Acute Effects of a Maximal Cardiopulmonary Exercise Test on Cardiac Hemodynamic and Cerebrovascular Response and Their Relationship with Cognitive Performance in Individuals with Type 2 Diabetes
Abstract
1. Introduction
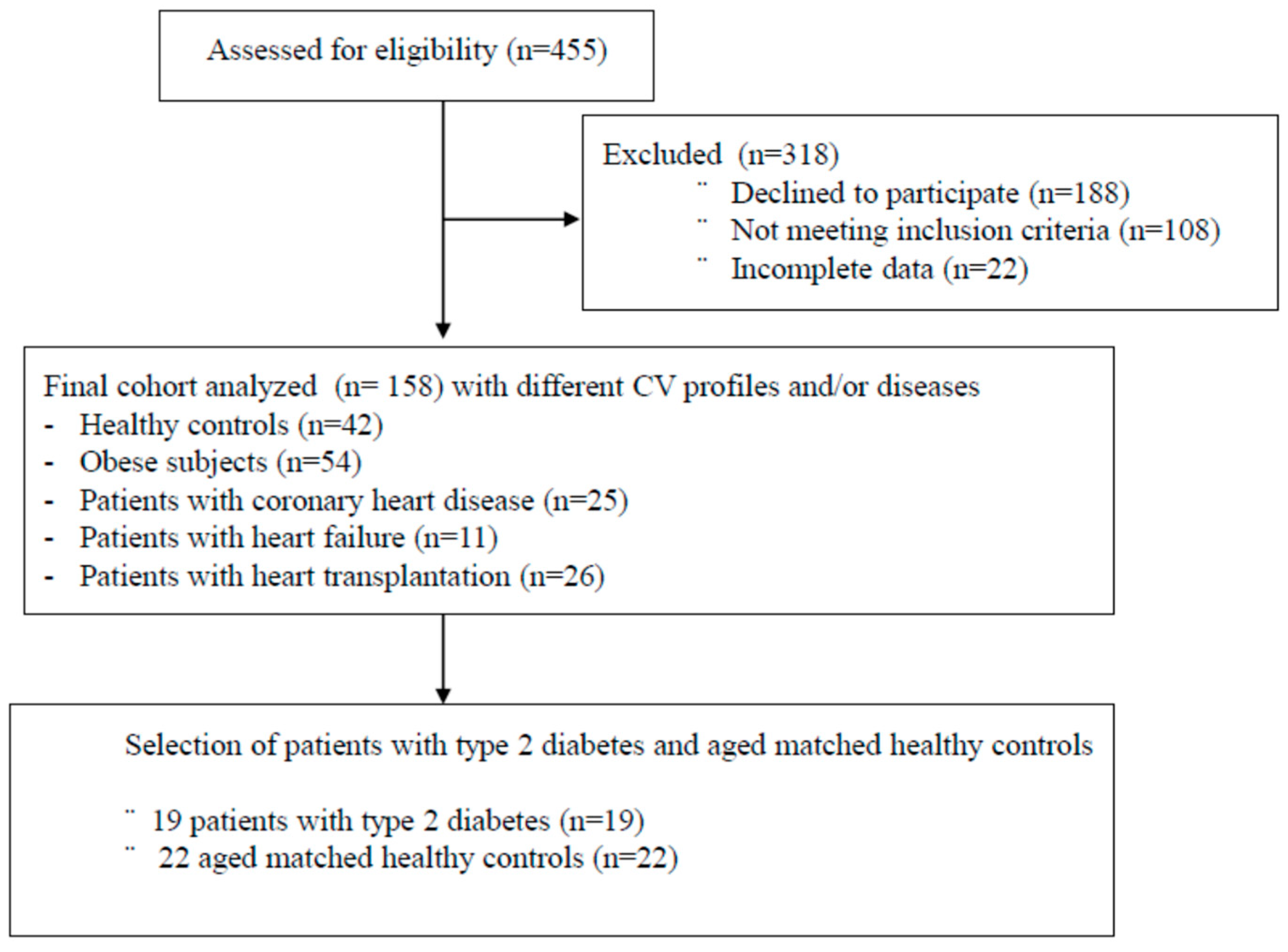
2. Material and Methods
2.1. Participants
2.2. Measurements
2.3. Maximal Cardiopulmonary Exercise Testing (CPET)
2.4. Cardiac Hemodynamics during CPET
2.5. Cerebral Oxygenation/Perfusion (NIRS)
2.6. Blood Samples Analyses and Neuropsychological Evaluation
2.7. Composite Scores for Cognition
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Cardiopulmonary Exercise Test and Cardiac Hemodynamic Parameters
3.3. Cerebral Oxygenation/Perfusion during Exercise and Recovery
3.4. Relationship between Cognitive Performance, Cardiorespiratory Fitness and Cerebral Hemodynamics
4. Discussion
4.1. Cardiopulmonary Function and Cardiac Hemodynamic at Maximal Exercise
4.2. Cerebral Hemodynamic during Exercise and Recovery
4.3. Cognitive Function
4.4. Relationship between Cognitive Performance, Cardiorespiratory Fitness and Cerebral Hemodynamics
5. Limitations and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federation, I.D. IDF Atlas. Available online: https://diabetesatlas.org/ (accessed on 1 March 2023).
- Diabetes Canada. Report from the Canadian Diabetes Association: Diabetes in Canada, 2016; Diabetes Canada: Ottawa, ON, Canada, 2016. [Google Scholar]
- Gedebjerg, A.; Almdal, T.P.; Berencsi, K.; Rungby, J.; Nielsen, J.S.; Witte, D.R.; Friborg, S.; Brandslund, I.; Vaag, A.; Beck-Nielsen, H.; et al. Prevalence of micro- and macrovascular diabetes complications at time of type 2 diabetes diagnosis and associated clinical characteristics: A cross-sectional baseline study of 6958 patients in the Danish DD2 cohort. J. Diabetes Its Complicat. 2018, 32, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Emslie-Smith, A.M.; Gardner, I.D.; Morris, A.D. ABC of arterial and venous disease: Vascular complications of diabetes. BMJ 2000, 320, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimaki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Srikanth, V.; Sinclair, A.J.; Hill-Briggs, F.; Moran, C.; Biessels, G.J. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020, 8, 535–545. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Sarkar, P.; Banu, S.; Bhattacharya, S.; Bala, A.; Sur, D. Pathophysiology associated with Diabetes Induced Tauopathy and Development of Alzheimer’s Disease. Curr. Diabetes Rev. 2023, 19, 37–39. [Google Scholar]
- Auroprajna, P.; Naik, B.M.; Sahoo, J.P.; Keerthi, G.S.; Pavanya, M.; Pal, G.K. Association of Sympathovagal Imbalance with Cognitive Impairment in Type 2 Diabetes in Adults. Can. J. Diabetes 2018, 42, 44–50. [Google Scholar] [CrossRef]
- Vianna, L.C.; Deo, S.H.; Jensen, A.K.; Holwerda, S.W.; Zimmerman, M.C.; Fadel, P.J. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H681–H687. [Google Scholar] [CrossRef]
- Palta, P.; Schneider, A.L.; Biessels, G.J.; Touradji, P.; Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 2014, 20, 278–291. [Google Scholar] [CrossRef]
- Vincent, C.; Hall, P.A. Executive Function in Adults with Type 2 Diabetes: A Meta-Analytic Review. Psychosom. Med. 2015, 77, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Sadanand, S.; Balachandar, R.; Bharath, S. Memory and executive functions in persons with type 2 diabetes: A meta-analysis. Diabetes Metab. Res. Rev. 2016, 32, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Beare, R.; Moran, C.; Phan, T.; Wang, W.; Srikanth, V.K. Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: A longitudinal study. Diabetologia 2019, 62, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.; Pennington, K.; Jones, A.; Bridle, C.; Smith, M.F.; Curtis, F. Effects of exercise, cognitive, and dual-task interventions on cognition in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232958. [Google Scholar] [CrossRef]
- Bherer, L.; Langeard, A.; Kaushal, N.; Vrinceanu, T.; Desjardins-Crepeau, L.; Langlois, F.; Kramer, A.F. Physical Exercise Training Effect and Mediation Through Cardiorespiratory Fitness on Dual-Task Performances Differ in Younger-Old and Older-Old Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 219–228. [Google Scholar] [CrossRef]
- Gayda, M.; Gremeaux, V.; Bherer, L.; Juneau, M.; Drigny, J.; Dupuy, O.; Lapierre, G.; Labelle, V.; Fortier, A.; Nigam, A. Cognitive function in patients with stable coronary heart disease: Related cerebrovascular and cardiovascular responses. PLoS ONE 2017, 12, e0183791. [Google Scholar] [CrossRef]
- Besnier, F.; Berube, B.; Gagnon, C.; Olmand, M.; Ribeiro, P.A.B.; Nigam, A.; Juneau, M.; Blondeau, L.; White, M.; Gremeaux, V.; et al. Cardiorespiratory Fitness Mediates Cognitive Performance in Chronic Heart Failure Patients and Heart Transplant Recipients. Int. J. Environ. Res. Public Health 2020, 17, 8591. [Google Scholar] [CrossRef]
- Oussaidene, K.; Prieur, F.; Tagougui, S.; Abaidia, A.; Matran, R.; Mucci, P. Aerobic fitness influences cerebral oxygenation response to maximal exercise in healthy subjects. Respir. Physiol. Neurobiol. 2015, 205, 53–60. [Google Scholar] [CrossRef]
- Gayda, M.; Lapierre, G.; Dupuy, O.; Fraser, S.; Bherer, L.; Juneau, M.; Gremeaux, V.; Nigam, A. Cardiovascular and cerebral hemodynamics during exercise and recovery in obese individuals as a function of their fitness status. Physiol. Rep. 2017, 5, e13321. [Google Scholar] [CrossRef]
- Boidin, M.; Handfield, N.; Ribeiro, P.A.B.; Desjardins-Crepeau, L.; Gagnon, C.; Lapierre, G.; Gremeaux, V.; Lalonge, J.; Nigam, A.; Juneau, M.; et al. Obese but Fit: The Benefits of Fitness on Cognition in Obese Older Adults. Can. J. Cardiol. 2020, 36, 1747–1753. [Google Scholar] [CrossRef]
- Kim, Y.S.; Davis, S.C.; Truijen, J.; Stok, W.J.; Secher, N.H.; van Lieshout, J.J. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension 2011, 57, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Seifert, T.; Brassard, P.; Rasmussen, P.; Vaag, A.; Nielsen, H.B.; Secher, N.H.; van Lieshout, J.J. Impaired cerebral blood flow and oxygenation during exercise in type 2 diabetic patients. Physiol. Rep. 2015, 3, e12430. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; George, C.J.; Izzetoglu, M.; Wang, C. The effect of diabetes on prefrontal cortex activation patterns during active walking in older adults. Brain Cogn. 2018, 125, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Lezak, M.D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2012; 1161p. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- McComb, E.; Tuokko, H.; Brewster, P.; Chou, P.H.; Kolitz, K.; Crossley, M.; Simard, M. Mental alternation test: Administration mode, age, and practice effects. J. Clin. Exp. Neuropsychol. 2011, 33, 234–241. [Google Scholar] [CrossRef]
- Delis, D.K.E.; Kramer, J. The Delis-Kaplan Executive Function System: Technical Manual; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Bokov, P.; Delclaux, C. The impact of obesity on respiratory function. Rev. Mal. Respir. 2019, 36, 1057–1063. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Sciuto, P.; Natali, A. Type 2 diabetes and reduced exercise tolerance: A review of the literature through an integrated physiology approach. Cardiovasc. Diabetol. 2020, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.A.; Wilkins, G.T.; Cotter, J.D.; Lamberts, R.R.; Lal, S.; Baldi, J.C. HIIT Improves Left Ventricular Exercise Response in Adults with Type 2 Diabetes. Med. Sci. Sports Exerc. 2019, 51, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Ainslie, P.N. Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 2017, 102, 1356–1371. [Google Scholar] [CrossRef]
- Tagougui, S.; Fontaine, P.; Leclair, E.; Aucouturier, J.; Matran, R.; Oussaidene, K.; Descatoire, A.; Prieur, F.; Mucci, P.; Vambergue, A.; et al. Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care 2015, 38, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Lefferts, W.K.; Weiner, C.M.; Mascone, S.E.; Augustine, J.A.; Heffernan, K.S.; Lefferts, E.C. Influence of sex and presence of cardiovascular risk factors on relations between cardiorespiratory fitness and cerebrovascular hemodynamics. J. Appl. Physiol. (1985) 2022, 133, 1019–1030. [Google Scholar] [CrossRef]
- Brugniaux, J.V.; Marley, C.J.; Hodson, D.A.; New, K.J.; Bailey, D.M. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J. Cereb. Blood Flow Metab. 2014, 34, 1873–1876. [Google Scholar] [CrossRef]
- Drigny, J.; Gremeaux, V.; Dupuy, O.; Gayda, M.; Bherer, L.; Juneau, M.; Nigam, A. Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: A pilot study. J. Rehabil. Med. 2014, 46, 1050–1054. [Google Scholar] [CrossRef]
- Cavuoto, L.A.; Maikala, R.V. Obesity and the Role of Short Duration Submaximal Work on Cardiovascular and Cerebral Hemodynamics. PLoS ONE 2016, 11, e0153826. [Google Scholar] [CrossRef]
- Cavuoto, L.A.; Maikala, R.V. Role of obesity on cerebral hemodynamics and cardiorespiratory responses in healthy men during repetitive incremental lifting. Eur. J. Appl. Physiol. 2015, 115, 1905–1917. [Google Scholar] [CrossRef]
- Zhao, F.; Tomita, M.R.; Dutta, A. Functional near-infrared spectroscopy of prefrontal cortex during memory encoding and recall in elderly with type 2 diabetes mellitus. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 3323–3326. [Google Scholar] [CrossRef]
- Zhao, R.R.; Mavros, Y.; Meiklejohn, J.; Anderberg, K.A.; Singh, N.; Kay, S.; Baker, M.K.; Wang, Y.; Climstein, M.; O’Sullivan, A.; et al. Effect of High Intensity Power Training on Cognitive Function in Older Adults with Type 2 Diabetes: Secondary Outcomes of the GREAT2DO Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.H.; Wang, Z.; Feng, L.Y.; Ni, G.X. Effect of Exercise on the Cognitive Function of Older Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 876935. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Hogan, D.B.; Eskes, G.A.; Longman, R.S.; Poulin, M.J. Cerebrovascular reserve: The link between fitness and cognitive function? Exerc. Sport Sci. Rev. 2012, 40, 153–158. [Google Scholar] [CrossRef] [PubMed]

| HC n = 23 | T2D n = 19 | ||||||
|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | p Value | |
| Age | 61.83 | ± | 10.54 | 61.95 | ± | 6.60 | 0.964 |
| Sex (female, %) | 4 (17.4%) | 5 (26.3%) | 0.483 | ||||
| Height (cm) | 171.00 | ± | 8.73 | 169.79 | ± | 9.96 | 0.677 |
| body mass (kg) | 71.66 | ± | 7.58 | 90.96 | ± | 14.22 | <0.001 |
| BMI (kg/m2) | 24.51 | ± | 1.93 | 31.67 | ± | 5.50 | <0.001 |
| Waist circumference (cm) | 89.43 | ± | 6.40 | 111.42 | ± | 14.36 | <0.001 |
| Fat free mass (kg) | 56.83 | ± | 8.69 | 59.92 | ± | 10.57 | 0.305 |
| Fat mass (%) | 21.00 | ± | 6.24 | 33.70 | ± | 8.56 | <0.001 |
| Fat mass (kg) | 14.87 | ± | 3.78 | 30.84 | ± | 9.35 | <0.001 |
| Medication | |||||||
| Beta-blocker | 0 (0%) | 3 (16%) | |||||
| ACE inhibitor | 0 (0%) | 9 (47%) | |||||
| ARBs | 0 (0%) | 2 (11%) | |||||
| Calcium channel blocker | 0 (0%) | 5 (26%) | |||||
| Antidiabetic agent | 0 (0%) | 9 (47%) | |||||
| Cardiovascular risk factors | |||||||
| Smoking (n, %) | 0 (0%) | 3 (15.8%) | 0.048 | ||||
| Hypertension (n, %) | 0 (0%) | 12 (63.2%) | <0.001 | ||||
| Dyslipidemias (n, %) | 2 (8.7%) | 15 (78.9%) | <0.001 | ||||
| Overweight: BMI > 25 and ≤ 30 kg·m2 | 8 (34.8%) | 7 (36.8%) | 0.889 | ||||
| Obesity: BMI > 30 kg·m2 | 0 (0%) | 11 (57.9%) | <0.001 | ||||
| Blood sample | |||||||
| Fasting glycemia (mmol/L) | 5.10 | ± | 0.54 | 7.72 | ± | 1.48 | <0.001 |
| Total cholesterol (mmol/L) | 5.01 | ± | 0.86 | 4.23 | ± | 1.10 | 0.018 |
| HDL-C (mmol/L) | 1.53 | ± | 0.50 | 1.12 | ± | 0.26 | 0.003 |
| LDL-C (mmol/L) | 3.08 | ± | 0.68 | 2.31 | ± | 0.97 | 0.013 |
| Triglycerides (mmol/L) | 0.90 | ± | 0.40 | 1.90 | ± | 1.00 | <0.001 |
| Fasting insulin (pmol/L) | 47.30 | ± | 69.07 | 110.71 | ± | 65.97 | 0.001 |
| HC n = 23 | T2D n = 19 | ||||||
|---|---|---|---|---|---|---|---|
| Cardiorespiratory Fitness | Mean | ± | SD | Mean | ± | SD | p Value |
| Time to exhaustion (sec) | 666.00 | ± | 177.20 | 589.20 | ± | 143.30 | 0.1467 |
| Power at VT1 (watts) | 154.80 | ± | 52.97 | 107.50 | ± | 31.94 | 0.0033 |
| O2 at VT1 (mL/min) | 2006.00 | ± | 564.50 | 1562.00 | ± | 348.30 | 0.0090 |
| Power increment (w/min) | 15.48 | ± | 2.69 | 13.53 | ± | 2.93 | 0.0418 |
| O2max (mL/kg/min) | 36.89 | ± | 7.66 | 22.84 | ± | 4.89 | <0.001 |
| O2max (mL/kg FFM/min) | 46.43 | ± | 7.63 | 34.50 | ± | 5.65 | <0.001 |
| O2max (mL/min) | 2666.91 | ± | 682.34 | 2057.95 | ± | 450.04 | 0.002 |
| O2 Plateau (n and %) | 20 (86%) | 16 (68%) | 0.1450 | ||||
| R.E.R | 1.17 | ± | 0.07 | 1.16 | ± | 0.08 | 0.431 |
| Emax (L/min) | 105 | ± | 39.2 | 75.5 | ± | 20.9 | 0.015 |
| PETCO2max (mmHg) | 34.33 | ± | 4.47 | 38.03 | ± | 4.53 | 0.014 |
| HR rest (bpm) | 65.13 | ± | 12.26 | 74.21 | ± | 14.65 | 0.015 |
| HR max (bpm) | 158.96 | ± | 14.20 | 144.74 | ± | 24.12 | 0.031 |
| Systolic blood pressure (mmHg) | 119.30 | ± | 7.90 | 127.74 | ± | 14.18 | 0.028 |
| Diastolic blood pressure (mmHg) | 71.91 | ± | 7.32 | 75.37 | ± | 8.69 | 0.169 |
| Maximal systolic blood pressure (mmHg) | 183.61 | ± | 19.09 | 204.95 | ± | 26.21 | 0.004 |
| Maximal diastolic blood pressure (mmHg) | 79.13 | ± | 10.49 | 79.79 | ± | 10.50 | 0.840 |
| HR at 1 min of recovery (bpm) | 139.22 | ± | 16.24 | 120.58 | ± | 23.90 | 0.005 |
| Maximal power output (watts) | 214.52 | ± | 68.21 | 149.06 | ± | 43.44 | 0.002 |
| Cardiac impedance at maximal exercise | |||||||
| Cardiac output (L/min) | 15.86 | ± | 2.12 | 12.82 | ± | 4.16 | 0.008 |
| Cardiac Index (L/min/m2) | 8.70 | ± | 1.09 | 6.27 | ± | 2.09 | <0.001 |
| Stroke volume (mL) | 101.04 | ± | 11.41 | 97.39 | ± | 31.57 | 0.636 |
| Stroke volume index (mL/m2) | 54.75 | ± | 4.45 | 47.03 | ± | 15.03 | 0.043 |
| Left cardiac work index (kg/m/m2) | 12.77 | ± | 2.10 | 9.90 | ± | 3.47 | 0.025 |
| Contractility Index | 147.35 | ± | 55.20 | 106.68 | ± | 45.99 | 0.024 |
| C(a- O2) (mL/100 mL) | 16.71 | ± | 3.25 | 17.12 | ± | 4.48 | 0.732 |
| Systemic vascular resistance index (Dyn·s/cm5·m2) | 583.35 | ± | 90.36 | 826.21 | ± | 308.21 | 0.005 |
| Systemic vascular resistance (Dyn·s/cm5) | 1071.09 | ± | 157.06 | 1986.21 | ± | 1297.65 | <0.001 |
| NIRS at Exercise and during Recovery | HC n = 23 | T2DM n = 19 | p | ||||
|---|---|---|---|---|---|---|---|
| AUC exercise tHb | 6.57 | ± | 9.79 | 3.87 | ± | 4.35 | 0.245 |
| AUC exercise HHb | 0.53 | ± | 2.77 | −0.34 | ± | 2.30 | 0.279 |
| AUC exercise O2Hb | 6.03 | ± | 10.42 | 4.21 | ± | 3.79 | 0.444 |
| AUC exercise HbDiff | 5.50 | ± | 11.70 | 4.56 | ± | 4.52 | 0.318 |
| AUC recovery tHb | 18.31 | ± | 11.95 | 19.70 | ± | 13.66 | 0.731 |
| AUC recovery HHb | 4.26 | ± | 5.59 | 1.07 | ± | 3.97 | 0.048 |
| AUC recovery O2Hb | 16.27 | ± | 15.00 | 17.60 | ± | 11.18 | 0.757 |
| AUC recovery HbDiff | 13.52 | ± | 20.28 | 17.56 | ± | 12.75 | 0.465 |
| AUC: Area Under the Curve | |||||||
| HC n = 23 | T2M n = 19 | ||||||
|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | p Value | |
| MMSE | 28.94 | ± | 1.16 | 28.94 | ± | 1.20 | 0.902 |
| GDS | 2.35 | ± | 2.78 | 2.35 | ± | 3.67 | 0.340 |
| Forward | 6.83 | ± | 0.92 | 6.47 | ± | 0.94 | 0.258 |
| Backward | 5.56 | ± | 1.50 | 5.35 | ± | 1.46 | 0.688 |
| TMT-A | 36.76 | ± | 12.27 | 38.46 | ± | 12.33 | 0.685 |
| TMT-B | 76.23 | ± | 19.20 | 83.45 | ± | 31.03 | 0.415 |
| Stroop 1 | 29.61 | ± | 5.67 | 29.77 | ± | 5.20 | 0.934 |
| Stroop 2 | 20.91 | ± | 3.37 | 21.61 | ± | 3.39 | 0.552 |
| Stroop 3 | 53.35 | ± | 9.66 | 63.06 | ± | 13.62 | 0.021 |
| Stroop 4 | 56.42 | ± | 13.83 | 69.38 | ± | 17.96 | 0.024 |
| Rey first recall | 11.94 | ± | 2.41 | 9.76 | ± | 3.31 | 0.036 |
| Rey diff | 10.67 | ± | 2.72 | 9.71 | ± | 2.80 | 0.311 |
| Rey 1–15 total words | 52.61 | ± | 9.23 | 46.41 | ± | 9.75 | 0.062 |
| DSST | 69.72 | ± | 11.42 | 62.65 | ± | 13.32 | 0.100 |
| Cognitive Z score | |||||||
| Working memory | 0.12 | ± | 0.79 | −0.13 | ± | 0.76 | 0.349 |
| Processing speed | −0.02 | ± | 0.44 | −0.06 | ± | 0.53 | 0.353 |
| Verbal memory | 0.27 | ± | 0.80 | −0.29 | ± | 0.98 | 0.069 |
| Executive function | −0.40 | ± | 0.60 | 0.18 | ± | 0.71 | 0.016 |
| HC n = 23 | T2DM n = 19 | |||
|---|---|---|---|---|
| Executive Function | R | p | R | p |
| BMI (kg/m2) | −0.019 | 0.941 | −0.274 | 0.305 |
| Waist circumference (cm) | 0.081 | 0.750 | −0.274 | 0.304 |
| Fat free mass (kg) | −0.305 | 0.218 | −0.121 | 0.655 |
| Fat mass (%) | 0.528 | 0.024 | 0.049 | 0.857 |
| Fasting glycemia (mmol/L) | 0.053 | 0.840 | 0.447 | 0.083 |
| triglycerides (mmol/L) | −0.172 | 0.510 | 0.219 | 0.415 |
| Fasting insulin (pmol/L) | −0.377 | 0.317 | −0.114 | 0.711 |
| O2peak (mL/kg FFM/min) | −0.519 | 0.027 | −0.300 | 0.260 |
| VT1 (mL/kg FFM/min) | −0.524 | 0.026 | −0.316 | 0.293 |
| Circulatory Power (mL/min/kg/mmHg) | −0.534 | 0.023 | −0.261 | 0.328 |
| Cardiac Index (L/min/m2) | −0.466 | 0.051 | −0.168 | 0.534 |
| Left cardiac work index (kg/m/m2) | −0.305 | 0.219 | −0.253 | 0.345 |
| Systemic vascular resistance index (Dyn·s/cm5·m2) | 0.370 | 0.131 | 0.081 | 0.766 |
| tHb AUC effort | −0.486 | 0.041 | −0.500 | 0.049 |
| HHb AUC effort | 0.456 | 0.057 | −0.311 | 0.242 |
| O2hb AUC effort | −0.620 | 0.006 | −0.416 | 0.109 |
| HbDiff AUC effort | −0.573 | 0.013 | −0.205 | 0.447 |
| tHb AUC recup | −0.265 | 0.287 | −0.682 | 0.005 |
| HHb AUC recup | 0.514 | 0.029 | 0.055 | 0.846 |
| O2hb AUC recup | −0.550 | 0.018 | −0.686 | 0.005 |
| HbDiff AUC recup | −0.650 | 0.004 | −0.675 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besnier, F.; Gagnon, C.; Monnet, M.; Dupuy, O.; Nigam, A.; Juneau, M.; Bherer, L.; Gayda, M. Acute Effects of a Maximal Cardiopulmonary Exercise Test on Cardiac Hemodynamic and Cerebrovascular Response and Their Relationship with Cognitive Performance in Individuals with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2023, 20, 5552. https://doi.org/10.3390/ijerph20085552
Besnier F, Gagnon C, Monnet M, Dupuy O, Nigam A, Juneau M, Bherer L, Gayda M. Acute Effects of a Maximal Cardiopulmonary Exercise Test on Cardiac Hemodynamic and Cerebrovascular Response and Their Relationship with Cognitive Performance in Individuals with Type 2 Diabetes. International Journal of Environmental Research and Public Health. 2023; 20(8):5552. https://doi.org/10.3390/ijerph20085552
Chicago/Turabian StyleBesnier, Florent, Christine Gagnon, Meghann Monnet, Olivier Dupuy, Anil Nigam, Martin Juneau, Louis Bherer, and Mathieu Gayda. 2023. "Acute Effects of a Maximal Cardiopulmonary Exercise Test on Cardiac Hemodynamic and Cerebrovascular Response and Their Relationship with Cognitive Performance in Individuals with Type 2 Diabetes" International Journal of Environmental Research and Public Health 20, no. 8: 5552. https://doi.org/10.3390/ijerph20085552
APA StyleBesnier, F., Gagnon, C., Monnet, M., Dupuy, O., Nigam, A., Juneau, M., Bherer, L., & Gayda, M. (2023). Acute Effects of a Maximal Cardiopulmonary Exercise Test on Cardiac Hemodynamic and Cerebrovascular Response and Their Relationship with Cognitive Performance in Individuals with Type 2 Diabetes. International Journal of Environmental Research and Public Health, 20(8), 5552. https://doi.org/10.3390/ijerph20085552









