. [Google Scholar] [CrossRef]
- Owhin, S.O.; Abejegah, C.; Olatunde, L.; Akhideno, P.E.; Emorinken, A.; Adelabu, Y.; Boma, P.O.; Friday, S.; Isiaka, A.A.; Gbenga-Ayeni, F.; et al. The Impact and Morphology of Anaemia among Lassa Fever Patients Treated in a Dedicated Treatment Center in South West Nigeria. J. Trop. Med. Health 2020, 4, 148. [Google Scholar]
- Akpede, G.O.; Asogun, D.A.; Okogbenin, S.A.; Dawodu, S.O.; Momoh, M.O.; Dongo, A.E.; Ike, C.; Tobin, E.; Akpede, N.; Ogbaini-Emovon, E.; et al. Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center’s Experience and Its Implications. Front. Public Health 2019, 7, 170. [Google Scholar]
- Okokhere, P.; Colubri, A.; Azubike, C.; Iruolagbe, C.; Osazuwa, O.; Tabrizi, S.; Chin, E.; Asad, S.; Ediale, E.; Rafiu, M.; et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: A retrospective, observational cohort study. Lancet Infect. Dis. 2018, 18, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, A.; Van Griensven, J.; Van Herp, M.; Van den Bergh, R.; Nzomukunda, Y.; Prior, J.; Alders, P.; Jambai, A.; Zachariah, R. Constraints in the diagnosis and treatment of Lassa Fever and the effect on mortality in hospitalized children and women with obstetric conditions in a rural district hospital in Sierra Leone. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Dwalu, E.; Jetoh, R.W.; Shobayo, B.I.; Pewu, I.; Taweh, F.; Wilson-Sesay, H.W.; Akpan, G.E.; Shannon, F.; Joseph, B.O.; Umeokonkwo, C.D.; et al. Trend of Lassa fever cases and factors associated with mortality in Liberia, 2016–2021: A secondary data analysis. Pan Afr. Med. J. 2024, 47, 22. [Google Scholar] [CrossRef] [PubMed]
- Shehu, N.Y.; Gomerep, S.S.; Isa, S.E.; Iraoyah, K.O.; Mafuka, J.; Bitrus, N.; Dachom, M.C.; Ogwuche, J.E.; Onukak, A.E.; Onyedibe, K.I.; et al. Lassa Fever 2016 Outbreak in Plateau State, Nigeria—The Changing Epidemiology and Clinical Presentation. Front. Public Health 2018, 6, 232. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Ayenale, M.; Akhigbe, I.; Akerele, L.O.; Isibor, E.; Idialu, J.; Aideloje, F.O.; Emuebonam, E.; Aire, C.; Adomeh, D.I.; et al. Acute kidney injury and mortality in pediatric Lassa fever versus the question of dialysis. Int. J. Infect. Dis. 2021, 103, 124–131. [Google Scholar] [CrossRef]
- Samuels, R.J.; Moon, T.D.; Starnes, J.R.; Alhasan, F.; Gbakie, M.; Goba, A.; Koroma, V.; Momoh, M.; Sandi, J.D.; Garry, R.F.; et al. Lassa Fever among Children in Eastern Province, Sierra Leone: A 7-year Retrospective Analysis (2012–2018). Am. J. Trop. Med. Hyg. 2021, 104, 585–592. [Google Scholar] [CrossRef]
- Okogbenin, S.; Okoeguale, J.; Akpede, G.; Colubri, A.; Barnes, K.G.; Mehta, S.; Eifediyi, R.; Okogbo, F.; Eigbefoh, J.; Momoh, M.; et al. Retrospective Cohort Study of Lassa Fever in Pregnancy, Southern Nigeria. Emerg. Infect. Dis. 2019, 25, 1494–1500. [Google Scholar] [CrossRef]
- Chika-Igwenyi, N.M.; Harrison, R.E.; Psarra, C.; Gil-Cuesta, J.; Gulamhusein, M.; Onwe, E.O.; Onoh, R.C.; Unigwe, U.S.; Ajayi, N.A.; Nnadozie, U.U.; et al. Early onset of neurological features differentiates two outbreaks of Lassa fever in Ebonyi State, Nigeria, during 2017–2018. PLoS Negl. Trop. Dis. 2021, 15, e0009169. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.E.; Ogah, O.E.; Ojide, C.K.; Odeh, E.C.; Abu, A.M.; Chika-Igwenyi, N.M.; Nwidi, D.U.; Unigwe, U.S.; Ajayi, N.A.; Eke, M.A.S.; et al. Prevalence and outcome of Lassa fever among hospitalized patients in Ebonyi State, Nigeria, 2018–2019. Virus Res. 2020, 285, 198000. [Google Scholar] [CrossRef]
- Ilori, E.A.; Furuse, Y.; Ipadeola, O.B.; Dan-Nwafor, C.C.; Abubakar, A.; Womi-Eteng, O.E.; Ogbaini-Emovon, E.; Okogbenin, S.; Unigwe, U.; Ogah, E.; et al. Epidemiologic and Clinical Features of Lassa Fever Outbreak in Nigeria, January 1–May 6, 2018. Emerg Infect Dis. 2019, 25, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Duvignaud, A.; Jaspard, M.; Etafo, I.C.; Gabillard, D.; Serra, B.; Abejegah, C.; Le Gal, C.; Abidoye, A.T.; Doutchi, M.; Owhin, S.; et al. Lassa fever outcomes and prognostic factors in Nigeria (LASCOPE): A prospective cohort study. Lancet Glob. Health 2021, 9, e469–e478. [Google Scholar] [CrossRef]
- Buba, M.I.; Dalhat, M.M.; Nguku, P.M.; Waziri, N.; Mohammad, J.O.; Bomoi, I.M.; Onyiah, A.P.; Onwujei, J.; Balogun, M.S.; Bashorun, A.T.; et al. Mortality Among Confirmed Lassa Fever Cases During the 2015–2016 Outbreak in Nigeria. Am. J. Public Health 2018, 108, 262–264. [Google Scholar] [CrossRef]
- Ilesanmi, O.S.; Ayodeji, O.O.; Adedosu, N.A.; Ojo, O.E.; Abejegah, C.; Jegede, T.O.; Adebayo, T.; Ayeni, I.; Olatunde, L.; Ahmed, L. Mortality among confirmed Lassa Fever cases in Ondo State, Nigeria, January 2017–March 2019. J. Commun. Health Res. 2022, 11, 5–11. [Google Scholar]
- Kenmoe, S.; Tchatchouang, S.; Ebogo-Belobo, J.T.; Ka’e, A.C.; Mahamat, G.; Guiamdjo Simo, R.E.; Bowo-Ngandji, A.; Demeni Emoh, C.P.; Che, E.; Tchami Ngongang, D.; et al. Systematic review and meta-analysis of the epidemiology of Lassa virus in humans, rodents, and other mammals in sub-Saharan Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008589. [Google Scholar] [CrossRef]
- Doohan, P.; Jorgensen, D.; Naidoo, T.M.; McCain, K.; Hicks, J.T.; McCabe, R.; Bhatia, S.; Charniga, K.; Cuomo-Dannenburg, G.; Hamlet, A.; et al. Lassa fever outbreaks, mathematical models, and disease parameters: A systematic review and meta-analysis. Lancet Glob. Health 2024, 12, e1962–e1972. [Google Scholar] [CrossRef]
- Murphy, H.L.; Ly, H. Pathogenicity and virulence mechanisms of Lassa virus and its animal modeling, diagnostic, prophylactic, and therapeutic developments. Virulence 2021, 12, 2989–3014. [Google Scholar] [CrossRef]
- Kayem, N.D.; Benson, C.; Aye, C.Y.L.; Barker, S.; Tome, M.; Kennedy, S.; Ariana, P.; Horby, P. Lassa fever in pregnancy: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 385–396. [Google Scholar] [CrossRef]
- Horton, L.E.; Cross, R.W.; Hartnett, J.N.; Engel, E.J.; Sakabe, S.; Goba, A.; Momoh, M.; Sandi, J.D.; Geisbert, T.W.; Garry, R.F.; et al. Endotheliopathy and Platelet Dysfunction as Hallmarks of Fatal Lassa Fever. Emerg. Infect. Dis. 2020, 11, 2625. [Google Scholar] [CrossRef]
- Okokhere, P.O.; Bankole, I.A.; Akpede, G.O. Central nervous system manifestations of Lassa fever in Nigeria and the effect on mortality. J. Neurol. Sci. 2013, 333, e604. [Google Scholar] [CrossRef]
- Chime, E.N.; Chime, P.E.; Nwosu, J.N. Hearing Loss in Lassa Fever: A Systematic Review. Open J. Prev. Med. 2022, 12, 239–247. [Google Scholar] [CrossRef]
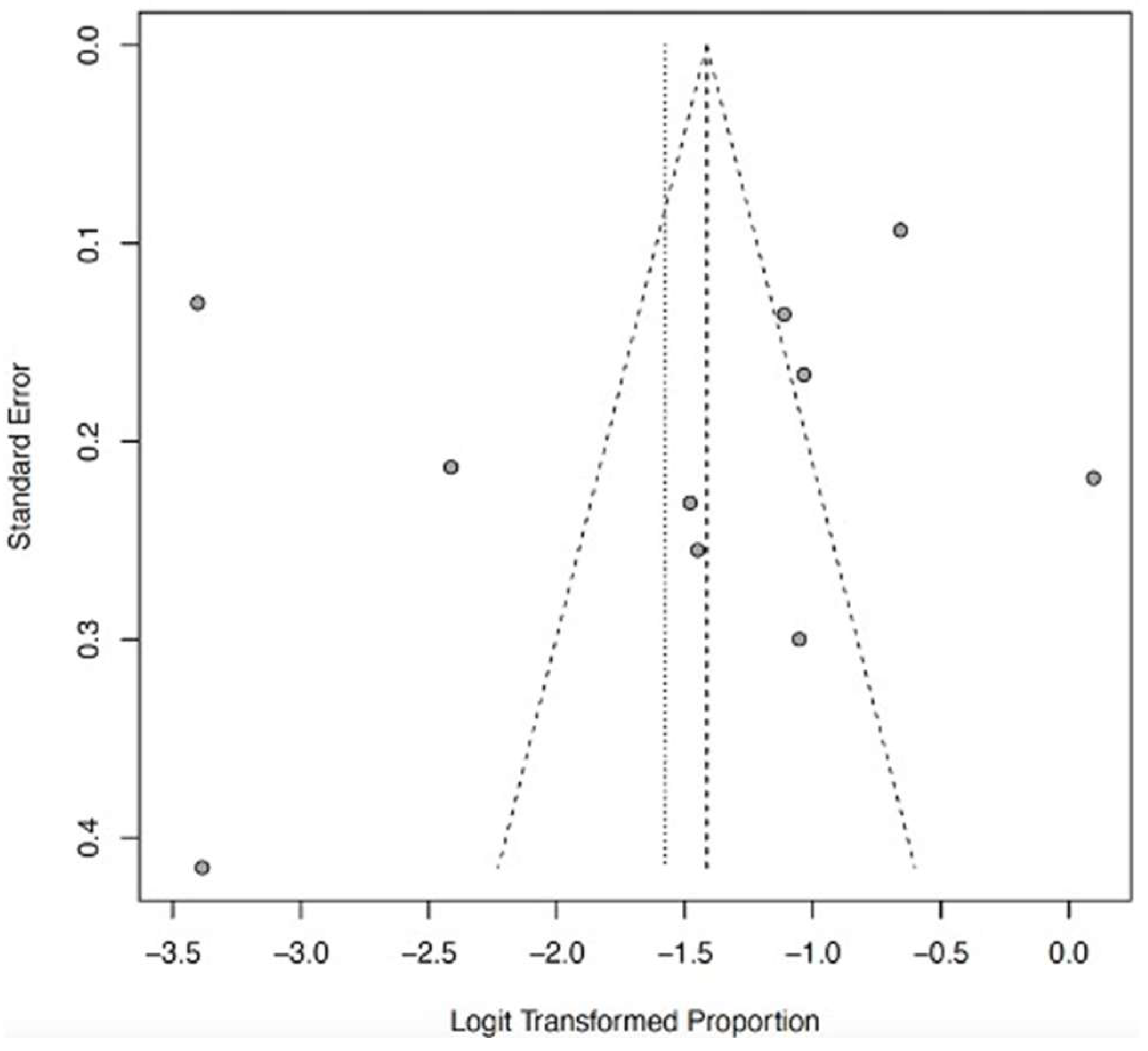
- Debray, T.P.A.; Moons, K.G.M.; Riley, R.D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]





| S/N | First Author’s Name | Publication Year | Title | Study Design | Sample Size | Study Setting | Mean/ Median * Age (Years) | Gender | Country of Study (City) | Quality Assessment Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Duvignaud et al. [17] | 2024 | Presentation and outcomes of Lassa fever in children in Nigeria: a prospective cohort study (LASCOPE) | Prospective cohort | 124 | Rural | - | M = 62 F = 62 | Nigeria (Owo) | 10 |
| 2 | Orji et al. [18] | 2022 | Positivity rate, Predictors, and Outcome of Paediatric Lassa Fever Disease (LFD) in a Lassa Fever Endemic State, Southeast Nigeria | Prospective cohort | 24 | Rural | 53 | M = 9 F = 15 | Nigeria (Abakaliki) | 10 |
| 3 | Saleh et al. [19] | 2020 | Exposure incidents and outcome of Lassa fever virus (LASV) infection among healthcare workers in Nigeria, 2019 | Retrospective cohort | 19 | Rural and urban | 38 * | M = 9 F = 10 | Nigeria | 10 |
| 4 | Abdu et al. [20] | 2022 | Factors affecting outcome in reverse transcriptase-polymerase chain reaction-positive Lassa fever patients with acute kidney injury: a retrospective analysis | Retrospective cohort | 187 | Urban | 37.3 | M = 130 F = 57 | Nigeria (Bauchi) | 10 |
| 5 | Owhin et al. [21] | 2020 | The Impact and Morphology of Anaemia among Lassa Fever Patients Treated in a Dedicated Treatment Centre in South West Nigeria | Retrospective observational | 100 | Rural | 33.9 | M = 54 F = 46 | Nigeria (Owo) | 10 |
| 6 | Akpede et al. [22] | 2019 | Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center’s Experience and Its Implications. | Retrospective cohort | 1594 | Rural | - | - | Nigeria (Irrua) | 10 |
| 7 | Okokhere et al. [23] | 2018 | Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. | Observational cohort | 291 | Rural | 35 | M = 170 F = 121 | Nigeria (Irrua) | 9 |
| 8 | Dahmane et al. [24] | 2014 | Constraints in the diagnosis and treatment of Lassa Fever and the effect on mortality in hospitalized children and women with obstetric conditions in a rural district hospital in Sierra Leone. | Retrospective cohort | 36 | Rural | - | M = 20 F = 16 | Nigeria (Owo) | 10 |
| 9 | Dwalu et al. [25] | 2024 | Trend of Lassa fever cases and factors associated with mortality in Liberia, 2016–2021: a secondary data analysis | Retrospective cohort | 192 | Rural | 21 * | M = 88 F = 104 | Liberia, (Bo district) | 10 |
| 10 | Shehu et al. [26] | 2018 | Lassa fever 2016 outbreak in Plateau State, Nigeria—The changing epidemiology and clinical presentation | Retrospective cohort | 11 | Rural and Urban | 31 * | M = 6 F = 5 | Nigeria (Jos) | 10 |
| 11 | Adetunji et al. [27] | 2021 | Acute kidney injury and mortality in paediatric Lassa fever versus the question of access to dialysis | Retrospective cohort | 58 | Rural | 6.4 | M = 34 F = 24 | Nigeria (Irrua) | 9 |
| 12 | Samuels et al. [28] | 2020 | Lassa Fever among Children in Eastern Province, Sierra Leone: A 7-year Retrospective Analysis (2012–2018). | Retrospective cohort | 57 | Rural | - | M = 36 F = 21 | Sierra Leone (Kenema) | 10 |
| 13 | Okogbenin et al. [29] | 2019 | Retrospective Cohort Study of Lassa Fever in Pregnancy, Southern Nigeria | Retrospective cohort | 30 | Rural | 28.1 | M = 0 F = 30 | Nigeria (Irrua) | 10 |
| 14 | Chika-Igwenyi et al. [30] | 2021 | Early onset of neurological features differentiates two outbreaks of Lassa fever in Ebonyi State, Nigeria, during 2017–2018 | Retrospective cohort | 70 | Rural | 36.3; 35.6 | M = 38; 7 F = 31; 7 | Nigeria (Abakaliki) | 10 |
| 15 | Nwafor et al. [31] | 2020 | Prevalence and outcome of Lassa fever among hospitalized patients in Ebonyi State, Nigeria, 2018–2019 | Retrospective cohort | 113 | Rural | 32.9 | - | Nigeria (Abakaliki) | 10 |
| 16 | Ilori et al. [32] | 2019 | Epidemiologic and Clinical Features of Lassa Fever Outbreak in Nigeria, January 1–May 6, 2018 | Retrospective cohort | 414 | Rural and Urban | 32 * | M = 157 F = 257 | Nigeria | 10 |
| 17 | Duvignaud et al. [33] | 2021 | Lassa fever outcomes and prognostic factors in Nigeria (LASCOPE): a prospective cohort study | Prospective cohort | 534 | Rural | 32 * | M = 258 F = 252 | Nigeria (Owo) | 10 |
| 18 | Buba et al. [34] | 2018 | Mortality Among Confirmed Lassa Fever Cases During the 2015–2016 Outbreak in Nigeria | Retrospective cohort | 47 | Rural | 31.4 | M = 30 F = 17 | Nigeria | 10 |
| 19 | Ilesanmi et al. [35] | 2022 | Mortality among confirmed Lassa Fever cases in Ondo State, Nigeria, January 2017–March 2019: A cross-sectional study | Retrospective cohort | 276 | Rural and Urban | 34 * | - | Nigeria (Owo) | 10 |
| S/N | First Author’s Name | Year of Publication | Title | Clinical Outcomes | |||
|---|---|---|---|---|---|---|---|
| Recovery (%) | Death (%) | Complications | Lost to Follow-Up (%) | ||||
| 1 | Duvignaud et al. 2024 [17] | 2024 | Presentation and outcomes of Lassa fever in children in Nigeria: a prospective cohort study (LASCOPE) | 120 (96.8) | 4 (3.2) | Bleeding, septic shock, respiratory dysfunction, and acute kidney injury | - |
| 2 | Orji et al. [18] | 2022 | Positivity rate, Predictors, and Outcome of Paediatric Lassa fever Disease (LFD) in a Lassa fever Endemic State, Southeast Nigeria | 17 (70.8) | 7 (29.1) | Bleeding, acute kidney injury, and deafness | - |
| 3 | Saleh et al. [19] | 2020 | Exposure incidents and outcome of Lassa fever virus (LASV) infection among healthcare workers in Nigeria, 2019 | 17 (89.4) | 2 (10.6) | - | - |
| 4 | Abdu et al. [20] | 2022 | Factors affecting outcome in reverse transcriptase-polymerase chain reaction-positive Lassa fever patients with acute kidney injury: a retrospective analysis | 134 (70.2) | 57 (29.8) | Bleeding, acute kidney injury, septic shock | - |
| 5 | Owhin et al. [21] | 2020 | The Impact and Morphology of Anaemia among Lassa Fever Patients Treated in a Dedicated Treatment Centre in Southwest Nigeria | 0 (0) | 0 (0) | Anemia, acute kidney injury, bleeding | - |
| 6 | Akpede et al. [22] | 2019 | Caseload and Case Fatality of Lassa Fever in Nigeria, 2001–2018: A Specialist Center’s Experience and Its Implications. | 8695 (96) | 362 (4.0) | - | - |
| 7 | Okokhere et al. [23] | 2018 | Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. | 216 (76) | 68 (24.0) | Anemia, acute kidney injury, bleeding, CNS dysfunction (coma, seizure; irrational talk/behavior, altered sensorium, tremors, and disorientation/confusion, which suggest encephalitis, meningitis, or encephalopathy, dizziness, lethargy, drowsiness) | 7 (2.4) |
| 8 | Dahmane et al. [24] | 2014 | Constraints in the diagnosis and treatment of Lassa Fever and the effect on mortality in hospitalized children and women with obstetric conditions in a rural district hospital in Sierra Leone. | 14 (38.9) | 22 (61.1) | Abnormal bleeding | - |
| 9 | Dwalu et al. [25] | 2024 | Trend of Lassa fever cases and factors associated with mortality in Liberia, 2016–2021: a secondary data analysis | 407 (55.2) | 330 (44.8) | - | - |
| 10 | Shehu et al. [26] | 2018 | Lassa fever 2016 outbreak in Plateau State, Nigeria—The changing epidemiology and clinical presentation | 7 (63.6) | 4 (36.4) | - | - |
| 11 | Adetunji et al. [27] | 2021 | Acute kidney injury and mortality in paediatric Lassa fever versus the question of access to dialysis | 41 (71.9) | 16 (28.1) | Acute kidney injury, abnormal bleeding, encephalopathy, septic shock | 1 (1.7) |
| 12 | Samuels et al. [28] | 2020 | Lassa Fever among Children in Eastern Province, Sierra Leone: A 7-year Retrospective Analysis (2012–2018). | 108 (37) | 184 (63.0) | Abnormal bleeding, CNS dysfunction (confusion or altered sensorium) | - |
| 13 | Okogbenin et al. [29] | 2019 | Retrospective Cohort Study of Lassa Fever in Pregnancy, Southern Nigeria | 19 (63.3) | 11 (36.7) | Intrauterine fetal death (IUFD) | - |
| 14 | Chika-Igwenyi et al. [30] | 2021 | Early onset of neurological features differentiates the two outbreaks of Lassa fever in Ebonyi state, Nigeria, during 2017–2018 | 223 (76.6) | 68 (23.4) | Acute kidney injury | - |
| 15 | Nwafor et al. [31] | 2020 | Prevalence and outcome of Lassa fever among hospitalized patients in Ebonyi State, Nigeria, 2018–2019 | 71 (62.8) | 42 (37.2) | - | - |
| 16 | Ilori et al. [32] | 2019 | Epidemiologic and Clinical Features of Lassa Fever Outbreak in Nigeria, January 1–May 6, 2018 | 317 (74.9) | 106 (25.1) | Abnormal bleeding, CNS dysfunction (myalgia, unconsciousness, disorientation) | - |
| 17 | Duvignaud et al. [33] | 2021 | Lassa fever outcomes and prognostic factors in Nigeria (LASCOPE): a prospective cohort study | 448 (87.8) | 62 (12.2) | Abnormal bleeding, acute kidney injury, CNS dysfunction (seizure, delirium, meningeal syndrome, focal deficiency, aphasia) | - |
| 18 | Buba et al. [34] | 2018 | Mortality Among Confirmed Lassa Fever Cases During the 2015–2016 Outbreak in Nigeria | 19 (40.4) | 28 (59.6) | - | - |
| 19 | Ilesanmi et al. [35] | 2022 | Mortality among confirmed Lassa Fever cases in Ondo State, Nigeria, January 2017–March 2019: A cross-sectional study | 246 (89.1) | 30 (10.9) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okwuraiwe, A.P.; Onyeaghala, C.A.; Ozoude, O.T.; Suleiman, M.O.; Abdu-Aguye, S.N.; Ezekwelu, N.J.; Oyeniyi, T.A.; Jegede, A.O.; Egwudo, A.E.; Okeke, O.P.; et al. Clinical Outcomes of Severe Lassa Fever in West Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2025, 22, 1504. https://doi.org/10.3390/ijerph22101504
Okwuraiwe AP, Onyeaghala CA, Ozoude OT, Suleiman MO, Abdu-Aguye SN, Ezekwelu NJ, Oyeniyi TA, Jegede AO, Egwudo AE, Okeke OP, et al. Clinical Outcomes of Severe Lassa Fever in West Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2025; 22(10):1504. https://doi.org/10.3390/ijerph22101504
Chicago/Turabian StyleOkwuraiwe, Azuka Patrick, Chizaram Anselm Onyeaghala, Obiageli Theresa Ozoude, Muritala Odidi Suleiman, Samirah Nndwan Abdu-Aguye, Nkolika Jacinta Ezekwelu, Tolulope Amos Oyeniyi, Ayodapo Oluwadare Jegede, Adaeze Elfrida Egwudo, Oluchukwu Perpetual Okeke, and et al. 2025. "Clinical Outcomes of Severe Lassa Fever in West Africa: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 22, no. 10: 1504. https://doi.org/10.3390/ijerph22101504
APA StyleOkwuraiwe, A. P., Onyeaghala, C. A., Ozoude, O. T., Suleiman, M. O., Abdu-Aguye, S. N., Ezekwelu, N. J., Oyeniyi, T. A., Jegede, A. O., Egwudo, A. E., Okeke, O. P., Abodunrin, O. R., Akinsolu, F. T., & Sobande, O. O. (2025). Clinical Outcomes of Severe Lassa Fever in West Africa: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 22(10), 1504. https://doi.org/10.3390/ijerph22101504







