Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review
Abstract
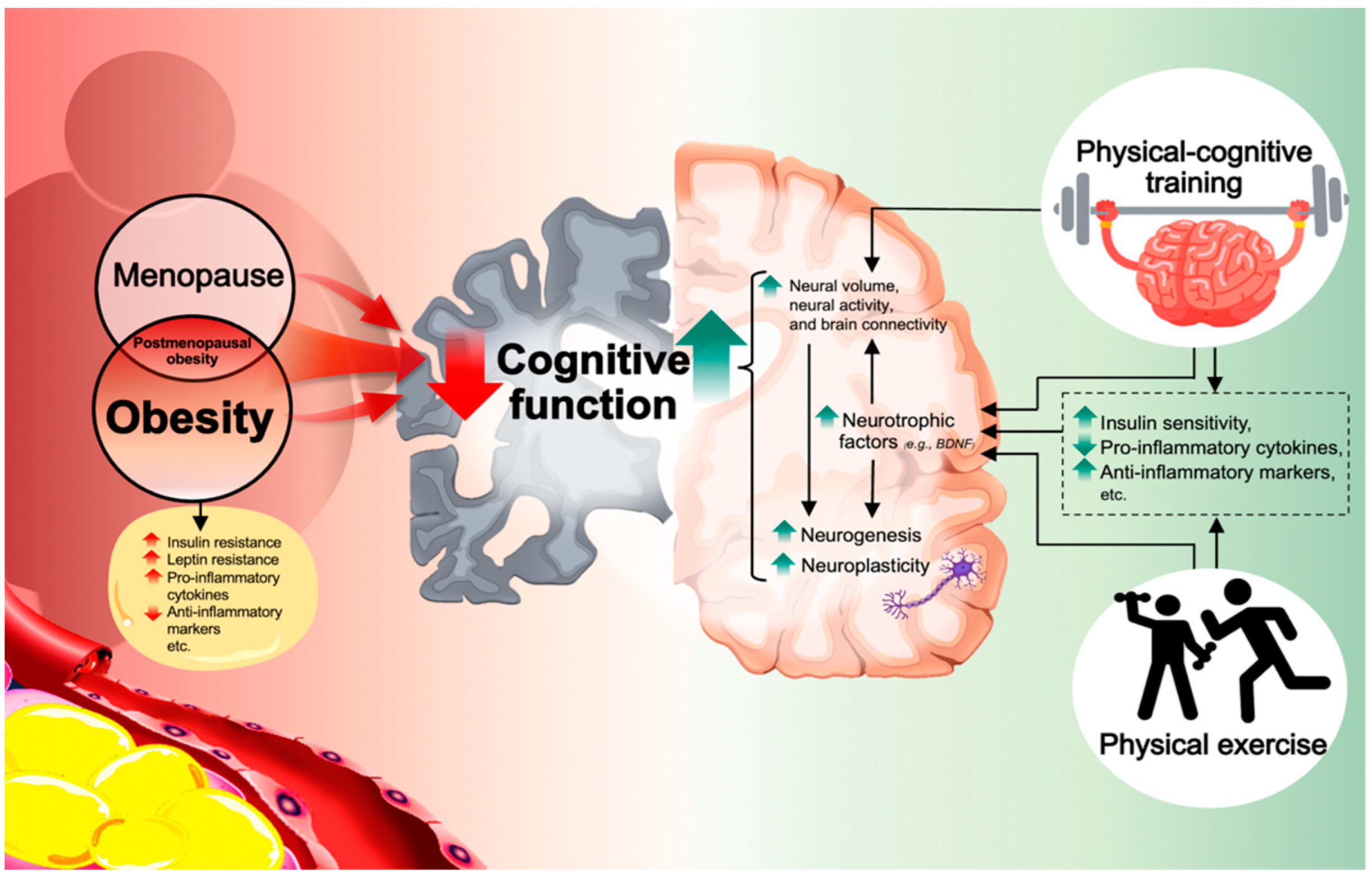
1. Introduction
2. Physical Exercise and Cognitive Benefits-Selected Studies
2.1. Evidence in Older Adults with Obesity
2.2. Evidence in Postmenopausal with Overweight and Obesity
3. Associated Physiological Mechanisms of Physical Exercise Induced-Cognitive Improvement in Obese Individuals—Selected Studies
4. Physical–cognitive Intervention and Cognitive Benefits-Selected Studies
5. Associated Physiological Mechanisms of Physical and Cognitive Exercise-Induced Cognitive Improvement in Obese Individuals—Selected Studies
6. Technology-Based Interventions for Health and Cognitive Benefits
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Malenfant, J.H.; Batsis, J.A. Obesity in the geriatric population—A global health perspective. J. Glob. Health Rep. 2019, 3, e2019045. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Lee, A.; Cardel, M.; Donahoo, W.T.; Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; et al. Social and Environmental Factors Influencing Obesity; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Zatońska, K.; Psikus, P.; Basiak-Rasała, A.; Stępnicka, Z.; Gaweł-Dąbrowska, D.; Wołyniec, M.; Gibka, J.; Szuba, A.; Połtyn-Zaradna, K. Obesity and Chosen Non-Communicable Diseases in PURE Poland Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 2701. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr. 2005, 82, 923–934. [Google Scholar] [CrossRef]
- Pasquali, R.; Casanueva, F.; Haluzik, M.; van Hulsteijn, L.; Ledoux, S.; Monteiro, M.P.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. Eur. J. Endocrinol. 2020, 182, G1–G32. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, A.; Gunderson, P.; Barrett-Connor, E.; Quesenberry, J.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ (Clin. Res.) 2005, 330, 1360. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kareholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Quesenberry, C.P., Jr.; Zhou, J.; Yaffe, K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr. Alzheimer Res. 2007, 4, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Beam, R.; Kaneshiro, C.; Jang, Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences between women and men in incidence rates of dementia and alzheimer’s disease. J. Alzheimers Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Chêne, G.; Beiser, A.; Au, R.; Preis, S.R.; Wolf, P.A.; Dufouil, C.; Seshadri, S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015, 11, 310–320. [Google Scholar] [CrossRef] [PubMed]
- De Franciscis, P.; Barbieri, M.; Leo, S.; Dalise, A.M.; Sardu, C.; Marfella, R.; Colacurci, N.; Paolisso, G.; Rizzo, M.R. Serum adiponectin levels are associated with worse cognitive function in postmenopausal women. PLoS ONE 2017, 12, e0186205. [Google Scholar] [CrossRef]
- Kaur, S.; Gonzales, M.M.; Tarumi, T.; Villalpando, A.; Alkatan, M.; Pyron, M.; Tanaka, H.; Haley, A.P. Serum brain-derived neurotrophic factor mediates the relationship between abdominal adiposity and executive function in middle age. J. Int. Neuropsychol. Soc. 2016, 22, 493–500. [Google Scholar] [CrossRef]
- Benito-León, J.; Mitchell, A.J.; Hernández-Gallego, J.; Bermejo-Pareja, F. Obesity and impaired cognitive functioning in the elderly: A population-based cross-sectional study (NEDICES). Eur. J. Neurol. 2013, 20, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Pratchayasakul, W.; Sa-Nguanmoo, P.; Sivasinprasasn, S.; Pintana, H.; Tawinvisan, R.; Sripetchwandee, J.; Kumfu, S.; Chattipakorn, N.; Chattipakorn, S.C. Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm. Behav. 2015, 72, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.; Pike, C.J. Menopause, obesity and inflammation: Interactive risk factors for Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M. The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Front. Neurosci. 2018, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Luukkonen, R.; Batty, G.D.; Ferrie, J.E.; Pentti, J.; Nyberg, S.T.; Shipley, M.J.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018, 14, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Parvatha, N.; Neelambikai, N. Effect of body mass index and waist hip ratio on cognitive performance in pre- and post-menopausal women. Indian J. Appl. Res. 2018, 8, 30–32. [Google Scholar]
- Kumar, N. Effect of abdominal obesity and serum lipid markers on cognitive functions in postmenopausal indian women. World J. Pharm. Pharm. Sci. 2014, 3, 2106–2111. [Google Scholar]
- Feinkohl, I.; Lachmann, G.; Brockhaus, W.R.; Borchers, F.; Piper, S.K.; Ottens, T.H.; Nathoe, H.M.; Sauer, A.M.; Dieleman, J.M.; Radtke, F.M.; et al. Association of obesity, diabetes and hypertension with cognitive impairment in older age. Clin. Epidemiol. 2018, 10, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Chu, C.H.; Chen, F.T.; Hung, T.M.; Etnier, J.L. Combined Effects of Physical Activity and Obesity on Cognitive Function: Independent, Overlapping, Moderator, and Mediator Models. Sport. Med. 2017, 47, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kang, S. Regular leisure-time physical activity is effective in boosting neurotrophic factors and alleviating menopause symptoms. Int. J. Environ. Res. Public Health 2020, 17, 8624. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Seib, C.; Rasmussen, L. Can physical activity prevent physical and cognitive decline in postmenopausal women? A systematic review of the literature. Maturitas 2014, 79, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Sirois-Leclerc, H.; Tulloch, H. The impact of long-term physical activity interventions for overweight/obese postmenopausal women on adiposity indicators, physical capacity, and mental health outcomes: A systematic review. J. Obes. 2016, 2016, 6169890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yin, S.; Lang, M.; He, R.; Li, J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016, 31, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Inzitari, M.; Greenlee, A.; Hess, R.; Perera, S.; Studenski, S.A. Attitudes of postmenopausal women toward interactive video dance for exercise. J. Womens Health (Larchmt) 2009, 18, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chan, Y.; Ren, L.; Yan, H. Obesity reduces cognitive and motor functions across the lifespan. Neural Plast. 2016, 2016, 2473081. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.; Cherbuin, N.; Pumpa, K.; Smee, D.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sport. Med. 2017, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Voss, M.; Prakash, R.; Basak, C.; Szabo-Reed, A.; Chaddock, L.; Kim, J.; Heo, S.; Alves, H.; Phillips, S.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Jedrziewski, K.; Ewbank, C.; Wang, H.; Trojanowski, Q. Exercise and cognition: Results from the National Long Term Care Survey. Alzheimers Dement. 2010, 6, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Shah, K.; Waters, D.L.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am. J. Clin. Nutr. 2014, 100, 189–198. [Google Scholar] [CrossRef]
- Boidin, M.; Handfield, N.; Ribeiro, P.; Desjardins-Crépeau, L.; Gagnon, C.; Lapierre, G.; Gremeaux, V.; Lalongé, J.; Nigam, A.; Juneau, M.; et al. Obese but Fit: The benefits of fitness on cognition in obese older adults. Can. J. Cardiol. 2020, 36, 1747–1753. [Google Scholar] [CrossRef]
- Coll-Padrós, N.; León, M.; Valech, N.; Ros, E.; Vidal, J.; Estruch, R.; Fitó, M.; Salas-Salvadó, J.; Corella, D.; Molinuevo, J.L.; et al. Physical activity is associated with better global cognition and frontal function in overweight/obese older adults with metabolic syndrome. Eur. Rev. Aging Phys. Act. 2019, 16, 23. [Google Scholar] [CrossRef]
- De Camargo Smolarek, A.; Boiko Ferreira, L.H.; Schoenfeld, B.; Ribeiro Cordeiro, G.; Alessi, A.; Laat, E.; Mascarenhas, L.; Carvalho Perin, S.; Zandoná, B.; Souza, W.; et al. Cognitive performance changes after a 12-week strength training program in overweight older women. J. Exerc. Physiol. Online 2019, 22, 1–9. [Google Scholar]
- Dominguez-Sanchez, A.; Bustos-Cruz, H.; Velasco-Orjuela, P.; Quintero, P.; Tordecilla-Sanders, A.; Correa-Bautista, E.; Triana-Reina, R.; Garcia-Hermoso, A.; Gonzalez-Ruiz, K.; Pena-Guzman, A.; et al. Acute effects of high intensity, resistance, or combined protocol on the increase of level of neurotrophic factors in physically inactive overweight adults: The BrainFit Study. Front. Physiol. 2018, 9, 741. [Google Scholar] [CrossRef]
- Khoo, J.; Dhamodaran, S.; Chen, D.; Yap, Y.; Chen, Y.; Tian, H. Exercise-induced weight loss is more effective than dieting for improving adipokine profile, insulin resistance, and inflammation in obese men. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Moller, E.; Horstmann, A.; Busse, F.; Lepsien, J.; Bluher, M.; Stumvoll, M.; Villringer, A.; Pleger, B. Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Front. Hum. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Feter, N.; Alt, R.; Dias, M.G.; Rombaldi, A.J. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci. Sports 2019, 34, 293–304. [Google Scholar] [CrossRef]
- Alizadeh, M.; Dehghanizade, J. The effect of functional training on level of brain-derived neurotrophic factor and functional performance in women with obesity. Physiol. Behav. 2022, 251, 113798. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Kerdphoo, S.; Petsophonsakul, P.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011, 88, 619–627. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front. Endocrinol. 2018, 9, 496. [Google Scholar] [CrossRef]
- Shih, K.-C.; Kwok, C. Exercise reduces body fat and improves insulin sensitivity and pancreatic β-cell function in overweight and obese male Taiwanese adolescents. BMC Pediatr. 2018, 18, 80. [Google Scholar] [CrossRef]
- Park, H.S.; Park, S.S.; Kim, C.J.; Shin, M.S.; Kim, T.W. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients 2019, 11, 1603. [Google Scholar] [CrossRef]
- Kang, E.B.; Cho, J.Y. Effects of treadmill exercise on brain insulin signaling and β-amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats. J. Exerc. Nutr. Biochem. 2014, 18, 89–96. [Google Scholar] [CrossRef]
- Colberg, S.R.; Somma, C.T.; Sechrist, S.R. Physical activity participation may offset some of the negative impact of diabetes on cognitive function. J. Am. Med. Dir. Assoc. 2008, 9, 434–438. [Google Scholar] [CrossRef]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of physical exercise on adiponectin, leptin, and inflammatory markers in childhood obesity: Systematic review and meta-analysis. Child. Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The impact of exercise training on inflammatory markers in postmenopausal women: A systemic review and meta-analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Lindbergh, C.A.; VandeBunte, A.; Neuhaus, J.; Schneider, J.A.; Buchman, A.S.; Honer, W.G.; Bennett, D.A. Microglial correlates of late life physical activity: Relationship with synaptic and cognitive aging in older adults. J. Neurosci. 2022, 42, 288–298. [Google Scholar] [CrossRef]
- Nichol, K.; Poon, W.; Parachikova, A.; Cribbs, D.; Glabe, C.; Cotman, C. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflamm. 2008, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Lexell, J.; Deierborg, T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil. Neural Repair 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.M.; Pereira, J.R.; de Andrade, L.P.; Garuffi, M.; Talib, L.L.; Forlenza, O.V.; Cancela, J.M.; Cominetti, M.R.; Stella, F. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr. Alzheimer Res. 2014, 11, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef] [PubMed]
- Lauenroth, A.; Ioannidis, A.E.; Teichmann, B. Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 2016, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.; Chamari, K.; Mucci, P.; Massé-Biron, J.; Préfaut, C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sport. Med. 2002, 23, 415–421. [Google Scholar] [CrossRef]
- Guo, W.; Zang, M.; Klich, S.; Kawczyński, A.; Smoter, M.; Wang, B. Effect of combined physical and cognitive interventions on executive functions in OLDER Adults: A meta-analysis of outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6166. [Google Scholar] [CrossRef]
- Torre, M.M.; Temprado, J.J. A review of combined training studies in older adults according to a new categorization of conventional interventions. Front. Aging Neurosci. 2021, 13, 808539. [Google Scholar] [CrossRef] [PubMed]
- Staiano, A.E.; Abraham, A.A.; Calvert, S.L. Competitive versus cooperative exergame play for African American adolescents’ executive function skills: Short-term effects in a long-term training intervention. Dev. Psychol. 2012, 48, 337–342. [Google Scholar] [CrossRef]
- García-Garro, P.A.; Hita-Contreras, F.; Martínez-Amat, A.; Achalandabaso-Ochoa, A.; Jiménez-García, J.D.; Cruz-Díaz, D.; Aibar-Almazán, A. Effectiveness of a pilates training program on cognitive and functional abilities in postmenopausal women. Int. J. Environ. Res. Public Health 2020, 17, 3580. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-A.; Wu, S.-S.; Han, H.-R.; Park, J.-J.; Park, S.; Cho, K.-I. Effects of exergaming in postmenopausal women with high cardiovascular risk: A randomized controlled trial. Clin. Cardiol. 2020, 43, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N.; Corkery, A.T. Exercise improves vascular function, but does this translate to the brain? Brain Plast. 2018, 4, 65–79. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, T.D.; van den Heuvel, O.A.; Berendse, H.W.; van der Werf, Y.D.; Vriend, C. The effects of cognitive training on brain network activity and connectivity in aging and neurodegenerative diseases: A systematic review. Neuropsychol. Rev. 2020, 30, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Hanley, C.; Arciero, P.J.; Brickman, A.M.; Nimon, J.P.; Okuma, N.; Westen, S.C.; Merz, M.E.; Pence, B.D.; Woods, J.A.; Kramer, A.F.; et al. Exergaming and older adult cognition: A cluster randomized clinical trial. Am. J. Prev. Med. 2012, 42, 109–119. [Google Scholar] [CrossRef]
- Anderson-Hanley, C.; Barcelos, N.M.; Zimmerman, E.A.; Gillen, R.W.; Dunnam, M.; Cohen, B.D.; Yerokhin, V.; Miller, K.E.; Hayes, D.J.; Arciero, P.J.; et al. The aerobic and cognitive exercise study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): Neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 2018, 10, 76. [Google Scholar] [CrossRef]
- Bherer, L.; Gagnon, C.; Langeard, A.; Lussier, M.; Desjardins-Crépeau, L.; Berryman, N.; Bosquet, L.; Vu, T.T.M.; Fraser, S.; Li, K.Z.H.; et al. Synergistic effects of cognitive training and physical exercise on dual-task performance in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Hanley, C.; Stark, J.; Wall, K.M.; VanBrakle, M.; Michel, M.; Maloney, M.; Barcelos, N.; Striegnitz, K.; Cohen, B.D.; Kramer, A.F. The interactive Physical and Cognitive Exercise System (iPACES™): Effects of a 3-month in-home pilot clinical trial for mild cognitive impairment and caregivers. Clin. Interv. Aging 2018, 13, 1565–1577. [Google Scholar] [CrossRef]
- Rahe, J.; Becker, J.; Fink, G.R.; Kessler, J.; Kukolja, J.; Rahn, A.; Rosen, J.B.; Szabados, F.; Wirth, B.; Kalbe, E. Cognitive training with and without additional physical activity in healthy older adults: Cognitive effects, neurobiological mechanisms, and prediction of training success. Front. Aging Neurosci. 2015, 7, 187. [Google Scholar] [CrossRef]
- Fabel, K.; Wolf, S.A.; Ehninger, D.; Babu, H.; Leal-Galicia, P.; Kempermann, G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 2009, 3, 50. [Google Scholar] [CrossRef]
- Langdon, K.D.; Corbett, D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil. Neural Repair 2012, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-Olmo, M.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Mat Ludin, A.F.; Chen, L.L.; Hossain, H.; Abdul, H., II; Sameeha, M.J.; Tahir, A.R.M. Motivations, barriers and exercise preferences among female undergraduates: A need assessment analysis. PLoS ONE 2022, 17, e0264158. [Google Scholar] [CrossRef]
- Baillot, A.; Chenail, S.; Barros Polita, N.; Simoneau, M.; Libourel, M.; Nazon, E.; Riesco, E.; Bond, D.S.; Romain, A.J. Physical activity motives, barriers, and preferences in people with obesity: A systematic review. PLoS ONE 2021, 16, e0253114. [Google Scholar] [CrossRef]
- Stanmore, E.; Stubbs, B.; Vancampfort, D.; de Bruin, E.D.; Firth, J. The effect of active video games on cognitive functioning in clinical and non-clinical populations: A meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2017, 78, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Duruturk, N. Effect of telerehabilitation applied during COVID-19 isolation period on physical fitness and quality of life in overweight and obese individuals. Int. J. Obes. 2022, 46, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Qiu, S.; Luo, D.; Wang, L.; Lu, Y.; Li, M. Mobile Application Interventions and Weight Loss in Type 2 Diabetes: A Meta-Analysis. Obesity 2020, 28, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Toro-Ramos, T.; Lee, D.H.; Kim, Y.; Michaelides, A.; Oh, T.J.; Kim, K.M.; Jang, H.C.; Lim, S. Effectiveness of a Smartphone Application for the Management of Metabolic Syndrome Components Focusing on Weight Loss: A Preliminary Study. Metab. Syndr. Relat. Disord. 2017, 15, 465–473. [Google Scholar] [CrossRef]
- Romeo, A.; Edney, S.; Plotnikoff, R.; Curtis, R.; Ryan, J.; Sanders, I.; Crozier, A.; Maher, C. Can smartphone apps increase physical activity? Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e12053. [Google Scholar] [CrossRef] [PubMed]
- Beleigoli, A.M.; Andrade, A.Q.; Cançado, A.G.; Paulo, M.N.; Diniz, M.D.F.H.; Ribeiro, A.L. Web-based digital health interventions for weight loss and lifestyle habit changes in overweight and obese adults: Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e298. [Google Scholar] [CrossRef] [PubMed]
- Di Lorito, C.; Bosco, A.; Rai, H.; Craven, M.; McNally, D.; Todd, C.; Booth, V.; Cowley, A.; Howe, L.; Harwood, R.H. A systematic literature review and meta-analysis on digital health interventions for people living with dementia and Mild Cognitive Impairment. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Cacciante, L.; Pietà, C.D.; Rutkowski, S.; Cieślik, B.; Szczepańska-Gieracha, J.; Agostini, M.; Kiper, P. Cognitive telerehabilitation in neurological patients: Systematic review and meta-analysis. Neurol. Sci. 2022, 43, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B.; Bier, J.-C.; Van Hove, O.; Sheldon, S.; Samadoulougou, S.; Kirakoya-Samadoulougou, F.; Klass, M. Age-associated capacity to progress when playing cognitive mobile games: Ecological retrospective observational study. JMIR Serious Games 2020, 8, e17121. [Google Scholar] [CrossRef]
- Best, J.R. Exergaming in youth: Effects on physical and cognitive health. Z. Psychol. 2013, 221, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.M.; Charles, D.; Porter-Armstrong, A.; McNeill, M.D.; McDonough, S.M.; McCormack, B. Gaming for health: A systematic review of the physical and cognitive effects of interactive computer games in older adults. J. Appl. Gerontol. 2015, 34, 166–189. [Google Scholar] [CrossRef] [PubMed]
- González-Palau, F.; Franco, M.; Bamidis, P.; Losada, R.; Parra, E.; Papageorgiou, S.G.; Vivas, A.B. The effects of a computer-based cognitive and physical training program in a healthy and mildly cognitive impaired aging sample. Aging Ment. Health 2014, 18, 838–846. [Google Scholar] [CrossRef]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.A.; Bamidis, P.D. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: An eLORETA Controlled Study on Resting States. Neural Plast. 2015, 2015, 172192. [Google Scholar] [CrossRef] [PubMed]
- Phirom, K.; Kamnardsiri, T.; Sungkarat, S. Beneficial effects of interactive physical-cognitive game-based training on fall risk and cognitive performance of older adults. Int. J. Environ. Res. Public Health 2020, 17, 6079. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhu, Z.; Wu, B.; McConnell, E.S. Technology-based cognitive training and rehabilitation interventions for individuals with mild cognitive impairment: A systematic review. BMC Geriatr. 2018, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jo, E.-A.; Ji, H.; Kim, K.-H.; Park, J.-J.; Kim, B.H.; Cho, K.I. Exergaming improves executive functions in patients with metabolic syndrome: Randomized controlled trial. JMIR Serious Games 2019, 7, e13575. [Google Scholar] [CrossRef] [PubMed]
- De Croon, R.; Geuens, J.; Verbert, K.; Vanden Abeele, V. A systematic review of the effect of gamification on adherence across disciplines. In Proceedings of the HCI in Games: Experience Design and Game Mechanics: Third International Conference, Virtual Event, 24–29 July 2021. [Google Scholar]
- Baranowski, T.; Buday, R.; Thompson, D.I.; Baranowski, J. Playing for real: Video games and stories for health-related behavior change. Am. J. Prev. Med. 2008, 34, 74–82. [Google Scholar] [CrossRef]
- Valenzuela, T.; Okubo, Y.; Woodbury, A.; Lord, S.R.; Delbaere, K. Adherence to Technology-Based Exercise Programs in Older Adults: A Systematic Review. J. Geriatr. Phys. Ther. 2018, 41, 49–61. [Google Scholar] [CrossRef]
- Campelo, A.M.; Katz, L. Older Adults’ Perceptions of the Usefulness of Technologies for Engaging in Physical Activity: Using Focus Groups to Explore Physical Literacy. Int. J. Environ. Res. Public Health 2020, 17, 1144. [Google Scholar] [CrossRef]
| Authors | Study Design | Population Characteristics | Methods (Focused on Exercise Intervention and Control) | Cognitive Assessments | Main Results |
|---|---|---|---|---|---|
| Evidence in older adults with obesity | |||||
| Napoli et al., 2014 [37] | Experimental study (RCT) Groups: Control Exercise Diet Diet-exercise | Gender (women) = 67 (63%) Age = 69.8 ± 4 years BMI = 37.2 ± 5 kg/m2 | Intervention: exercise training program (Multicomponent of exercise; aerobic exercise (65–85% of HRmax), resistance exercise (65% of 1 RM, 1–2 sets of 8–12 repetitions, gradually increased the intensity to 80% of 1 RM, 2–3 sets of 6–8 repetitions), balance exercise, 90 min/session, 3 sessions/week, 1 year) Control: no exercise | The Modified Mini-Mental State Examination, the Trail Making Test, and the Word List Fluency Test | Exercise group demonstrated better global cognitive function and verbal fluency domain than control group |
| Boidin et al., 2020 [38] | Cross-sectional study Groups: Higher-fit obese Lower-fit obese Non-obese | Gender (women) = 19 (30%) Age = 62 ± 6 years BMI = 29.7 ± 3.9 kg/m2 | Participants were classified by their aerobic fitness relative to lean body mass (peak VO2/LBM) | Neuropsychological test battery | Higher-fit obese had better working memory and executive function than lower-fit obese participants |
| Coll-Padrós et al., 2019 [39] | Cross-sectional study Groups: No or low physical activity Regular physical activity | Gender (women) = 42 (51%) Age = 67.1 ± 4.7 years BMI = 31.8 ± 3.0 kg/m2 | Physical activity was determined by the Rapid Assessment of Physical Activity (RAPA) questionnaire | Neuropsychological test battery | Participants who engaged in regular physical activity had better global cognition, attention, cognitive flexibility, and working memory than no or low physical activity levels |
| Evidence in postmenopausal with overweight and obesity | |||||
| Kim and Kang, 2020 [25] | Experimental study Groups: Premenopausal Postmenopausal | Gender (women) = 52 (100%) Age = 52.68 ± 5.9 years BMI = 25.72 ± 3.6 kg/m2 | Intervention: exercise training program (Aerobic exercise; 50% of HRR and resistance exercise; 55–65% of 1 RM, 3 sets of 10–12 repetitions, 12 weeks) | The Attention Function Index (self-reported cognitive function) | No significantly improved perceived cognitive functioning in postmenopausal group after intervention |
| De Camargo Smolarek et al., 2019 [40] | Experimental study Groups: Control Exercise | Gender (women) = 21 (100%) Age = >60 years BMI = 31.05 ± 2.0 kg/m2 | Intervention: exercise training program (Resistance exercise; 60–70% of 1 RM, 12 weeks) Control: no exercise | The Montreal Cognitive Assessment | Global cognition improvement in exercise group after intervention |
| Authors | Study Design | Population Characteristics | Methods (Focused on Physical–Cognitive Intervention and Control) | Cognitive Assessments | Main Results |
|---|---|---|---|---|---|
| Evidence in obesity with and without postmenopausal condition | |||||
| Staiano et al., 2012 [64] | Experimental study Groups: Control Competitive exergame Cooperative- exergame | Gender (women) = 31 (57%) Age = 16.5 years BMI = 33.1 kg/m2 | Intervention: physical–cognitive training program (Exergame (the Nintendo Wii EA Sports Active exergame), 30 min/sessions, ~5 sessions/week, 10 weeks) Control: no exercise | The Delis-Kaplan Executive Function System (D-KEFS) | Competitive exergame group demonstrated better executive function than cooperative exergame group and control group |
| Garcia-Garro et al., 2020 [65] | Experimental study (RCT) Groups: Control Pilates | Gender (women) = 110 (100%) Age = 68.2 ± 8.4 years BMI = 29.4 ± 4.5 kg/m2 | Intervention: physical–cognitive training program (Pilates exercise (mind-body training), 60 min/session, 2 sessions/week, 12 weeks) Control: no exercise | The Mini-Mental State Examination, Isaacs test, and the Trail Making Test | Pilates group demonstrated better verbal fluency and executive function than control group |
| Jo et al., 2020 [66] | Experimental study (RCT) Groups: Control Treadmill Exergame | Gender (women) = 65 (100%) Age = 60.5 ± 10.8 years BMI = 27.3 ± 3.5 kg/m2 | Intervention: physical–cognitive training program (Exergame (the Exer Heart device), 42–82% of HRR, 40 min/session, 12 weeks) Control: no exercise | - | Exergaming improved cardiorespiratory fitness and endothelial function in a similar way to treadmill exercise, but it had better attendance and adherence rates |
| Authors | Study Design | Population Characteristics | Methods (Focused on Technology-Based Intervention and Control) | Cognitive Assessments | Main Results |
|---|---|---|---|---|---|
| Evidence in obesity, adults, and older adults | |||||
| Ozturk et al., 2022 [80] | Experimental study Groups: Control Telerehabilitation | Gender (women) = 21 (51%) Age = 41.0 ± 12.9 years BMI = 30.9 ± 3.0 kg/m2 | Intervention: telerehabilitation program (trunk stabilization exercises and breathing exercises), 45 min/sessions, 3 sessions/week, 6 weeks Control: no exercise | - | Exercise through telerehabilitation improved physical fitness and quality of life than control group |
| Toro-Ramos et al., 2017 [82] | Experimental study Groups: Control Intervention | Gender (women) = 46 (29%) Age = 37.4 ± 8.7 years BMI = 28.2 ± 3.4 kg/m2 | Intervention: smartphone application (the Noom app; lifestyle intervention, 15 weeks) Control: no exercise | - | After intervention, intervention group had lower body weight, lower %fat, and improved metabolic profiles compared to control group |
| Bonnechère et al., 2020 [87] | Retrospective observational study Groups: 18–24 years old 25–34 years old 35–44 years old 45–55 years old 55–64 years old ≥65 years old | Gender (women) = - Age: 18–24 years = 21.3 ± 2.2 years; 25–34 years = 30.6 ± 3.3 years; 35–44 years = 40.3 ± 4.2 years; 45–54 years = 49.4 ± 3.3 years; 55–64 years = 59.7 ± 3.5 years; ≥65 years = 70.5 ± 4.2 years BMI = - | Cognitive mobile games, 100 gaming sessions | Cognitive mobile game scores | Improved cognitive mobile game scores in all age populations |
| González-Palau et al., 2014 [90] | Experimental study Groups: Healthy MCI | Gender (women) = 40 (80.5%) Age = 73.4 ± 7.5 years BMI = - | Intervention: computer-based cognitive and physical training program (The Long Lasting Memories program), 60 min/session, 3 sessions/week, 12 weeks | Neuropsychological test battery | Intervention improved global cognition, verbal memory, and episodic memory in both MCI and healthy participants |
| Styliadis et al., 2015 [91] | Experimental study Groups: Passive control Active control Combined physical–cognitive training Cognitive training Physical training | Gender (women) = 45 (64%) Age = 70.61 ± 5.2 years BMI = - | Combined computerized physical and cognitive training: The Long Lasting Memories program (LLM), 10 h/week, 8 weeks Cognitive training: CT component of LLM, 1 h/session, 5 sessions/week, 8 weeks Physical training: PT component of LLM, 1 h/session, 5 sessions/week, 8 weeks Passive control: no exercise Active control: no exercise, watching documentaries | The Mini-Mental State Examination and electroencephalogram | Improvement of cognitive function was found in combined physical–cognitive group after training |
| Phirom et al., 2020 [92] | Experimental study Groups: Control Intervention | Gender (women) = 17 (85%) Age = 69.8 ± 3.78 years BMI = - | Intervention: physical–cognitive game-based training (the Xbox Kinect), 60 min/sessions, 3 sessions/week, 12 weeks Control: no exercise | The Montreal Cognitive Assessment | Intervention group demonstrated improvement in global cognitive function than control group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keawtep, P.; Wichayanrat, W.; Boripuntakul, S.; Chattipakorn, S.C.; Sungkarat, S. Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 13364. https://doi.org/10.3390/ijerph192013364
Keawtep P, Wichayanrat W, Boripuntakul S, Chattipakorn SC, Sungkarat S. Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(20):13364. https://doi.org/10.3390/ijerph192013364
Chicago/Turabian StyleKeawtep, Puntarik, Wanachaporn Wichayanrat, Sirinun Boripuntakul, Siriporn C. Chattipakorn, and Somporn Sungkarat. 2022. "Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 20: 13364. https://doi.org/10.3390/ijerph192013364
APA StyleKeawtep, P., Wichayanrat, W., Boripuntakul, S., Chattipakorn, S. C., & Sungkarat, S. (2022). Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review. International Journal of Environmental Research and Public Health, 19(20), 13364. https://doi.org/10.3390/ijerph192013364






