Drug-Resistant Tuberculosis in Rural Eastern Cape, South Africa: A Study of Patients’ Characteristics in Selected Healthcare Facilities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Data Collection Methods
2.4. Data Analysis Methods
2.5. Definition of Terms
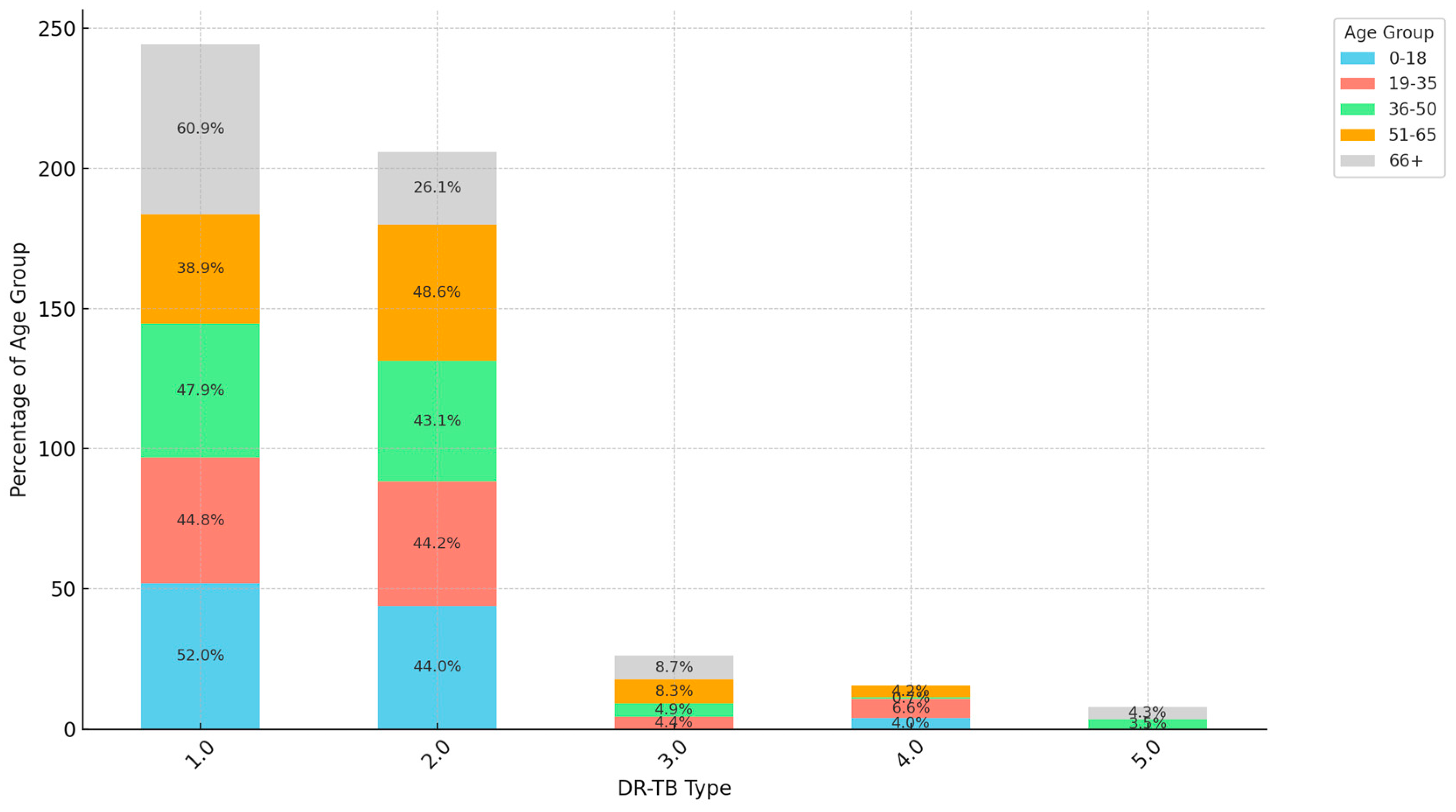
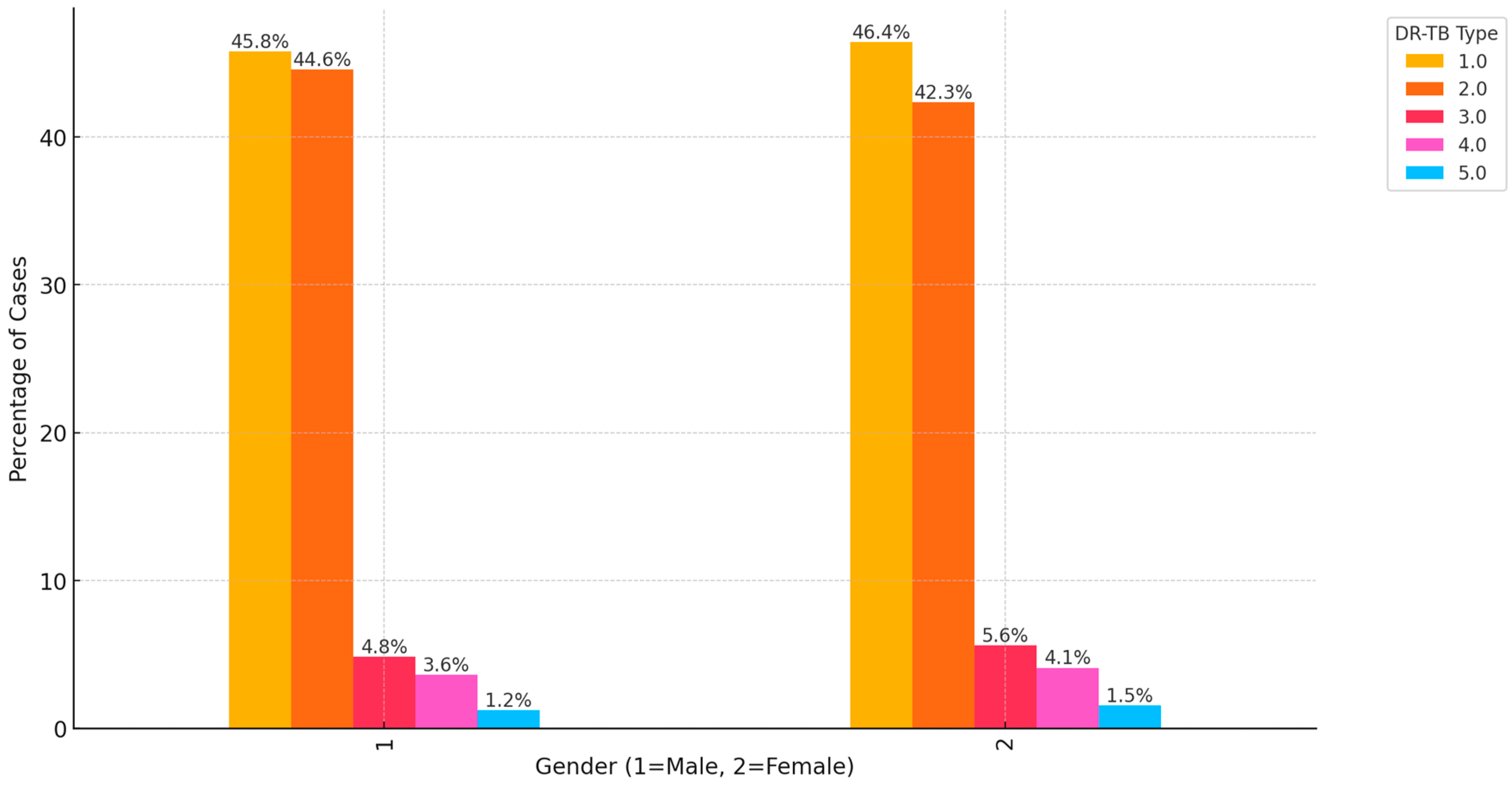
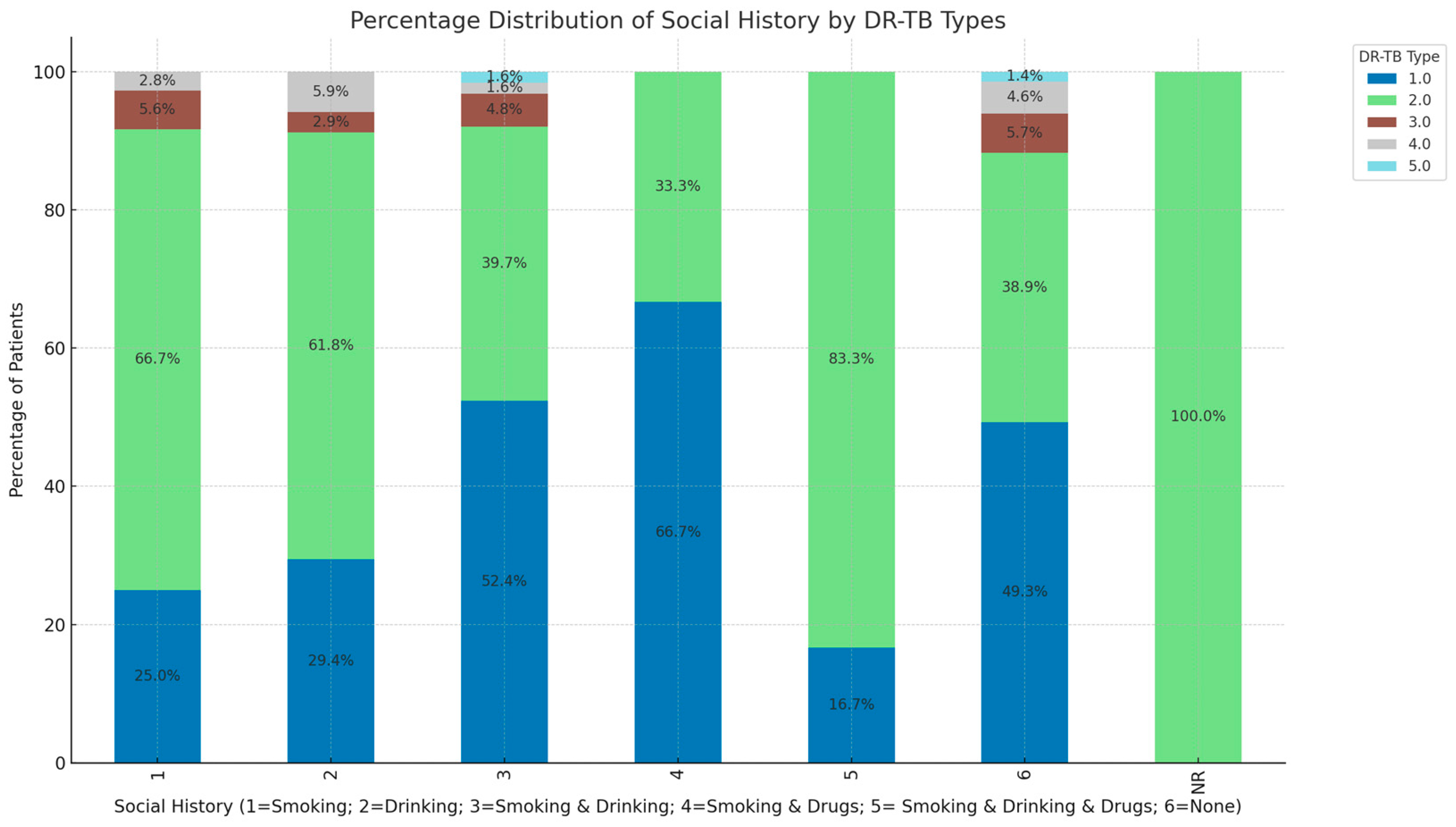
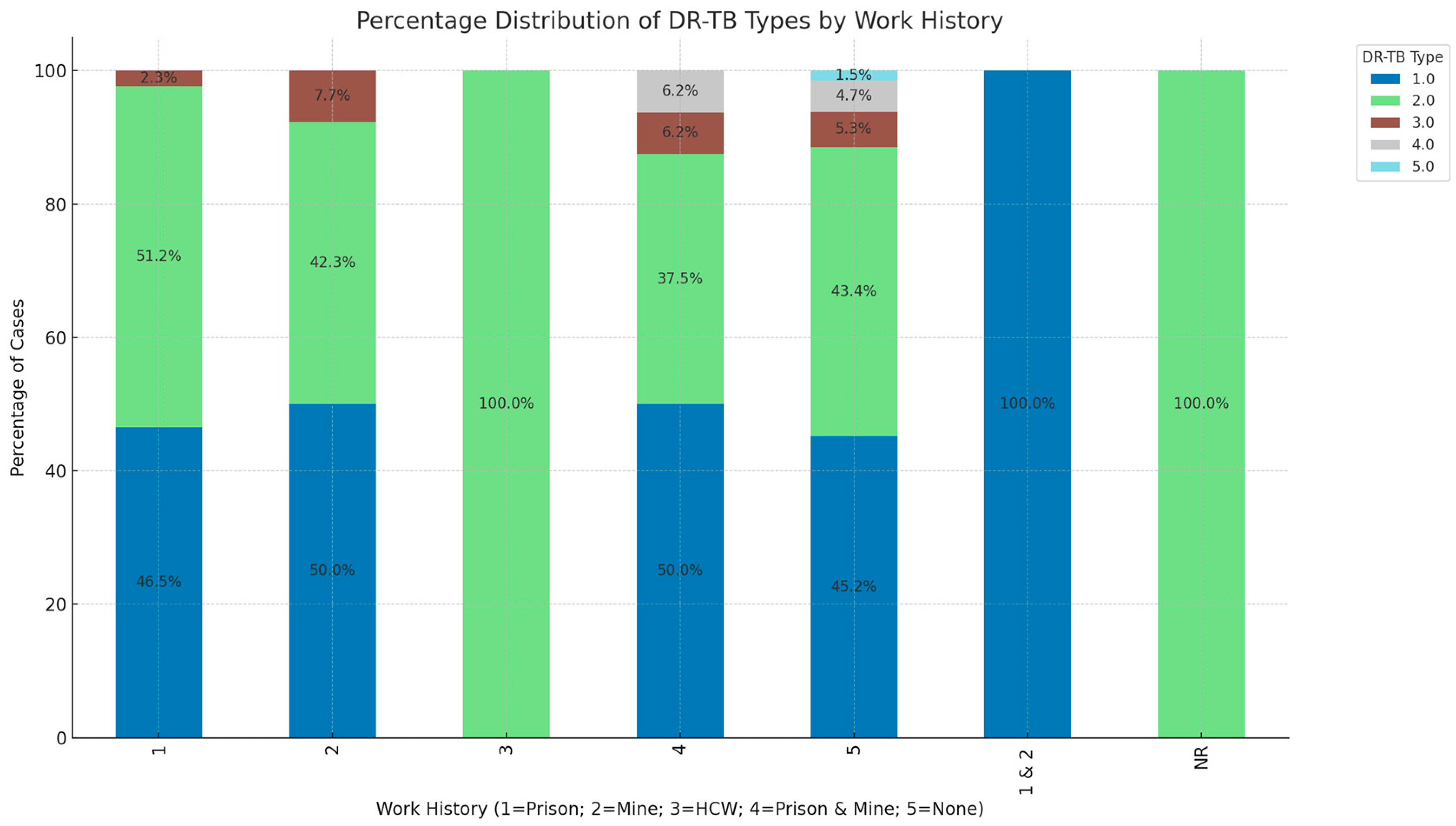
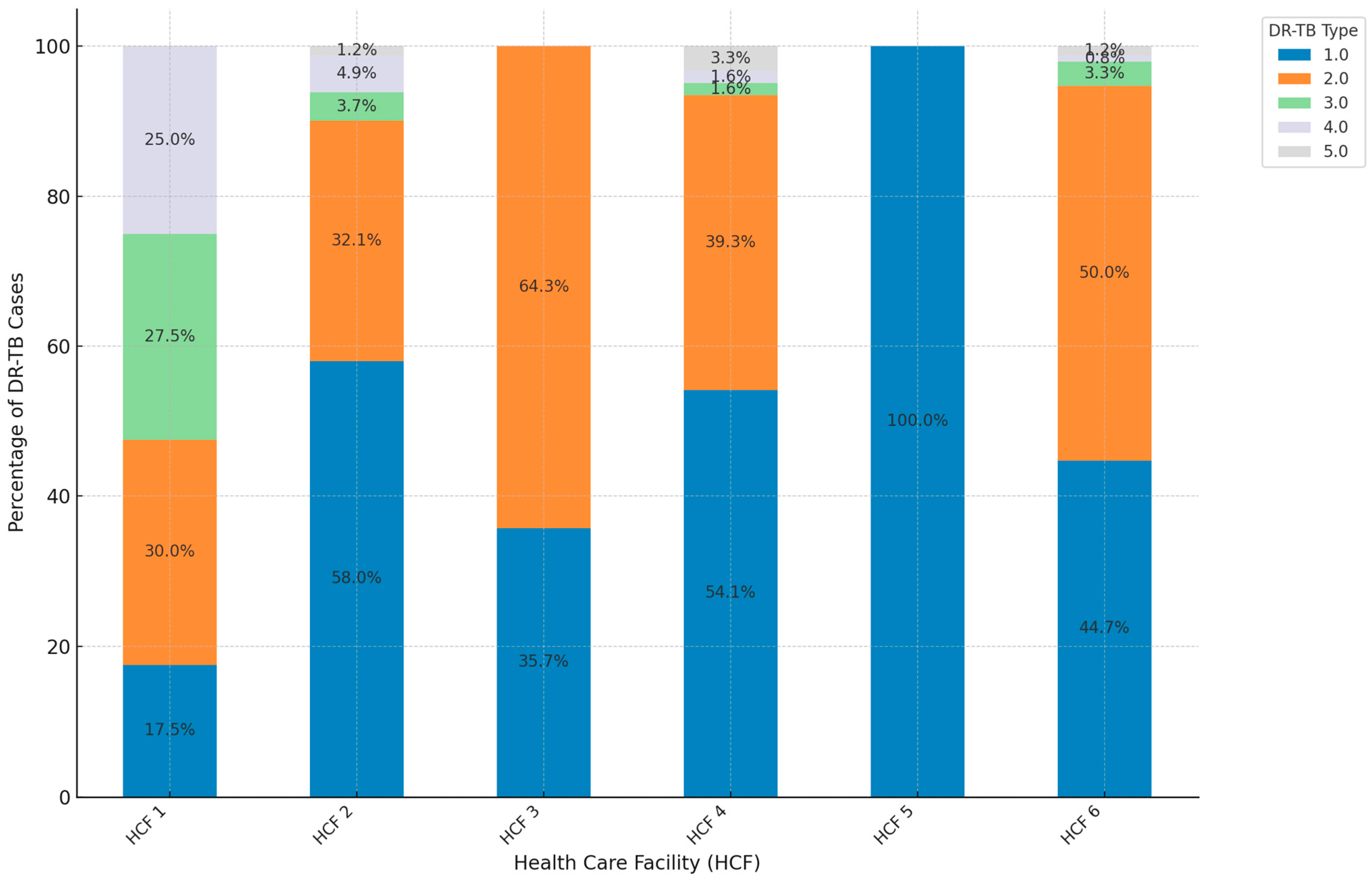
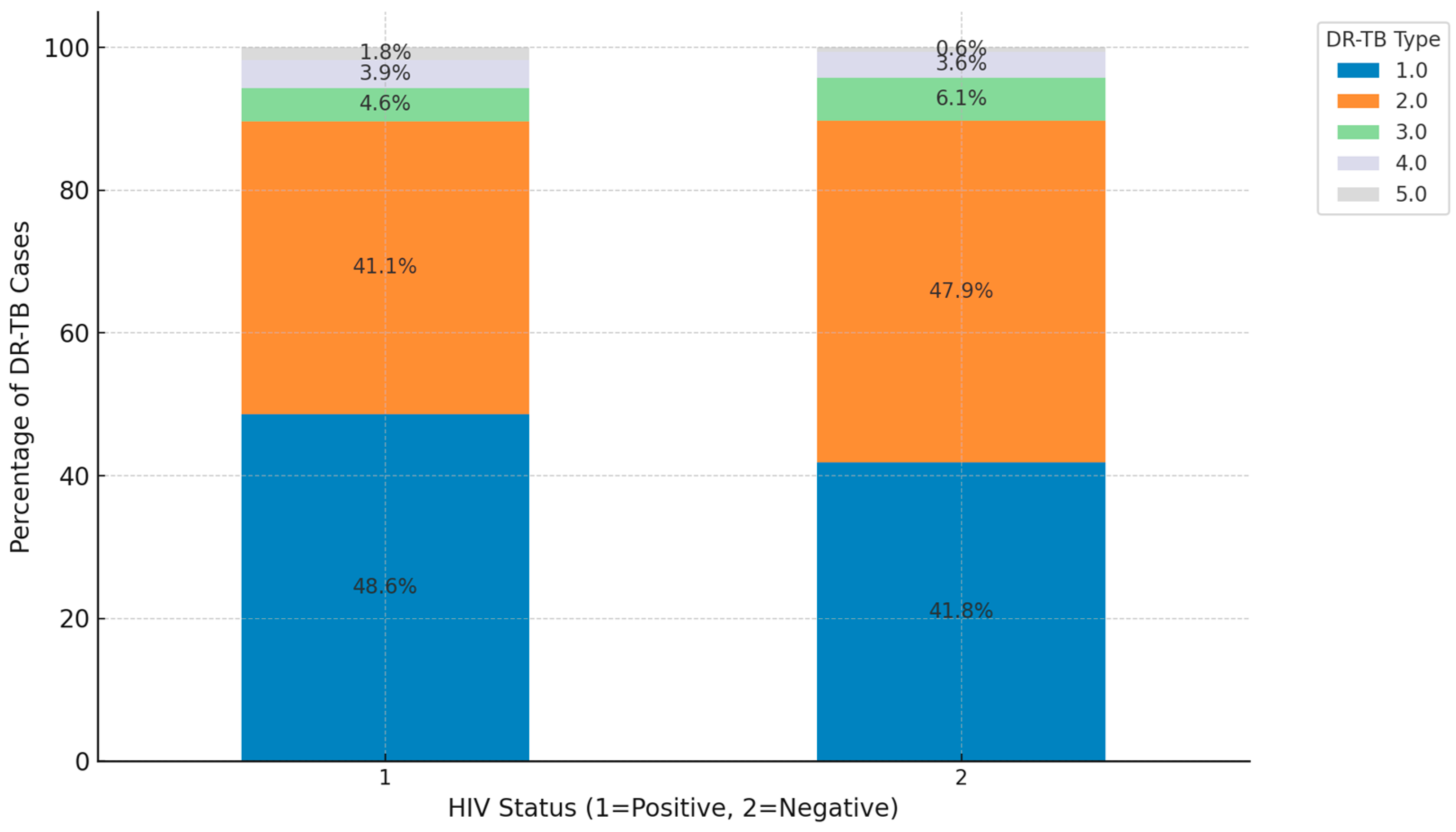
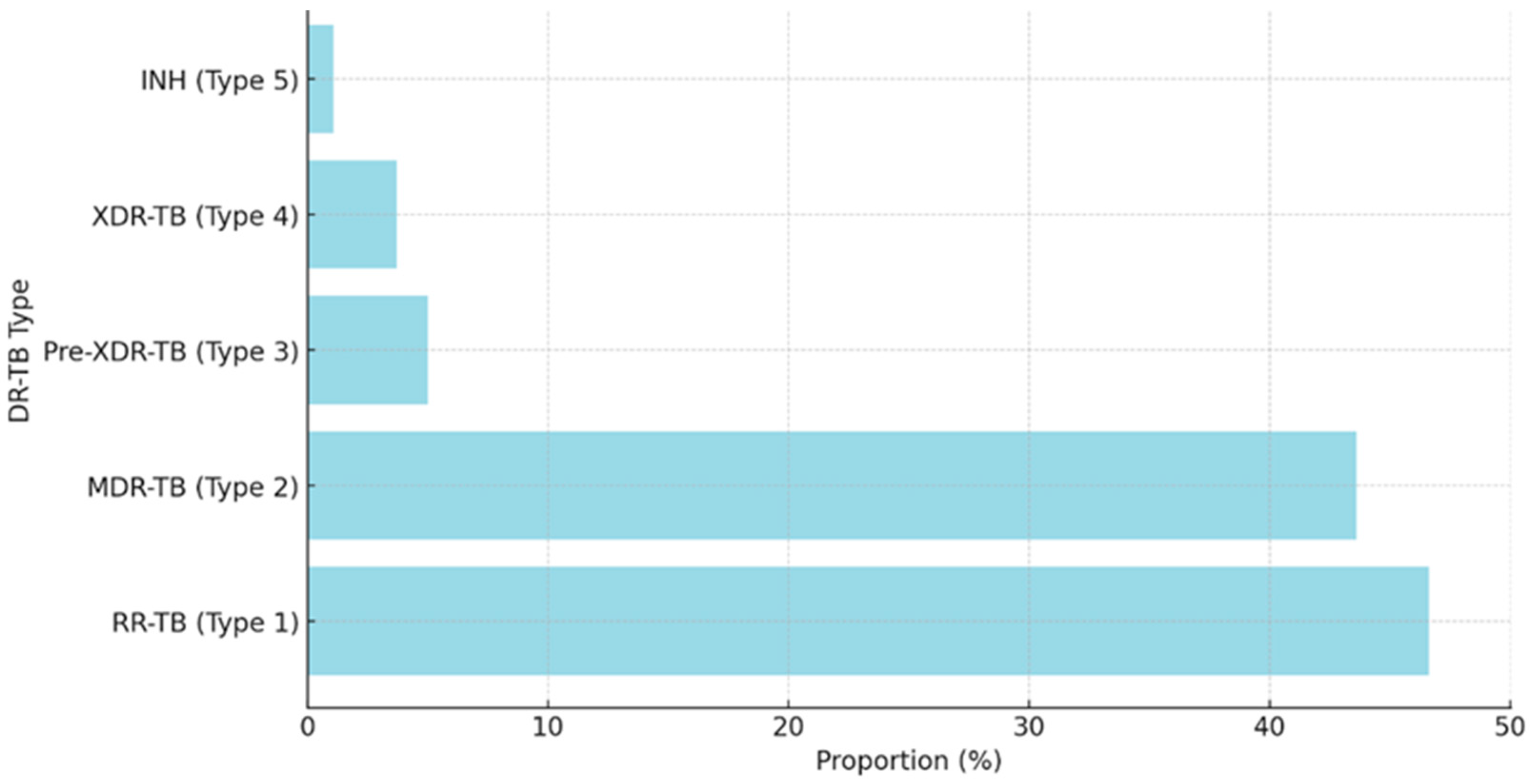
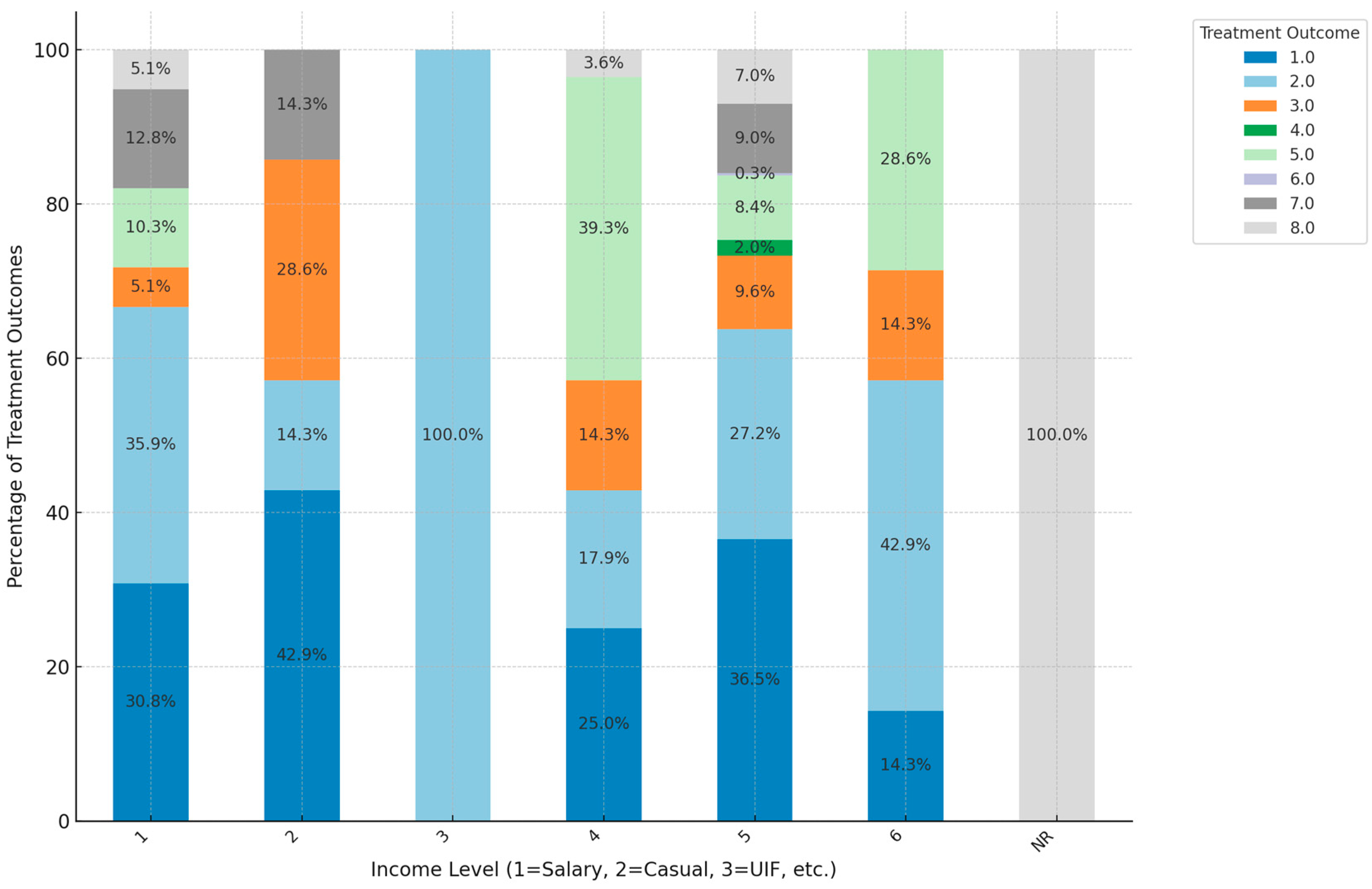
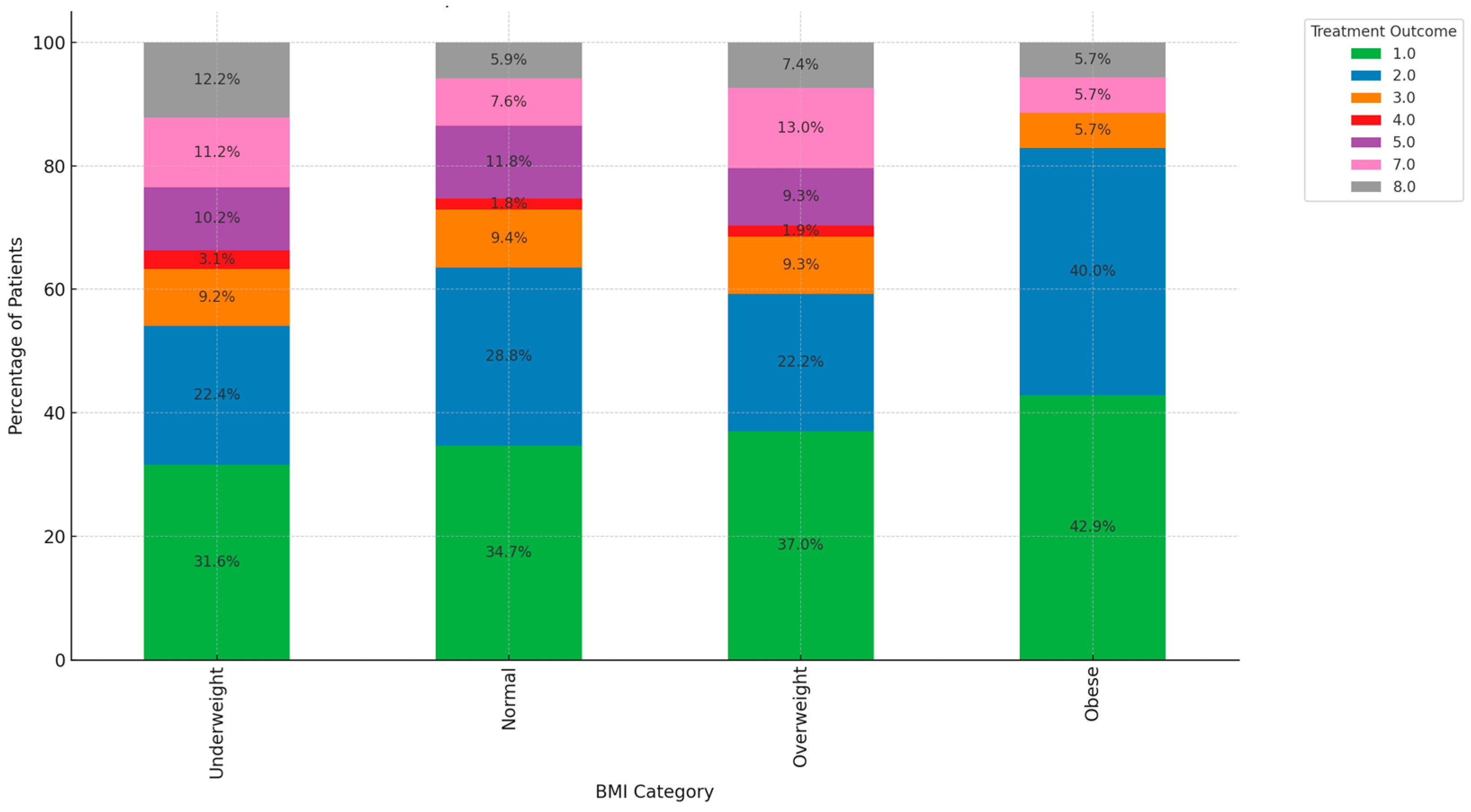
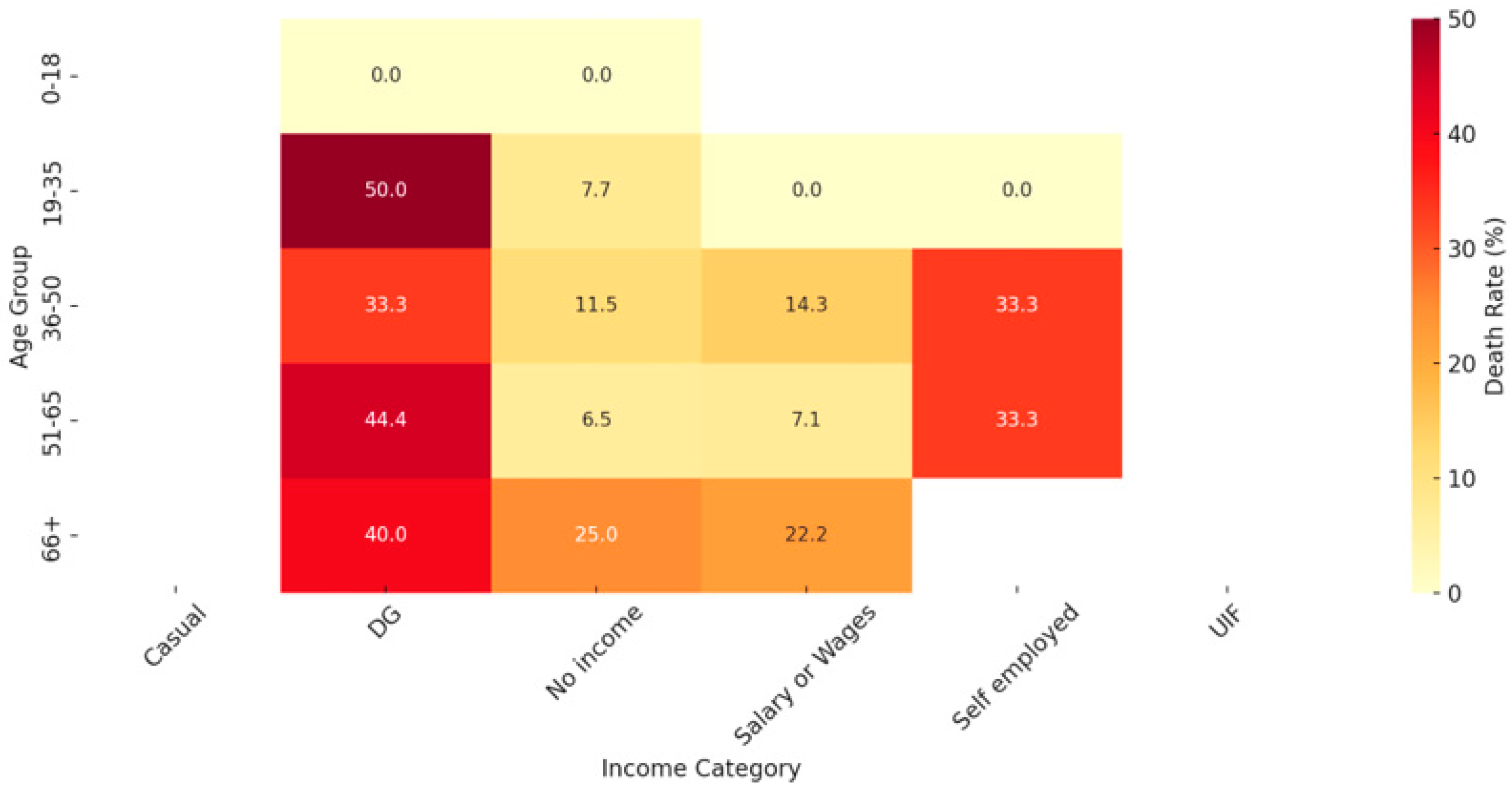
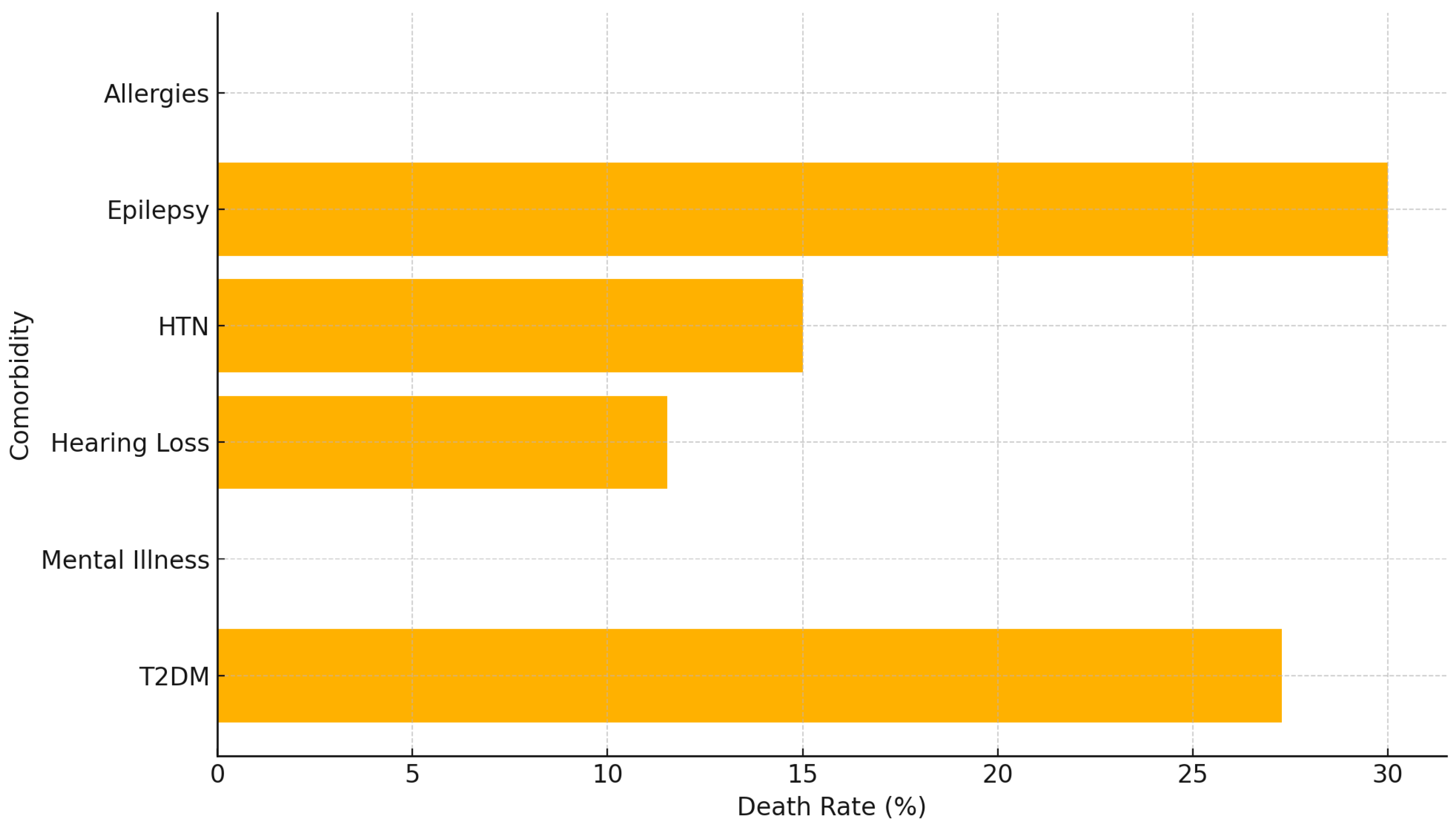
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 13 September 2024).
- Seloma, N.M.; Makgatho, M.E.; Maimela, E. Evaluation of drug-resistant tuberculosis treatment outcome in Limpopo province, South Africa. Afr. J. Prim. Health Care Fam. Med. 2023, 15, 3764. [Google Scholar] [CrossRef] [PubMed]
- The National Department of Health. The National Strategic Plan for HIV, TB, and STIs 2023-2028. Available online: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/NSP-HIV-TB-STIs-2023-2028-MARCH20_23-PRINT2.pdf (accessed on 15 August 2024).
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections: A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 74. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; De Gaetano, S.; Ponzo, E.; Biondo, C. Tackling drug-resistant tuberculosis: New challenges from the old pathogen Mycobacterium tuberculosis. Microorganisms 2023, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Shriwastav, U.K. Disseminated tuberculosis presenting as venous thromboembolism. J. Clin. Images Med. Case Rep. 2023, 4, e35575. [Google Scholar] [CrossRef]
- Suárez, I.; Fünger, S.M.; Kröger, S.; Rademacher, J.; Fätkenheuer, G.; Rybniker, J. The diagnosis and treatment of tuberculosis. Dtsch. Arztebl. Int. 2019, 116, 749. [Google Scholar] [CrossRef] [PubMed]
- Swalehe, H.M.; Obeagu, E.I. Tuberculosis: Current diagnosis and management. Elite J. Public Health 2024, 2, 23–33. [Google Scholar]
- Daftary, A.; Mondal, S.; Zelnick, J.; Friedland, G.; Seepamore, B.; Boodhram, R.; Amico, K.R.; Padayatchi, N.; O’Donnell, M.R. Dynamic needs and challenges of people with drug-resistant tuberculosis and HIV in South Africa: A qualitative study. Lancet Glob. Health 2021, 9, e479–e488. [Google Scholar] [CrossRef]
- Ismail, N.A.; Mvusi, L.; Nanoo, A.; Dreyer, A.; Omar, S.V.; Babatunde, S.; Molebatsi, T.; van der Walt, M.; Adelekan, A.; Deyde, V.; et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect. Dis. 2018, 18, 779–787. [Google Scholar] [CrossRef]
- National Institute of Communicable Diseases (NICD). Introduction of New Drugs and Drug Regimens for the Management of Drug-Resistant Tuberculosis in South Africa: Policy Framework. 2015. Available online: https://www.nicd.ac.za/assets/files/Introduction%20of%20new%20drugs%20and%20drug%20regimens%20for%20the%20management%20of%20drug%20resistant%20TB%20in%20SA%20-%202015.pdf (accessed on 15 August 2024).
- Oga-Omenka, C.; Tseja-Akinrin, A.; Sen, P.; Mac-Seing, M.; Agbaje, A.; Menzies, D.; Zarowsky, C. Factors influencing diagnosis and treatment initiation for multidrug-resistant/rifampicin-resistant tuberculosis in six sub-Saharan African countries: A mixed-methods systematic review. BMJ Glob. Health 2020, 5, e002280. [Google Scholar] [CrossRef]
- Chanda, E. The clinical profile and outcomes of drug-resistant tuberculosis in Central Province of Zambia. BMC Infect. Dis. 2024, 24, 364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dickson, L.; Le Roux, S.R.; Mitrani, L.; Hill, J.; Jassat, W.; Cox, H.; Mlisana, K.; Black, J.; Loveday, M.; Grant, A.; et al. Organisation of care for people receiving drug-resistant tuberculosis treatment in South Africa: A mixed methods study. BMJ Open 2023, 17, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organisation. Global Tuberculosis Report 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 13 September 2024).
- Cox, V.; McKenna, L.; Acquah, R.; Reuter, A.; Wasserman, S.; Vambe, D.; Ustero, P.; Udwadia, Z.; Trivino-Duran, L.; Tommasi, M.; et al. Clinical perspectives on treatment of rifampicin-resistant/multidrug-resistant TB. Int. J. Tuberc. Lung Dis. 2020, 24, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Kielmann, K.; Vidal, N.; Riekstina, V.; Krutikov, M.; van der Werf, M.J.; Biraua, E.; Duric, P.; Moore, D.A.J. “Treatment is of primary importance, and social assistance is secondary”: A qualitative study on the organisation of tuberculosis (TB) care and patients’ experience of starting and staying on TB treatment in Riga, Latvia. PLoS ONE 2018, 13, e0203937. [Google Scholar] [CrossRef] [PubMed]
- Dhande, V.S.; Mankar, A.V.; Dahake, S.W.; Navare, V. Clinico-epidemiological profile and adherence to antitubercular treatment of tuberculosis patients in a city of Maharashtra. Int. J. Community Med. Public Health 2020, 7, 3644–3648. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Worku, T.; Gamtesa, D.F.; Alebel, A. Predictors of mortality in patients with drug-resistant tuberculosis: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0253848. [Google Scholar] [CrossRef]
- Stuckler, D.; Basu, S.; McKee, M.; Lurie, M. Mining and risk of tuberculosis in sub-Saharan Africa. Am. J. Public Health 2011, 101, 524–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basu, S.; Stuckler, D.; McKee, M. The Health Impacts of the Economic Crisis in the European Union. Eur. J. Public Health 2019, 29, 390–391. [Google Scholar] [CrossRef]
- Appiah, M.A.; Arthur, J.A.; Asampong, E.; Kamau, E.M.; Gborgblorvor, D.; Solaga, P.; Dako-Gyeke, P. Health service providers’ perspective on barriers and strategies to tuberculosis treatment adherence in Obuasi Municipal and Obuasi East District in the Ashanti region, Ghana: A qualitative study. Discov. Health Syst. 2024, 3, 19. [Google Scholar] [CrossRef]
- Nidoi, J.; Muttamba, W.; Walusimbi, S.; Imoko, J.F.; Lochoro, P.; Ictho, J.; Mugenyi, L.; Sekibira, R.; Turyahabwe, S.; Byaruhanga, R.; et al. Impact of socio-economic factors on tuberculosis treatment outcomes in north-eastern Uganda: A mixed methods study. BMC Public Health 2021, 21, 2167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nyasulu, P.S.; Ngasama, E.; Tamuzi, J.L.; Sigwadhi, L.N.; Ozougwu, L.U.; Nhandara, R.B.C.; Ayele, B.T.; Umanah, T.; Ncayiyana, J. Effect of HIV status and antiretroviral treatment on treatment outcomes of tuberculosis patients in a rural primary healthcare clinic in South Africa. PLoS ONE 2022, 17, e0274549. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS Fact Sheet. Tuberculosis and HIV. Available online: https://www.unaids.org/sites/default/files/media_asset/tb-and-hiv_en.pdf (accessed on 16 June 2024).
- Parsons, L.M.; Somoskövi, Á.; Gutierrez, C.; Lee, E.; Paramasivan, C.N.; Abimiku, A.; Spector, S.; Roscigno, G.; Nkengasong, J. Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin. Microbiol. Rev. 2011, 24, 314–350. [Google Scholar] [CrossRef] [PubMed]
- Dlatu, N.; Longo-Mbenza, B.; Apalata, T. Predictors of tuberculosis incidence and the effects of multiple deprivation indices on tuberculosis management in OR Tambo district over a 5-year period. PLoS ONE 2022, 17, e0264811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colebunders, R.; Apers, L.; Dieltiens, G.; Worodria, W. Tuberculosis in resource poor countries. BMJ 2007, 334, 105–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Marme, G.; Rutherford, S.; Harris, N. What tuberculosis infection control measures are effective in resource-constrained primary healthcare facilities? A systematic review of the literature. Rural Remote Health 2023, 23, 7175. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.M.; Hosu, M.C.; Iruedo, J.; Vasaikar, S.; Nokoyo, K.A.; Tsuro, U.; Apalata, T. Treatment outcomes and associated factors among tuberculosis patients from selected rural Eastern Cape hospitals: An ambidirectional study. Trop. Med. Infect. Dis. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paleckyte, A.; Dissanayake, O.; Mpagama, S.; Lipman, M.C.; McHugh, T.D. Reducing the risk of tuberculosis transmission for HCWs in high incidence settings. Antimicrob. Resist. Infect. Control 2021, 10, 106. [Google Scholar] [CrossRef]
- Atkins, S.; Heimo, L.; Carter, D.; Closa, M.R.; Vanleeuw, L.; Chenciner, L.; Wambi, P.; Sidney-Annerstedt, K.; Egere, U.; Verkuijl, S.; et al. The socioeconomic impact of tuberculosis on children and adolescents: A scoping review and conceptual framework. BMC Public Health 2022, 22, 2153. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, Z.; Li, X.; Xia, D.; Ma, J.; Dong, Y.; Zhang, X. Treatment outcomes and factors affecting unsuccessful outcome among new pulmonary smear positive and negative tuberculosis patients in Anqing, China: A retrospective study. BMC Infect. Dis. 2018, 18, 104. [Google Scholar] [CrossRef]
- Agazhu, H.W.; Assefa, Z.M.; Beshir, M.T.; Tadesse, H.; Mengstie, A.S. Treatment outcomes and associated factors among tuberculosis patients attending Gurage Zone Public Hospital, Southern Nations, Nationalities, and People’s Region, Ethiopia: An institution-based cross-sectional study. Front. Med. 2023, 10, 1105911. [Google Scholar] [CrossRef]
- Oyageshio, O.P.; Myrick, J.W.; Saayman, J.; van der Westhuizen, L.; Al-Hindi, D.R.; Reynolds, A.W.; Zaitlen, N.; Hoal, E.G.; Uren, C.; Möller, M.; et al. Strong effect of demographic changes on tuberculosis susceptibility in South Africa. PLoS Glob. Public Health 2024, 4, e0002643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, J.; Liu, Z.D.; Feng, Y.P.; Luo, S.R.; Jiang, Q.M. Assessment of effective anti-TB regimens and adverse outcomes related risk factors in the elderly and senile-aged TB patients. Infect. Drug Resist. 2023, 16, 3903–3915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shibabaw, A.; Gelaw, B.; Gebreyes, W.; Robinson, R.; Wang, S.H.; Tessema, B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS ONE 2020, 15, e0229040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Utpat, K.V.; Rajpurohit, R.; Desai, U. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among extra pulmonary (EP) multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai in pre Bedaquiline (BDQ) era. Lung India 2023, 40, 19–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adisa, R.; Ayandokun, T.T.; Ige, O.M. Knowledge about tuberculosis, treatment adherence and outcome among ambulatory patients with drug-sensitive tuberculosis in two directly-observed treatment centres in Southwest Nigeria. BMC Public Health 2021, 21, 677. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.L.; Oladimeji, K.E.; Goon, D.T. Socio-economic drivers of drug-resistant tuberculosis in Africa: A scoping review. BMC Public Health 2021, 21, 488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kootbodien, T.; Monyane, M.; Mothibe, N. The impact of socio-economic factors on tuberculosis treatment outcomes in South Africa: A retrospective cohort study. BMC Public Health 2018, 18, 1234. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, X.; Zhang, X.; Bai, J.; You, S.; Li, X.; Li, S.; Wang, Y.; Zhang, W.; Xu, Y. Global prevalence and burden of multidrug-resistant tuberculosis from 1990 to 2019. BMC Infect Dis. 2024, 24, 243. [Google Scholar] [CrossRef]
- Said, H.; Kachingwe, E.; Gardee, Y.; Bhyat, Z.; Ratabane, J.; Erasmus, L.; Lebaka, T.; van der Meulen, M.; Gwala, T.; Omar, S.; et al. Determining the risk-factors for molecular clustering of drug-resistant tuberculosis in South Africa. BMC Public Health 2023, 23, 2329. [Google Scholar] [CrossRef]
- Ataguba, J.E.; Akazili, J.; McIntyre, D. Socioeconomic-related health inequality in South Africa: Evidence from General Household Surveys. Int. J. Equity Health 2011, 10, 48. [Google Scholar] [CrossRef]
- Tadokera, R.; Bekker, L.G.; Kreiswirth, B.N.; Mathema, B.; Middelkoop, K. TB transmission is associated with prolonged stay in a low socio-economic, high burdened TB and HIV community in Cape Town, South Africa. BMC Infect. Dis. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cramm, J.M.; Koolman, X.; Møller, V.; Nieboer, A.P. Socio-economic status and self-reported tuberculosis: A multilevel analysis in a low-income township in the Eastern Cape, South Africa. J. Public Health Afr. 2011, 2, e34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, R.A.; Marconi, V.C.; Gandhi, R.T.; Kuritzkes, D.R.; Sunpath, H. Coadministration of lopinavir/ritonavir and rifampicin in HIV and tuberculosis co-infected adults in South Africa. PLoS ONE 2012, 7, e44793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adamu, A.L.; Aliyu, M.H.; Galadanci, N.A.; Musa, B.M.; Lawan, U.M.; Bashir, U.; Abubakar, I. The impact of rural residence and HIV infection on poor tuberculosis treatment outcomes in a large urban hospital: A retrospective cohort analysis. Int. J. Equity Health 2018, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Filha, N.T.; Li, J.; Phillips-Howard, P.A.; Quayyum, Z.; Kibuchi, E.; Mithu, M.I.H.; Vidyasagaran, A.; Sai, V.; Manzoor, F.; Karuga, R.; et al. The economics of healthcare access: A scoping review on the economic impact of healthcare access for vulnerable urban populations in low- and middle-income countries. Int. J. Equity Health 2022, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Harling, G.; Lima Neto, A.S.; Sousa, G.S.; Machado, M.M.T.; Castro, M.C. Determinants of tuberculosis transmission and treatment abandonment in Fortaleza, Brazil. BMC Public Health 2017, 17, 508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wingfield, T.; Tovar, M.A.; Huff, D.; Boccia, D.; Saunders, M.J.; Datta, S.; Montoya, R.; Ramos, E.; Lewis, J.J.; Gilman, R.H.; et al. Beyond pills and tests: Addressing the social determinants of tuberculosis. Clin. Med. 2016, 16 (Suppl. S6), s79–s91. [Google Scholar] [CrossRef]
- Roy, S.C.; Khanam, P.A.; Ahmed, B.N.; Islam, M.A.; Huq, A.F.; Saha, M.R. Assessment of treatment outcomes of multidrug-resistant tuberculosis in Bangladeshi population: A retrospective cohort study. J. Armed Forces Med. Coll. Bangladesh 2022, 18, 57–60. [Google Scholar] [CrossRef]
- Sy, K.T.L.; Leavitt, S.V.; Vos Md Dolby, T.; Bor, J.; Horsburgh, C.R.; Warren, R.M.; Streicher, E.M.; Jenkins, H.E.; Jacobson, K.R. Spatial heterogeneity of extensively drug resistant-tuberculosis in western cape province, South Africa. Sci. Rep. 2022, 12, 10844. [Google Scholar] [CrossRef]
- Vu, T.V. Economic complexity and health outcomes: A global perspective. Soc. Sci. Med. 2020, 265, 113480. [Google Scholar] [CrossRef]
- Schneider, M.; Hlophe, H. The impact of social grants on health outcomes in South Africa. S. Afr. J. Public Health 2016, 1, 12–20. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Rahman, A. Socio-economic determinants of tuberculosis treatment adherence: A cross-sectional study in urban settings. Int. J. Tuberc. Lung Dis. 2021, 25, 567–574. [Google Scholar] [CrossRef]
- Bouza, E.; Arribas, J.R.; Alejos, B.; Bernardino, J.I.; Coiras, M.; Coll, P.; Del Romero, J.; Fuster, M.J.; Górgolas, M.; Gutiérrez, A.; et al. Past and future of HIV infection. A document based on expert opinion. Rev. Esp. Quimioter. 2021, 35, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.; van Rensburg, A.J.; Engelbrecht, M.; Buffel, V.; Campbell, L.; Sommerland, N.; Rau, A.; Kigozi, G.; van Olmen, J.; Masquillier, C. How the HIV/TB co-epidemic, HIV stigma, and TB stigma syndemic impacts on the use of occupational health services for TB in South African hospitals: A structural equation modelling analysis of the baseline data from the HaTSaH Study (cluster RCT). BMJ Open 2022, 12, e045477. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; van Schalkwyk, C.; Naidoo, P.; Seddon, J.A.; Dunbar, R.; Dlamini, S.S.; Welte, A.; Hesseling, A.C.; Claassens, M.M. Mortality during tuberculosis treatment in South Africa using an 8-year analysis of the national tuberculosis treatment register. Sci. Rep. 2021, 11, 15894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, J.; Kim, J.S.; Kim, H.W.; Ko, Y.; Oh, J.Y.; Jeong, Y.-J.; Lee, E.H.; Yang, B.; Lee, K.M.; Ahn, J.H.; et al. Effects of underweight and overweight on mortality in patients with pulmonary tuberculosis. Front. Public Health 2023, 11, 1236099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peinado, J.; Lecca, L.; Jiménez, J.; Calderón, R.; Yataco, R.; Becerrra, M.; Murray, M. Association between overweight/obesity and multidrug-resistant tuberculosis. Rev. Peru. Med. Exp. Salud Publica 2023, 40, 59–66. [Google Scholar] [CrossRef]
- Diallo, A.; Diallo, B.D.; Camara, L.M.; Kounoudji, L.A.N.; Bah, B.; N’zabintawali, F.; Carlos-Bolumbu, M.; Diallo, M.H.; Sow, O.Y. Different profiles of body mass index variation among patients with multidrug-resistant tuberculosis: A retrospective cohort study. BMC Infect. Dis. 2020, 20, 315. [Google Scholar] [CrossRef]
- Limenh, L.W.; Kasahun, A.E.; Sendekie, A.K.; Seid, A.M.; Mitku, M.L.; Fenta, E.T.; Workye, M.; Simegn, W.; Ayenew, W. Tuberculosis treatment outcomes and associated factors among tuberculosis patients treated at healthcare facilities of Motta Town, Northwest Ethiopia: A five-year retrospective study. Sci. Rep. 2024, 14, 7695. [Google Scholar] [CrossRef]
- Cáceres, G.; Calderon, R.; Ugarte-Gil, C. Tuberculosis and comorbidities: Treatment challenges in patients with comorbid diabetes mellitus and depression. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Starshinova, A.; Nazarenko, M.; Belyaeva, E.; Chuzhov, A.; Osipov, N.; Kudlay, D. Assessment of comorbidity in patients with drug-resistant tuberculosis. Pathogens 2023, 12, 1394. [Google Scholar] [CrossRef]
- Seegert, A.B.; Patsche, C.B.; Sifna, A.; Gomes, V.F.; Wejse, C.; Storgaard, M.; Rudolf, F. Hypertension is associated with increased mortality in patients with tuberculosis in Guinea-Bissau. Int. J. Infect. Dis. 2021, 109, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Andriani, N.D.; Yuliani, R.D. Meta-analysis: Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in tuberculosis patients. J. Health Promot. Behav. 2021, 6, 233–249. [Google Scholar] [CrossRef]
- Elduma, A.H.; Mansournia, M.A.; Foroushani, A.R.; Ali, H.M.H.; Elegail, A.M.A.S.; Elsony, A.A.; Holakouie-Naieni, K. Assessment of the risk factors associated with multidrug-resistant tuberculosis in Sudan: A case-control study. Epidemiol. Health 2019, 41, e2019014. [Google Scholar] [CrossRef]
- Habibi, M.R.; Bakhtiar, A.; Indiastuti, D.N.; Meliana, R.Y. Diabetes mellitus and history of tuberculosis treatment as risk factors of developing multidrug-resistant tuberculosis at TB Polyclinic Dr. Soetomo General Hospital 2019-2020. J. Ilm. Univ. Batanghari Jambi 2022, 22, 537. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faye, L.M.; Hosu, M.C.; Apalata, T. Drug-Resistant Tuberculosis in Rural Eastern Cape, South Africa: A Study of Patients’ Characteristics in Selected Healthcare Facilities. Int. J. Environ. Res. Public Health 2024, 21, 1594. https://doi.org/10.3390/ijerph21121594
Faye LM, Hosu MC, Apalata T. Drug-Resistant Tuberculosis in Rural Eastern Cape, South Africa: A Study of Patients’ Characteristics in Selected Healthcare Facilities. International Journal of Environmental Research and Public Health. 2024; 21(12):1594. https://doi.org/10.3390/ijerph21121594
Chicago/Turabian StyleFaye, Lindiwe Modest, Mojisola Clara Hosu, and Teke Apalata. 2024. "Drug-Resistant Tuberculosis in Rural Eastern Cape, South Africa: A Study of Patients’ Characteristics in Selected Healthcare Facilities" International Journal of Environmental Research and Public Health 21, no. 12: 1594. https://doi.org/10.3390/ijerph21121594
APA StyleFaye, L. M., Hosu, M. C., & Apalata, T. (2024). Drug-Resistant Tuberculosis in Rural Eastern Cape, South Africa: A Study of Patients’ Characteristics in Selected Healthcare Facilities. International Journal of Environmental Research and Public Health, 21(12), 1594. https://doi.org/10.3390/ijerph21121594




