The Effect and Cost-Effectiveness of Offering a Combined Lifestyle Intervention for the Prevention of Cardiovascular Disease in Primary Care: Results of the Healthy Heart Stepped-Wedge Trial
Abstract
1. Introduction
2. Materials and Methods
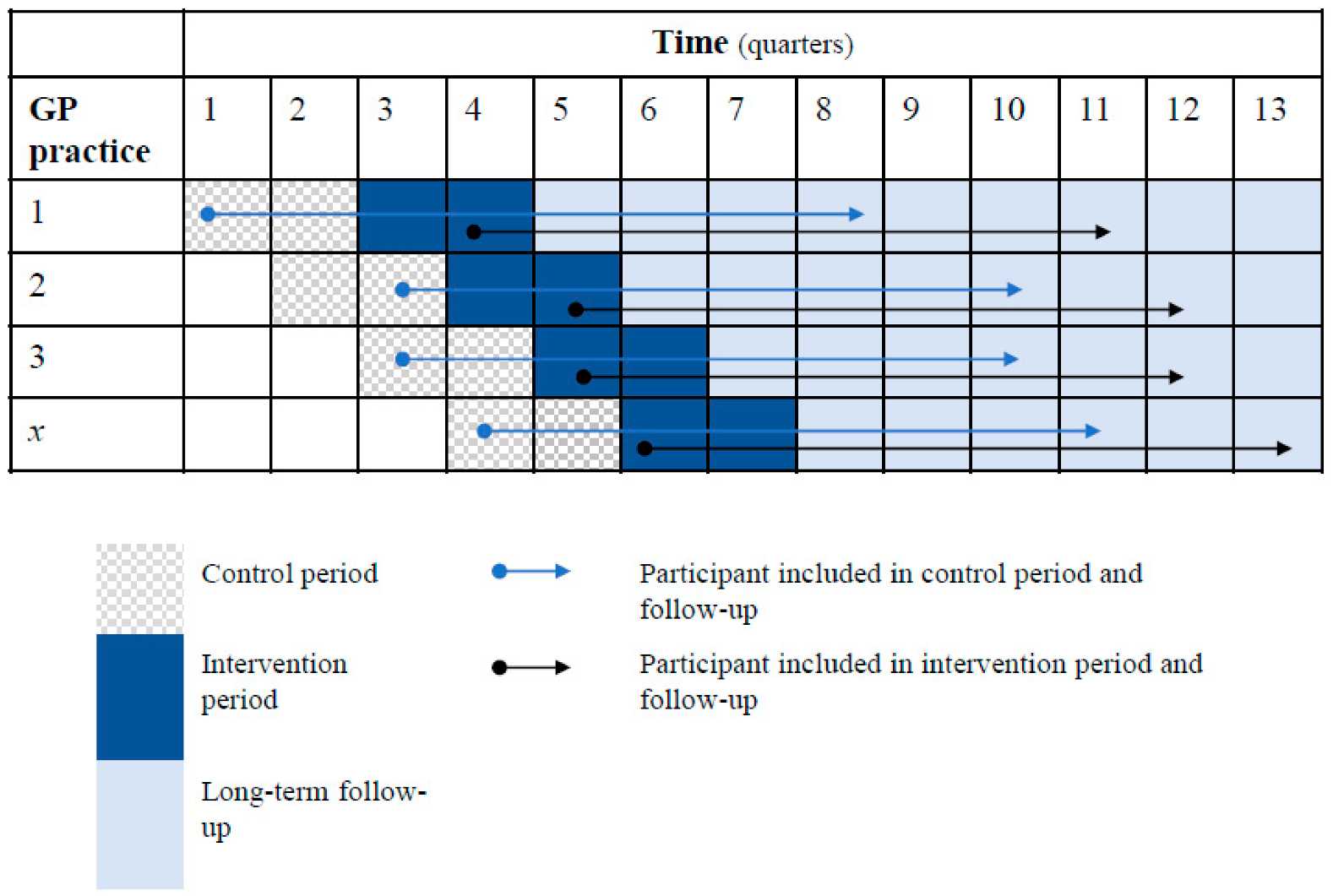
2.1. Study Design and Setting
2.2. Participants
2.2.1. Healthy Heart Lifestyle Programme
2.2.2. Outcomes and Sources Effectiveness Study
2.2.3. Utilities, Healthcare Use and Costs
2.2.4. Determinants
2.3. Statistical Analyses
2.3.1. Effectiveness Study
2.3.2. Economic Evaluation
2.3.3. Missing Data
3. Results
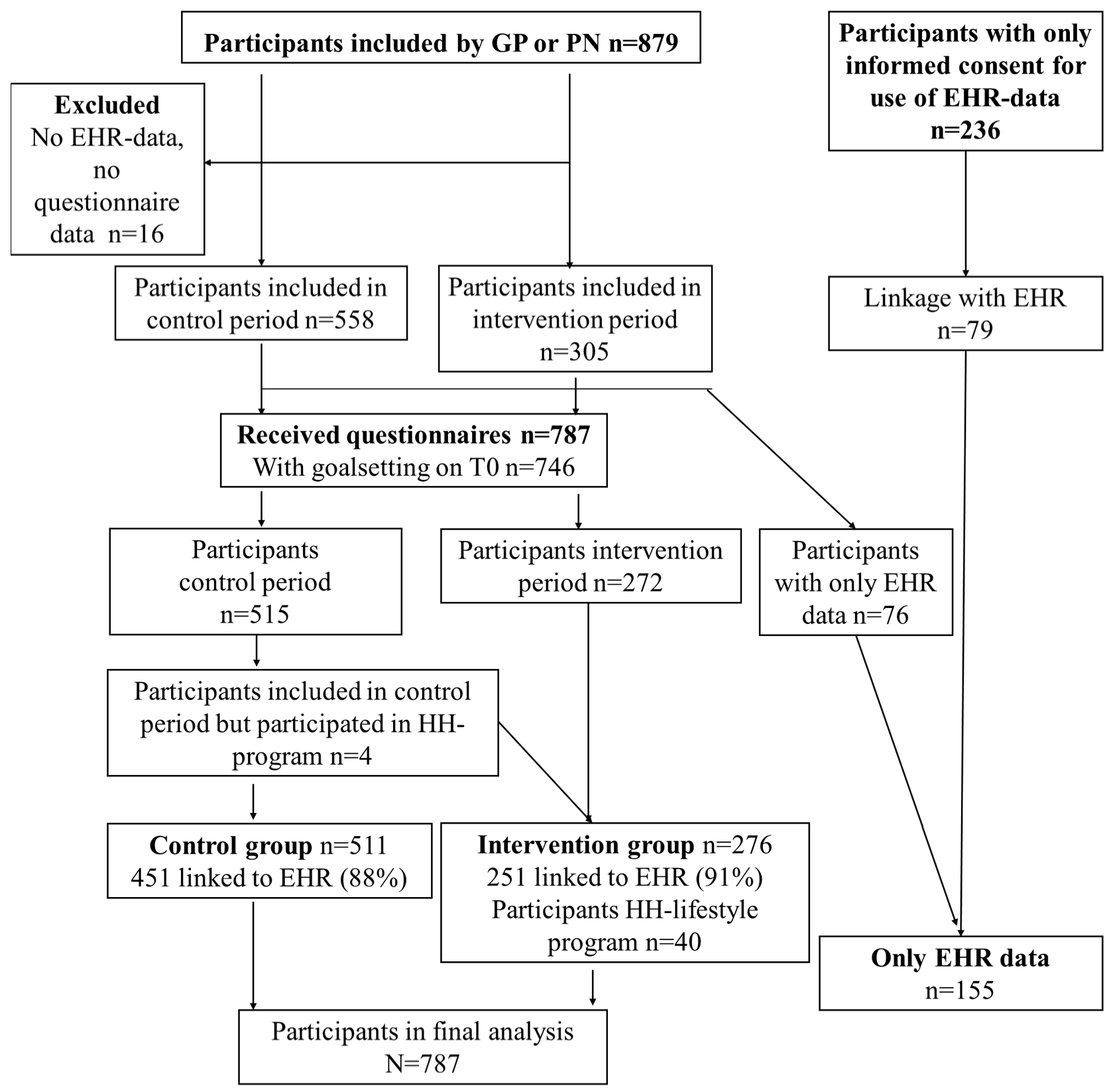
3.1. Recruitment and Characteristics
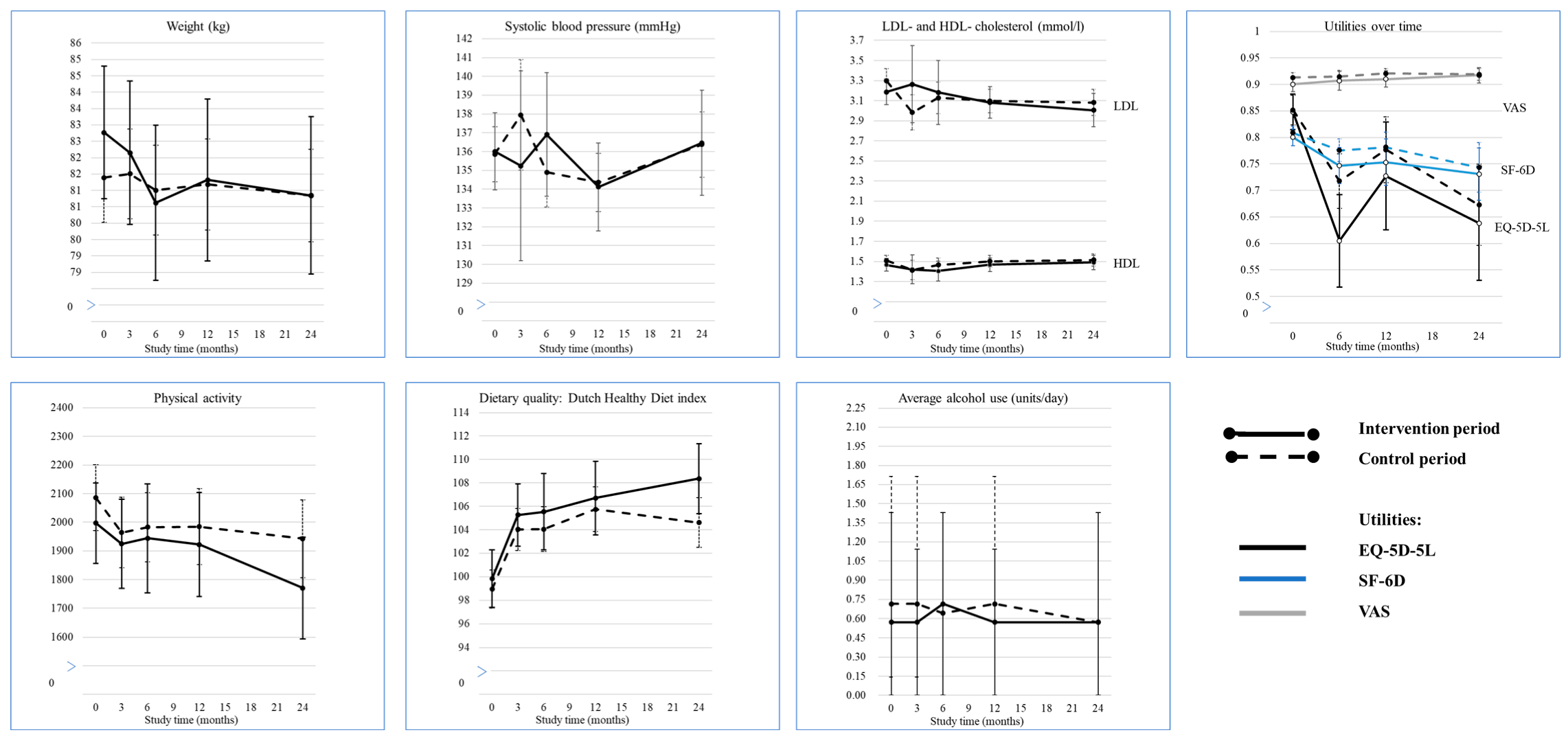
3.2. Cardiovascular and Lifestyle Behaviour Outcomes
3.3. Effect Modification of Sex and Age
3.4. Goalsetting and Outcomes
3.5. Economic Evaluation
3.6. Costs
3.7. In-Depth Analysis
3.7.1. Participants of the Healthy Heart Programme
3.7.2. Participants with Only EHR Data
4. Discussion
4.1. Comparison with Previous Literature
4.2. Limitations
4.3. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilderink, H.B.M.; Plasmans, M.H.D.; Poos, M.J.J.C.; Eysink, P.E.D.; Gijsen, R. Dutch DALYs, current and future burden of disease in the Netherlands. Arch. Public Health 2020, 78, 85. [Google Scholar] [CrossRef] [PubMed]
- Volksgezondheid en Zorg. Hart-en Vaatziekten: Trends en Zorguitgaven. Available online: https://www.volksgezondheidenzorg.info/onderwerp/hart-en-vaatziekten/cijfers-context/trends (accessed on 31 January 2022).
- WHO. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014.
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Pelizzari, P.M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study. Lancet 2020, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Sisti, L.G.; Dajko, M.; Campanella, P.; Shkurti, E.; Ricciardi, W.; de Waure, C. The effect of multifactorial lifestyle interventions on cardiovascular risk factors: A systematic review and meta-analysis of trials conducted in the general population and high risk groups. Prevent. Med. 2018, 109, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Van Rinsum, C.; Gerards, S.; Rutten, G.; Philippens, N.; Janssen, E.; Winkens, B.; Van de Goor, I.; Kremers, S. The Coaching on Lifestyle (CooL) Intervention for Overweight and Obesity: A Longitudinal Study into Participants’ Lifestyle Changes. Int. J. Environ. Res. Public Health 2018, 15, 680. [Google Scholar] [CrossRef]
- Schutte, B.A.; Haveman-Nies, A.; Preller, L. One-Year Results of the BeweegKuur Lifestyle Intervention Implemented in Dutch Primary Healthcare Settings. Biomed Res. Int. 2015, 2015, 484823. [Google Scholar] [CrossRef]
- Duijzer, G.; Haveman-Nies, A.; Jansen, S.C.; Beek, J.T.; van Bruggen, R.; Willink, M.G.J.; Hiddink, G.J.; Feskens, E.J.M. Effect and maintenance of the SLIMMER diabetes prevention lifestyle intervention in Dutch primary healthcare: A randomised controlled trial. Nutr. Diabetes 2017, 7, e268. [Google Scholar] [CrossRef]
- Berendsen, B.A.J.; Kremers, S.P.J.; Savelberg, H.H.C.M.; Schaper, N.C.; Hendriks, M.R.C. The implementation and sustainability of a combined lifestyle intervention in primary care: Mixed method process evaluation. BMC Fam. Pract. 2015, 16, 37. [Google Scholar] [CrossRef]
- van Rinsum, C.; Gerards, S.; Rutten, G.; Johannesma, M.; van de Goor, I.; Kremers, S. The implementation of the coaching on lifestyle (CooL) intervention: Lessons learnt. BMC Health Serv. Res. 2019, 19, 667. [Google Scholar] [CrossRef]
- Oosterhoff, M.; de Weerdt, A.; Feenstra, T.; de Wit, A. Jaarrapportage monitor GLI 2022. Stand van zaken gecombineerde leefstijlinterventie. In Annual Report—Monitor Combined Lifestyle Intervention 2022. Combined Lifestyle Intervention Progress Report; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Badenbroek, I.F.; Nielen, M.M.J.; Hollander, M.; Stol, D.M.; de Wit, N.J.; Schellevis, F.G. Characteristics and motives of non-responders in a stepwise cardiometabolic disease prevention program in primary care. Eur. J. Public Health 2021, 31, 991–996. [Google Scholar] [CrossRef]
- Nierkens, V.; Hartman, M.A.; Nicolaou, M.; Vissenberg, C.; Beune, E.J.; Hosper, K.; van Valkengoed, I.G.; Stronks, K. Effectiveness of cultural adaptations of interventions aimed at smoking cessation, diet, and/or physical activity in ethnic minorities. A systematic review. PloS ONE 2013, 8, e73373. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-van der Bruggen, M.A.M.; Bos, G.t.; Bemelmans, W.J.; Hoogenveen, R.T.; Vijgen, S.M.; Baan, C.A. Lifestyle Interventions Are Cost-Effective in People With Different Levels of Diabetes Risk: Results from a modeling study. Diabetes Care 2007, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- van Wier, M.F.; Lakerveld, J.; Bot, S.D.; Chinapaw, M.J.; Nijpels, G.; van Tulder, M.W. Economic evaluation of a lifestyle intervention in primary care to prevent type 2 diabetes mellitus and cardiovascular diseases: A randomized controlled trial. BMC Fam. Pract. 2013, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Lou, I.; Zabaleta-Del-Olmo, E.; Casajuana-Closas, M.; Sánchez-Viñas, A.; Parody-Rúa, E.; Bolíbar, B.; Iracheta-Todó, M.; Bulilete, O.; López-Jiménez, T.; Pombo-Ramos, H.; et al. Cost-effectiveness analysis of a multiple health behaviour change intervention in people aged between 45 and 75 years: A cluster randomized controlled trial in primary care (EIRA study). Int. J. Behav. Nutr. Phys. Act 2021, 18, 88. [Google Scholar] [CrossRef]
- Walther, D.; Curjuric, I.; Dratva, J.; Schaffner, E.; Quinto, C.; Schmidt-Trucksäss, A.; Eze, I.C.; Burdet, L.; Pons, M.; Gerbase, M.W.; et al. Hypertension, diabetes and lifestyle in the long-term—Results from a Swiss population-based cohort. Prevent. Med. 2017, 97, 56–61. [Google Scholar] [CrossRef]
- Hall, L.H.; Thorneloe, R.; Rodriguez-Lopez, R.; Grice, A.; Thorat, M.A.; Bradbury, K.; Kamble, M.W.; Okoli, G.N.; Powell, D.; Beeken, R.J. Delivering brief physical activity interventions in primary care: A systematic review. Br. J. Gen. Pract. 2021, 72, e209–e216. [Google Scholar] [CrossRef]
- Webb, T.L.; Sheeran, P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol. Bull. 2006, 132, 249–268. [Google Scholar] [CrossRef]
- Fredrix, M.; McSharry, J.; Flannery, C.; Dinneen, S.; Byrne, M. Goal-setting in diabetes self-management: A systematic review and meta-analysis examining content and effectiveness of goal-setting interventions. Psychol. Health 2018, 33, 955–977. [Google Scholar] [CrossRef]
- Cohen, D.J.; Clark, E.C.; Lawson, P.J.; Casucci, B.A.; Flocke, S.A. Identifying teachable moments for health behavior counseling in primary care. Patient Educ. Counsel. 2011, 85, e8–e15. [Google Scholar] [CrossRef]
- Wikström, K.; Lindström, J.; Tuomilehto, J.; Saaristo, T.E.; Helakorpi, S.; Korpi-Hyövälti, E.; Oksa, H.; Vanhala, M.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; et al. National diabetes prevention program (DEHKO): Awareness and self-reported lifestyle changes in Finnish middle-aged population. Public Health 2015, 129, 210–217. [Google Scholar] [CrossRef]
- Bonten, T.N.; Verkleij, S.M.; van der Kleij, R.M.; Busch, K.; van den Hout, W.B.; Chavannes, N.H.; Numans, M.E. Selective prevention of cardiovascular disease using integrated lifestyle intervention in primary care: Protocol of the Healthy Heart stepped-wedge trial. BMJ Open 2021, 11, e043829. [Google Scholar] [CrossRef]
- Wiersma, T.J.; Boukes, F.S.; Geijer, R.M.M.; Goudswaard, A.N. NHG-Standaard Cardiovasculair risicomanagement (eerste herziening). Huisarts Wet 2012, 55, 14–28. [Google Scholar]
- Versteegh, M.M.; Vermeulen, K.M.; Evers, S.M.A.A.; de Wit, G.A.; Prenger, R.; Stolk, E.A. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health 2016, 19, 343–352. [Google Scholar] [CrossRef]
- Brazier, J.E.; Roberts, J. The estimation of a preference-based measure of health from the SF-12. Med. Care 2004, 42, 851–859. [Google Scholar] [CrossRef]
- Stiggelbout, A.M.; Eijkemans, M.J.; Kiebert, G.M.; Kievit, J.; Leer, J.W.; De Haes, H.J. The ‘utility’ of the visual analog scale in medical decision making and technology assessment. Is it an alternative to the time trade-off? Int. J. Technol. Assess Health Care 1996, 12, 291–298. [Google Scholar] [CrossRef]
- Zorginstituut Nederland. Farmacotherapeutisch Kompas. Beschikbaar via. Available online: https://farmacotherapeutischkompas.nl (accessed on 3 February 2022).
- D.H.C.A. Available online: https://www.scal.nl/system/files/inline/Tarieven%20Laboratorium%20Klinische%20Chemie_5.pdf (accessed on 3 February 2022).
- Hakkaart-van Roijen, L.V.d.L.N.; Bouwmans, C.; Kanters, T.; Tan, S.S. Kostenhandleiding. Methodologie van Kostenonderzoek en Referentieprijzen voor Economische Evaluaties in de Gezondheidszorg in Opdracht van Zorginstituut Nederland. Geactualiseerde versie 2016. Available online: https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg (accessed on 4 February 2022).
- Leefbarometer 2018. Available online: https://data.overheid.nl/dataset/leefbaarometer-meting-2018#documentation (accessed on 4 February 2022).
- van Oostrom, S.H.; Picavet, H.S.; van Gelder, B.M.; Lemmens, L.C.; Hoeymans, N.; van Dijk, C.E.; Verheij, R.A.; Schellevis, F.G.; Baan, C.A. Multimorbidity and comorbidity in the Dutch population—Data from general practices. BMC Public Health 2012, 12, 715. [Google Scholar] [CrossRef]
- Fenwick, E.; Marshall, D.A.; Levy, A.R.; Nichol, G. Using and interpreting cost-effectiveness acceptability curves: An example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv. Res. 2006, 6, 52. [Google Scholar] [CrossRef]
- Zabaleta-Del-Olmo, E.; Casajuana-Closas, M.; López-Jiménez, T.; Pombo, H.; Pons-Vigués, M.; Pujol-Ribera, E.; Cabezas-Peña, C.; Llobera, J.; Martí-Lluch, R.; Vicens, C.; et al. Multiple health behaviour change primary care intervention for smoking cessation, physical activity and healthy diet in adults 45 to 75 years old (EIRA study): A hybrid effectiveness-implementation cluster randomised trial. BMC Public Health 2021, 21, 2208. [Google Scholar] [CrossRef]
- Faries, M.D. Why We Don’t “Just Do It”: Understanding the Intention-Behavior Gap in Lifestyle Medicine. Am. J. Lifestyle Med. 2016, 10, 322–329. [Google Scholar] [CrossRef]
- van de Vijver, P.L.; Wielens, H.; Slaets, J.P.J.; van Bodegom, D. Vitality club: A proof-of-principle of peer coaching for daily physical activity by older adults. Transl. Behav. Med. 2018, 8, 204–211. [Google Scholar] [CrossRef]
- Manios, Y.; Androutsos, O.; Lambrinou, C.P.; Cardon, G.; Lindstrom, J.; Annemans, L.; Mateo-Gallego, R.; de Sabata, M.S.; Iotova, V.; Kivela, J.; et al. A school- and community-based intervention to promote healthy lifestyle and prevent type 2 diabetes in vulnerable families across Europe: Design and implementation of the Feel4Diabetes-study. Public Health Nutr. 2018, 21, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- van der Heiden, W.; Lacroix, J.; Moll van Charante, E.; Beune, E. GPs’ views on the implementation of combined lifestyle interventions in primary care in the Netherlands: A qualitative study. BMJ Open 2022, 12, e056451. [Google Scholar] [CrossRef] [PubMed]
- Bukman, A.J.; Teuscher, D.; Meershoek, A.; Renes, R.J.; van Baak, M.A.; Feskens, E.J. Effectiveness of the MetSLIM lifestyle intervention targeting individuals of low socio-economic status and different ethnic origins with elevated waist-to-height ratio. Public Health Nutr. 2017, 20, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- van Bruggen, S.; Rauh, S.P.; Bonten, T.N.; Chavannes, N.H.; Numans, M.E.; Kasteleyn, M.J. Association between GP participation in a primary care group and monitoring of biomedical and lifestyle target indicators in people with type 2 diabetes: A cohort study (ELZHA cohort-1). BMJ Open 2020, 10, e033085. [Google Scholar] [CrossRef] [PubMed]
- Anne K Smit, R.C.V.; Rozemarijn, W.B.; Sanne, M.V.; Jessica, C.; de Jong, K.; Tobias, N. Bonten Implementation of a Group-Based Lifestyle Intervention Programme (Healthy Heart) in General Practices in the Netherlands: A Mixed-Methods Study. Data Avail. Request Author, 2022; unpublished. [Google Scholar]
- Kelly, L.; Harrison, M.; Richardson, N.; Carroll, P.; Robertson, S.; Keohane, A.; Donohoe, A. Reaching beyond the ‘worried well’: Pre-adoption characteristics of participants in ‘Men on the Move’, a community-based physical activity programme. J. Public Health 2019, 41, e192–e202. [Google Scholar] [CrossRef]
- The Hague in Numbers (Den Haag in Cijfers), Dashbord ‘Bevolking’. Available online: https://denhaag.incijfers.nl/dashboard/Overzichten/Bevolking/ (accessed on 5 February 2022).
- Centraal Bureau Voor de Statistiek, D.H.H. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81565NED/table?dl=78DCB (accessed on 17 February 2023).
- The Hague in Numbers (Den Haag in Cijfers), Dashbord ‘Roken’. Available online: https://gezondheidsgids.ggdhaaglanden.nl/mosaic/dashboard/roken/ (accessed on 5 February 2022).
- D.H.H. Centraal Bureau voor de Statistiek. Gezondheidmonitor Volwassenen en Ouderen. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/83674NED (accessed on 20 April 2022).
- Bosscher, R.J.; Smit, J.H. Confirmatory factor analysis of the General Self-Efficacy Scale. Behav. Res. Ther. 1998, 36, 339–343. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- van Lee, L.; Feskens, E.J.; Meijboom, S.; Hooft van Huysduynen, E.J.; van’t Veer, P.; de Vries, J.H. Evaluation of a screener to assess diet quality in the Netherlands. Br. J. Nutr. 2016, 115, 517–526. [Google Scholar] [CrossRef]
- Nederlands Huisartsen Genootschap; De Grauw, W.; De Leest, K.; Schenk, P.; Scherpbier-De Haan, N.; Tjin-A-Ton, J.; Tuut, M.; Van Balen, J. NHG-standaard Chronische Nierschade. TPO Prakt. 2018, 13, 26–29. [Google Scholar]
- Faria, R.; Gomes, M.; Epstein, D.; White, I.R. A Guide to Handling Missing Data in Cost-Effectiveness Analysis Conducted within Randomised Controlled Trials. PharmacoEconomics 2014, 32, 1157–1170. [Google Scholar] [CrossRef]
| Characteristic | Available Data (n) | Period (N = 787) | |||
|---|---|---|---|---|---|
| Intervention (n = 276) | Control (n = 511) | ||||
| n, Unless Otherwise Indicated | %, SD or IQR | n, Unless Otherwise Indicated | % or SD | ||
| Age (years) | 784 | 64.5 | 10.0 | 65.6 | 9.3 |
| Sex (women) | 785 | 159 | 58% | 283 | 56% |
| Origin (Dutch) | 743 | 225 | 86% | 434 | 90% |
| Educational level (low) | 724 | 151 | 59% | 283 | 61% |
| Household composition (cohabiting) | 744 | 194 | 74% | 355 | 74% |
| Job status | 744 | ||||
| Currently employed (<65 years) | 90 | 35% | 172 | 36% | |
| not employed (<65 years) | 41 | 16% | 58 | 12% | |
| retirement (>65 years) | 130 | 50% | 253 | 52% | |
| Neighbourhood deprivation score | 691 | ||||
| Weak | 27 | 11% | 57 | 13% | |
| Satisfactory | 30 | 13% | 80 | 18% | |
| Good–extremely good | 183 | 76% | 314 | 70% | |
| Comorbidity * | 690 | ||||
| Chronic comorbidity | 22 | 9.2% | 34 | 7.5% | |
| Hypercholesterolaemia | 88 | 37% | 145 | 32% | |
| Hypertension | 196 | 82% | 392 | 87% | |
| Macrovascular disease | 11 | 4.6% | 15 | 3.3% | |
| Impaired kidney function | 30 | 13% | 60 | 13% | |
| Medication usage (yes) | 691 | ||||
| Antihypertensive | 177 | 74% | 351 | 78% | |
| Lipid lowering | 93 | 39% | 200 | 44% | |
| Anti coagulants or thrombocyte aggregation inhibitors | 15 | 6.3% | 45 | 10% | |
| Psychiatric medication | 18 | 7.5% | 41 | 9.1% | |
| Body mass index (kg/m2) | 778 | 28.0 | 4.8 | 27.5 | 4.5 |
| Use of alcohol (yes) | 772 | 199 | 74% | 398 | 79% |
| Smoking status | 742 | ||||
| Never | 120 | 48% | 225 | 47% | |
| Previous | 107 | 43% | 213 | 45% | |
| Current | 23 | 9.2% | 39 | 8.2% | |
| General self-efficacy scale (scale 10–40) | 733 | 33 | (30–37) | 33 | (30–37) |
| Quality of life (scale 0–100) Perceived health, EQ-VAS score | 732 | 78.8 | 14.1 | 80.9 | 14.6 |
| Adherence to exercise guideline ** (yes) | 730 | ||||
| minutes of physical activity | 97 | 38% | 181 | 38% | |
| minutes of physical activity + strength and balance | 87 | 34% | 157 | 33% | |
| Utility *** | |||||
| EQ-5D-5L | 786 | 0.85 | 0.28 | 0.85 | 0.32 |
| SF-6D | 786 | 0.80 | 0.13 | 0.81 | 0.14 |
| VAS | 786 | 0.90 | 0.12 | 0.91 | 0.11 |
| Total health care costs (Euros) *** | 786 | EUR 651 | 1423 | EUR 671 | 1567 |
| Control Period Versus Intervention Period | Goal Versus No Goal at Baseline | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Control Period: Baseline | Intervention Period: Baseline | Between- Period Δ after Follow-Up: Adjusted β or Odds (95% CI) b | Goal (No) | Goal (Yes) | Between- Period Δ after Follow-Up: Adjusted β or Odds (95% CI) | ||||||
| Mean, Median or n a | SD, IQR or % | Mean, Median or n a | SD, IQR or % | 3–6 Months | 12–24 Months | Mean, Median or n e | SD, IQR or % | Mean, Median or n e | SD, IQR, or % | 3–6 Months | 12–24 Months | |
| Weight (kg) | 81.4 | 15.8 | 82.8 | 17.0 | −0.5 (−1.1; 0.05) | −0.13 (−1.0; 0.7) | 73.5 | 12.4 | 87.1 | 16.1 | −0.41 (−2.2; 0.2) | 0.75 (−1.7; 0.2) |
| Systolic blood pressure (mmHg) | 136 | 15 | 136 | 15 | 0.15 (−2.7; 3.0) | −0.35 (−2.3; 1.6) | n/a | n/a | n/a | n/a | ||
| LDL-cholesterol (mmol/L) | 3.3 | 1.0 | 3.2 | 0.9 | 0.07 (−0.2; 0.35) | 0.02 (-0.1; 0.2) | n/a | n/a | n/a | n/a | ||
| HDL-cholesterol | 1.5 | 0.4 | 1.5 | 0.4 | −0.03 (−0.10; 0.05) | −0.01 (−0.05; 0.3) | n/a | n/a | n/a | n/a | ||
| Minutes of total weekly physical activity | 2085 | 1297 | 1997 | 1138 | 38 (−97; 171) | −42 (−201; 116) | 1997 | 1168 | 2089 | 1270 | 58 (−75; 190) | 46 (−104; 195) |
| Dietary quality (DHD index, scale 0–150) | 99 | 18 | 100 | 20 | 0.92 (−1.0; 2.8) | 1.47 (−0.6; 3.6) | 100 | 18 | 99 | 18 | 3.1 (1.2; 4.9) | 1.9 (−0.2; 3.9) |
| Alcohol intake c | 0.81 (0.4; 1.5) | 0.66 (0.3; 1.7) | 0.84 (0.4; 1.9) | 2.17 (0.7; 7.1) | ||||||||
| No alcohol | 111 | 22% | 74 | 27% | 168 | 23% | 436 | 59% | ||||
| 0–1 | 201 | 39% | 112 | 41% | 50 | 31% | 242 | 58% | ||||
| 1–2 | 105 | 21% | 47 | 17% | 51 | 32% | 95 | 23% | ||||
| 2 or more | 83 | 16% | 36 | 13% | 54 | 34% | 59 | 14% | ||||
| Quit smoking during follow-up d | 43 | 8.4% | 19 | 6.9% | 2.54 (0.5; 14.2) | 1.07 (0.1; 11.6) | 32 | 52% | 30 | 48% | 0.42 (0.1; 2.5) | 0.30 (0.03; 3.2) |
| Number of CVRM-related contacts with practice in the past 6 months | 1 | 1–2 | 2 | 1–2 | 0.99 (0.9; 1.1) | 0.92 (0.8; 1.1) | n/a | n/a | n/a | n/a | ||
| Goal at Baseline (Yes) (N = 787) | Goal Achieved (Yes) after 6 Months a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Period | Intervention Period | Missing c | Mean Δ (95% CI) | Control Period | Intervention Period | Missing c | |||||
| Goal at Baseline (yes) | N, Mean, Median | %, SD, IQR | N, Mean, Median | %, SD, IQR | % | N, Mean, Median | %, SD, IQR | N, Mean, Median | %, SD, IQR | % | |
| Minutes physical activity per week | 297 | 62% | 172 | 66% | 5.8% | 127 | 61% | 65 | 68% | 25% | |
| Motivation | 7.8 | 1.3 | 8.03 | 1.1 | 0.23 (−0.01; 0.5) | ||||||
| Confidence | 7.2 | 1.5 | 7.55 | 1.4 | 0.32 (0.05; 0.6) | ||||||
| Diet quality | 294 | 62% | 162 | 62% | 6.0% | 147 | 75% | 69 | 73% | 26% | |
| Motivation | 7.5 | 1.5 | 7.81 | 1.3 | 0.30 (0.03; 0.6) | ||||||
| Confidence | 7.2 | 1.5 | 7.55 | 1.4 | 0.37 (0.1; 0.7) | ||||||
| Weight reduction | 274 | 57% | 181 | 69% | 5.8% | 57 | 28% | 42 | 45% | 26% | |
| Motivation | 7.8 | 1.5 | 8.12 | 1.4 | 0.29 (0.02; 0.6) | ||||||
| Confidence | 7.1 | 1.5 | 7.38 | 1.6 | 0.31 (0.02; 0.6) | ||||||
| Alcohol usage b | 101 | (26%) | 59 | (30%) | 6.1% | 83 | 83% | 34 | 77% | 28% | |
| Motivation | 7.1 | 1.7 | 7.4 | 1.6 | 0.26 (−0.3; 0.8) | ||||||
| Confidence | 7.3 | 1.7 | 7.9 | 1.3 | 0.53 (0.2; 1.0) | ||||||
| Quit smoking b | 24 | 55% | 6 | 30% | 5.7% | 4 | 24% | 1 | 50% | 69% | |
| Motivation | 8.0 | 7.3–9.0 | 9 | 8.5–10 | 1.17 (−0.8; 3.1) | ||||||
| Confidence | 6.0 | 5.0–7.0 | 7.5 | 6.8–9.3 | 2.17 (−0.04; 4.4) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieuwenhuijse, E.A.; Vos, R.C.; van den Hout, W.B.; Struijs, J.N.; Verkleij, S.M.; Busch, K.; Numans, M.E.; Bonten, T.N. The Effect and Cost-Effectiveness of Offering a Combined Lifestyle Intervention for the Prevention of Cardiovascular Disease in Primary Care: Results of the Healthy Heart Stepped-Wedge Trial. Int. J. Environ. Res. Public Health 2023, 20, 5040. https://doi.org/10.3390/ijerph20065040
Nieuwenhuijse EA, Vos RC, van den Hout WB, Struijs JN, Verkleij SM, Busch K, Numans ME, Bonten TN. The Effect and Cost-Effectiveness of Offering a Combined Lifestyle Intervention for the Prevention of Cardiovascular Disease in Primary Care: Results of the Healthy Heart Stepped-Wedge Trial. International Journal of Environmental Research and Public Health. 2023; 20(6):5040. https://doi.org/10.3390/ijerph20065040
Chicago/Turabian StyleNieuwenhuijse, Emma A., Rimke C. Vos, Wilbert B. van den Hout, Jeroen N. Struijs, Sanne M. Verkleij, Karin Busch, Mattijs E. Numans, and Tobias N. Bonten. 2023. "The Effect and Cost-Effectiveness of Offering a Combined Lifestyle Intervention for the Prevention of Cardiovascular Disease in Primary Care: Results of the Healthy Heart Stepped-Wedge Trial" International Journal of Environmental Research and Public Health 20, no. 6: 5040. https://doi.org/10.3390/ijerph20065040
APA StyleNieuwenhuijse, E. A., Vos, R. C., van den Hout, W. B., Struijs, J. N., Verkleij, S. M., Busch, K., Numans, M. E., & Bonten, T. N. (2023). The Effect and Cost-Effectiveness of Offering a Combined Lifestyle Intervention for the Prevention of Cardiovascular Disease in Primary Care: Results of the Healthy Heart Stepped-Wedge Trial. International Journal of Environmental Research and Public Health, 20(6), 5040. https://doi.org/10.3390/ijerph20065040






