Decision Analytic Modeling for Global Clinical Trial Planning: A Case for HIV-Positive Patients at High Risk for Mycobacterium tuberculosis Sepsis in Uganda
Abstract
1. Introduction
2. Methods
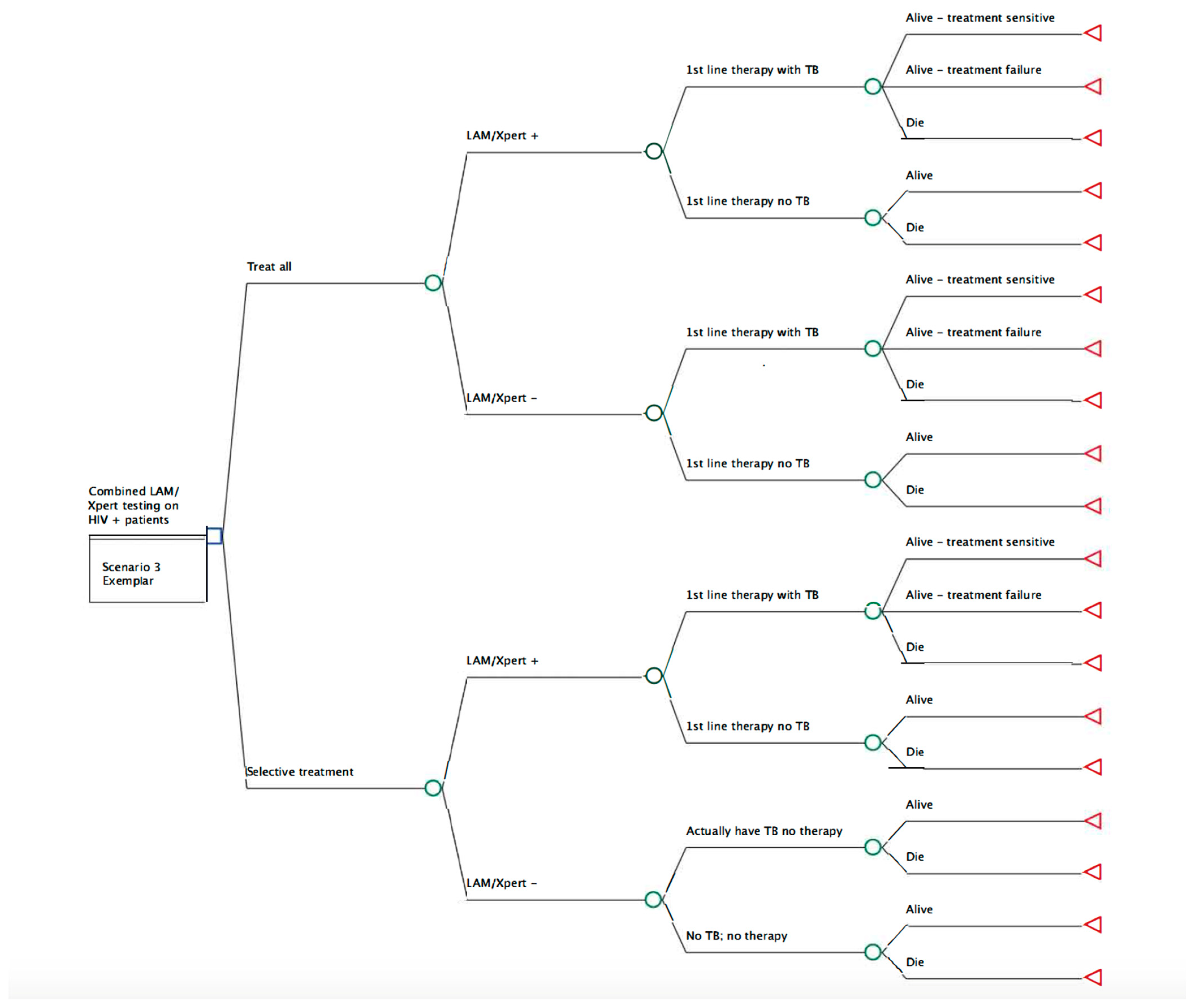
Study Model and Parameters
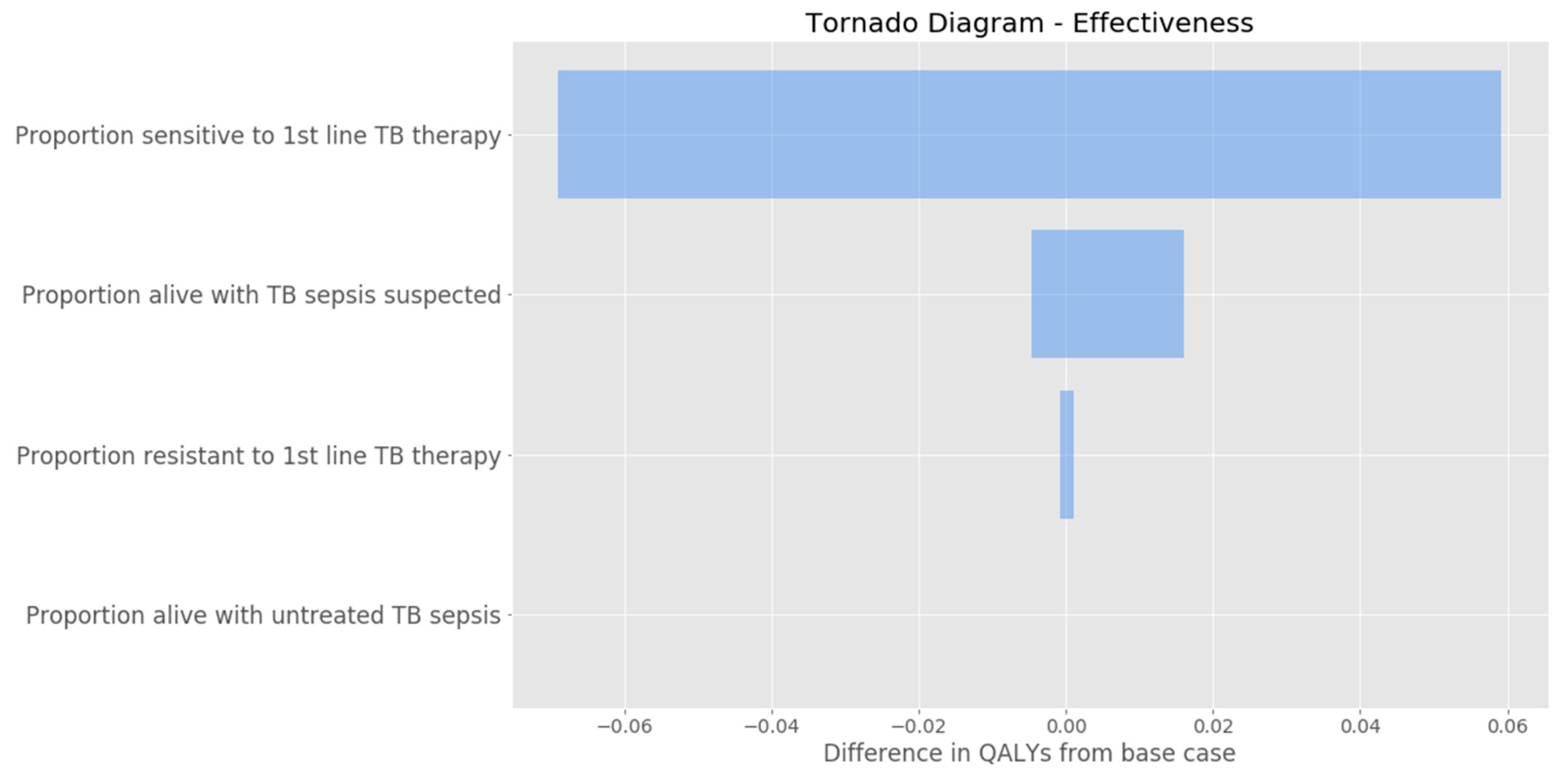
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019; Volume 1, Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed on 1 March 2023).
- Ford, N.; Matteelli, A.; Shubber, Z.; Hermans, S.; Meintjes, G.; Grinsztejn, B.; Waldrop, G.; Kranzer, K.; Doherty, M.; Getahun, H. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: A systematic review and meta-analysis. J. Int. AIDS Soc. 2016, 19, 20714. [Google Scholar] [CrossRef]
- Gupta, R.K.; Lucas, S.B.; Fielding, K.L.; Lawn, S.D. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. Aids 2015, 29, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Kerkhoff, A.D.; Burton, R.; Schutz, C.; Boulle, A.; Vogt, M.; Gupta-Wright, A.; Nicol, M.P.; Meintjes, G. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: A prospective cohort. BMC Med. 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Younis, H.; Kerschbaumer, I.; Moon, J.-Y.; Kim, R.S.; Blanc, C.J.; Chen, T.; Wood, R.; Lawn, S.; Achkar, J.M. Combining urine lipoarabinomannan with antibody detection as a simple non-sputum-based screening method for HIV-associated tuberculosis. PLoS ONE 2019, 14, e0218606. [Google Scholar] [CrossRef]
- Shah, M.; Dowdy, D.; Joloba, M.; Ssengooba, W.; Manabe, Y.C.; Ellner, J.; Dorman, S.E. Cost-effectiveness of novel algorithms for rapid diagnosis of tuberculosis in HIV-infected individuals in Uganda. Aids 2013, 27, 2883–2892. [Google Scholar] [CrossRef]
- Jacob, S.T.; Pavlinac, P.B.; Nakiyingi, L.; Banura, P.; Baeten, J.M.; Morgan, K.; Magaret, A.; Manabe, Y.; Reynolds, S.; Liles, W.C.; et al. Mycobacterium tuberculosis Bacteremia in a Cohort of HIV-Infected Patients Hospitalized with Severe Sepsis in Uganda–High Frequency, Low Clinical Sand Derivation of a Clinical Prediction Score. PLoS ONE 2013, 8, e70305. [Google Scholar] [CrossRef]
- WHO. Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extrapulmonary Tuberculosis among Adults and Adolescents: Recommendations for HIV-Prevalent and Resource-Constrained Settings. Available online: https://www.who.int/hiv/pub/tb/pulmonary/en/ (accessed on 3 March 2023).
- Thorlund, K.; Golchi, S.; Haggstrom, J.; Mills, E.J. Highly Efficient Clinical Trials Simulator (HECT): Software application for planning and simulating platform adaptive trials. Gates Open Res. 2019, 3, 780. [Google Scholar] [CrossRef]
- Jenniskens, K.; Lagerweij, G.R.; Naaktgeboren, C.A.; Hooft, L.; Moons, K.G.; Poldervaart, J.M.; Koffijberg, H.; Reitsma, J.B. Decision analytic modeling was useful to assess the impact of a prediction model on health outcomes before a randomized trial. J. Clin. Epidemiol. 2019, 115, 106–115. [Google Scholar] [CrossRef]
- Kerkhoff, A.D.; Wood, R.; Vogt, M.; Lawn, S.D. Prognostic Value of a Quantitative Analysis of Lipoarabinomannan in Urine from Patients with HIV-Associated Tuberculosis. PLoS ONE 2014, 9, e103285. [Google Scholar] [CrossRef]
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Reipold, E.I.; Ward, A.A.; Barr, D.; Macé, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef]
- Kohli, M.; Schiller, I.; Dendukuri, N.; Dheda, K.; Denkinger, C.M.; Schumacher, S.G.; Steingart, K.R. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance (Review) summary of findings for the main comparison. Cochrane Database Syst Rev. 2018, 8, CD012768. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.G.; Zijenah, L.S.; Chanda, D.; Clowes, P.; Lesosky, M.; Gina, P.; Mehta, N.; Calligaro, G.; Lombard, C.J.; Kadzirange, G.; et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: A pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016, 387, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Wright, A.; Corbett, E.L.; van Oosterhout, J.J.; Wilson, D.; Grint, D.; Alufandika-Moyo, M.; Peters, J.A.; Chiume, L.; Flach, C.; Lawn, S.D.; et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): A pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018, 392, 292–301. [Google Scholar] [CrossRef]
- Reddy, K.P.; Gupta-Wright, A.; Fielding, K.L.; Costantini, S.; Zheng, A.; Corbett, E.L.; Yu, L.; van Oosterhout, J.J.; Resch, S.C.; Wilson, D.P.; et al. Cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV in Africa: A microsimulation modelling study. Lancet Glob. Health 2019, 7, e200–e208. [Google Scholar] [CrossRef] [PubMed]
- Hysell, S. (Unpublished data from the Heysell University of Virginia Laboratory). Personal Communication, May 2020.
- Talmor, D.; Greenberg, D.; Howell, M.D.; Lisbon, A.; Novack, V.; Shapiro, N. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit. Care Med. 2008, 36, 1168–1174. [Google Scholar] [CrossRef]
- Speich, B.; Gloy, V.; Schur, N.; Ewald, H.; Hemkens, L.G.; Schwenkglenks, M.; Briel, M. A scoping review shows that several nonvalidated budget planning tools for randomized trials are available. J. Clin. Epidemiol. 2019, 117, 9–19. [Google Scholar] [CrossRef]
- Roberts, S.L.E.; Healey, A.; Sevdalis, N. Use of health economic evaluation in the implementation and improvement science fields—A systematic literature review. Implement. Sci. 2019, 14, 72. [Google Scholar] [CrossRef]
- Alffenaar, J.-W.C.; Gumbo, T.; Dooley, K.E.; Peloquin, C.A.; Mcilleron, H.; Zagorski, A.; Cirillo, D.M.; Heysell, S.K.; Silva, D.R.; Migliori, G.B. Integrating Pharmacokinetics and Pharmacodynamics in Operational Research to End Tuberculosis. Clin. Infect. Dis. 2019, 70, 1774–1780. [Google Scholar] [CrossRef]
- Steingart, K.R.; Schiller, I.; Horne, D.J.; Pai, M.; Boehme, C.C.; Dendukuri, N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (Review). Cochrane Database Syst. Rev. 2015, 1, 1–167. [Google Scholar] [CrossRef]
- Mukoka, M.; Twabi, H.H.; Msefula, C.; Semphere, R.; Ndhlovu, G.; Lipenga, T.; Sikwese, T.D.; Malisita, K.; Choko, A.; Corbett, E.L.; et al. Utility of Xpert MTB/RIF Ultra and digital chest radiography for the diagnosis and treatment of TB in people living with HIV: A randomised controlled trial (XACT-TB). Trans. R. Soc. Trop. Med. Hyg. 2023, 117, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Sanders, G.; Russell, L.; Siegel, J.; Ganiats, T. Cost-Effectiveness in Health and Medicine; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Foroutan, N.; Tarride, J.-E.; Xie, F.; Mills, F.; Levine, M. A Comparison of Pharmaceutical Budget Impact Analysis (BIA) Recommendations Amongst the Canadian Patented Medicine Prices Review Board (PMPRB), Public and Private Payers. Pharm.-Open 2019, 3, 437–451. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Abilities | Value (Range if Included in Sensitivity Analysis) | Source |
|---|---|---|
| Sensitivity of Urine TB-LAM | 53% | Peter et al. [15] Steingart et al. [15] |
| Specificity of Urine TB-LAM | 96% | Peter et al. [15] |
| Assay sensitivity and ability to obtain diagnostic yield of Sputum Xpert | 42% | Gupta-Wright et al./STAMP trial [16,17] |
| Assay specificity and ability to obtain diagnostic yield of Sputum Xpert | 99% | Gupta-Wright et al./STAMP trial [16,17] |
| Est. sensitivity of combined | 63.5% | Lawn et al. [4] Broger et al. [12] |
| Est. specificity of combined | 99% | Shah et al. (Supplement) [6] |
| Prevalence of TB in HIV + patients with CD4 | 50% | Shah et al. (Supplement) [6] Broger et al. [12] |
| Treatment success rate (sensitive) | 80% (66–92) | Shah et al. (Supplement) [6] |
| Treatment failure (resistance) | 6.5% (4–15) | Shah et al. (Supplement) [6] |
| Death in those given TB treatment | 13.5% | Unpublished data, Heysell et al. (2020) [18] |
| Death in those with untreated TB | 90% (75–100) | Shah et al. [6] |
| Death in TB suspect without TB | 20% (7–30) | Shah et al. [6] |
| Utilities | ||
| Disability weight TB with HIV infection | 0.399 | Shah et al. [6] |
| Disability weight TB treatment | 0.1 | Shah et al. [6] |
| Disability weight HIV on ART | 0.053 | Shah et al. [6] |
| Disability weight severe sepsis | 0.31 | Talmor et al. [19] |
| Costs | ||
| Urine LAM | USD 4.19 | Shah et al. [6] |
| Xpert MTB/Rif | USD 17.42 | Shah et al. [6] |
| Treatment costs TB treatment | USD 195 | Shah et al. [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keim-Malpass, J.; Heysell, S.K.; Thomas, T.A.; Lobo, J.M.; Mpagama, S.G.; Muzoora, C.; Moore, C.C. Decision Analytic Modeling for Global Clinical Trial Planning: A Case for HIV-Positive Patients at High Risk for Mycobacterium tuberculosis Sepsis in Uganda. Int. J. Environ. Res. Public Health 2023, 20, 5041. https://doi.org/10.3390/ijerph20065041
Keim-Malpass J, Heysell SK, Thomas TA, Lobo JM, Mpagama SG, Muzoora C, Moore CC. Decision Analytic Modeling for Global Clinical Trial Planning: A Case for HIV-Positive Patients at High Risk for Mycobacterium tuberculosis Sepsis in Uganda. International Journal of Environmental Research and Public Health. 2023; 20(6):5041. https://doi.org/10.3390/ijerph20065041
Chicago/Turabian StyleKeim-Malpass, Jessica, Scott K. Heysell, Tania A. Thomas, Jennifer M. Lobo, Stellah G. Mpagama, Conrad Muzoora, and Christopher C. Moore. 2023. "Decision Analytic Modeling for Global Clinical Trial Planning: A Case for HIV-Positive Patients at High Risk for Mycobacterium tuberculosis Sepsis in Uganda" International Journal of Environmental Research and Public Health 20, no. 6: 5041. https://doi.org/10.3390/ijerph20065041
APA StyleKeim-Malpass, J., Heysell, S. K., Thomas, T. A., Lobo, J. M., Mpagama, S. G., Muzoora, C., & Moore, C. C. (2023). Decision Analytic Modeling for Global Clinical Trial Planning: A Case for HIV-Positive Patients at High Risk for Mycobacterium tuberculosis Sepsis in Uganda. International Journal of Environmental Research and Public Health, 20(6), 5041. https://doi.org/10.3390/ijerph20065041







