Abstract
The COVID-19 pandemic severely affected people’s mental health all over the world. This review aims to present a comprehensive overview of the literature related to the effects of COVID-19 lockdown measures and COVID-19 infection on cognitive functioning in both healthy people and people with neurological conditions by considering only standardized tests. We performed a narrative review of the literature via two databases, PUBMED and SCOPUS, from December 2019 to December 2022. In total, 62 out of 1356 articles were selected and organized into three time periods: short-term (1–4 months), medium-term (5–8 months), and long-term (9–12 months), according to the time in which the tests were performed. Regardless of the time period, most studies showed a general worsening in cognitive performance in people with neurological conditions due to COVID-19 lockdown measures and in healthy individuals recovered from COVID-19 infection. Our review is the first to highlight the importance of considering standardized tests as reliable measures to quantify the presence of cognitive deficits due to COVID-19. Indeed, we believe that they provide an objective measure of the cognitive difficulties encountered in the different populations, while allowing clinicians to plan rehabilitation treatments that can be of great help to many patients who still, nowadays, experience post-COVID-19 symptoms.
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global epidemic that is still circulating across countries, leading to public health crises throughout the world [1,2,3]. To contain the speed of viral transmission, many national governments enacted different restrictive measures, such as social distancing, face coverings, avoidance of crowded places, testing, and tracing [4,5,6]. These measures were first limited to the most affected areas, but were rapidly extended to entire countries worldwide [7,8,9]. Regulations also consisted of lockdown measures aimed at further reducing exposure to contagion, which were implemented by the central and local authorities in different ways in China, European nations (such as Italy and Spain), and in the United States [10,11]. However, despite the active vaccination campaigns still in progress worldwide, it is difficult to achieve global control of the pandemic [6].
As it has been now well-documented, lockdown measures and infection due to COVID-19 have greatly affected people’s mental health resulting in severe psychological and cognitive consequences [12,13,14,15]. Indeed, higher levels of anxiety, depression, and stress have been recorded during the confinement period compared to the pre-COVID-19 emergency, disrupting the balance of daily activities and the perception of well-being in both healthy people [16,17,18,19,20] and people with neurological conditions [21,22,23,24]. Lockdown measures imposed during the COVID-19 pandemic also caused cognitive changes in different populations [20,25,26,27,28]. For instance, in Nogueira et al.’s study [28], a deterioration of cognitive flexibility and processing speed compared to pre-COVID-19 confinement was detected in a group of healthy subjects. Additionally, subjective cognitive decline complaints also significantly increased during the pandemic [28]. During COVID-19 lockdown, Pisano et al. [20] reported a decline in working and prospective memory assessed on standardized cognitive tests in a sample of young university students. At the same time, Baschi et al. [25] described a worsening of cognitive, behavioral, and motor symptoms in Parkinson’s (PD) and Mild Cognitive Impairment (MCI) patients. The negative impact of COVID-19 isolation on cognitive functioning was also reported by Chen et al.’s study [27]. In their study, Alzheimer’s (AD) and dementia with Lewy bodies (DLB) patients exhibited an accelerated cognitive decline and neuropsychiatric symptoms over a one-year follow-up period [27].
It is worth noting that 43% of individuals affected by COVID-19 infection, including asymptomatic cases, and approximately 80% of patients hospitalized due to COVID-19 may experience post-COVID-19 sequelae [29,30]. Fatigue and cognitive impairment, along with other enduring neuropsychiatric (e.g., depression) [31] and physical (e.g., dyspnea) manifestations, have been described as part of the ‘post-acute sequelae of SARS-CoV-2′ (i.e., symptoms persisting for at least four weeks following infection) [32], colloquially, also referred to as “long COVID” or “post-COVID” [33,34].
As for studies on COVID-19 lockdown, several studies have investigated the effects induced by COVID-19 infection on cognitive functioning in healthy and neurological populations [35,36,37,38] using either self-reported questionnaires or standardized tests.
In a New York cohort of 740 COVID-19 patients (50% managed in a community setting), Becker et al. [35] reported a deterioration in memory encoding (24% affected), category fluency (20%), processing speed (18%), and executive functions (16%) [35]. A prospective study by Frontera et al. [37] showed that patients with neurological complications during index hospitalization had significantly worse six-months functional and cognitive outcomes than those without. Importantly, the authors found that approximately 50% of COVID-19 patients reported cognitive deficits and 47% was unable to return to work after six months. In line with this evidence, Boesl et al. [36] administered a screening test and self-questionnaires to a sample of 100 patients who presented with persisting neurological symptoms 12 weeks after the acute infection with SARS-CoV-2. The residual neurological symptoms indicated the persistence of fatigue, headache, and pathological scores on the Montreal Cognitive Assessment Scale, a test used by healthcare providers to evaluate the presence of cognitive decline [39].
Given the above reported results, the scope of this review is to present a comprehensive overview of the literature related to the effects of COVID-19 lockdown measures and COVID-19 infection on cognitive functioning in healthy people and people with neurological conditions. To this end, we decided to investigate only studies which used standardized tests to assess cognitive decline. Indeed, since self-reported questionnaires are more susceptible to social desirability and self-reported bias, they might lead to inaccurate self-reports and erroneous study conclusions.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
We conducted this study using the scope reviews methodological framework. We searched for articles on cognitive effects of COVID-19 lockdown measures and COVID-19 infection among healthy people and people with neurological conditions on two databases: PubMed and Scopus. Four different searches were conducted using different keywords combined with the Boolean operator “AND” and “OR”. The search period was set from December 2019 to December 2022. Keywords included: (COVID-19 lockdown or confinement measures) AND (Cognitive deficits OR Memory deficits OR Language deficits OR Attention Deficits); (Long COVID-19 OR Post COVID-19 OR Cognitive Sequelae of COVID-19) AND (Cognitive deficits OR Memory deficits OR Language deficits OR Attention Deficits); (Long COVID-19 OR Post COVID-19 OR Cognitive Sequelae of COVID) AND (Parkinson OR Dementia OR Alzheimer OR Stroke); (COVID-19 lockdown OR confinement measures) AND (Parkinson OR Dementia OR Alzheimer OR Stroke).
Included articles met the following criteria: (i) only studies using standardized cognitive tests on the effects of the COVID-19 lockdown/confinement measures and on the effects of COVID-19 infection among healthy people and people with neurological conditions; (ii) only studies conducted with participants over 18 years of age; and (iii) only studies with samples larger than 20 participants (N = >20); (iv) only studies conducted between December 2019 and December 2022. We excluded non-COVID-19 articles and COVID-19 articles not related to the study. Articles were also excluded if they were reviews, single case studies or case series. After eliminating duplicates, all potentially relevant full texts were screened by the authors (AM, FP) independently of one another to exclude non-eligible items.
2.2. Data Extraction and Analysis
A total of 1356 articles were retrieved through database searching. After the removal of 398 duplicates, a total of 958 articles remained, out of which 661 articles were excluded by title or abstract for not dealing with our research topic, 19 were removed as reporting case series, and 60 were excluded as referring to reviews. A total of 218 articles were considered eligible for the study. After full text screening, another 156 articles were removed since four were single cases, 31 mixed neurological with healthy participants, 14 included less than 20 participants, two had only the abstract available, 56 were not related to cognitive sequelae of COVID-19, six were not clinical trials, 32 did not include standardized tests, three were longitudinal studies, thus, it was not possible to individuate a precise period of testing time, and eight did not report the time of testing (see Figure 1).
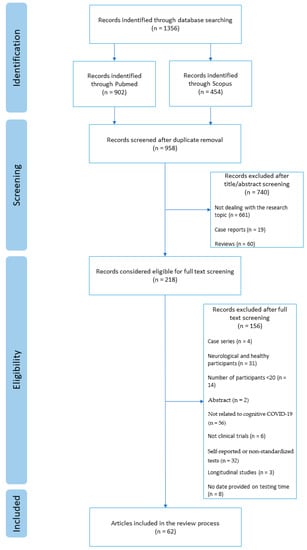
Figure 1.
Flow diagram of review process.
The selected 62 articles were rearranged according to the two principal aims of the review: (1) studies on the impact of COVID-19 lockdown measures on the cognitive functions (N = 16) of, respectively, (1a) people with neurological conditions (N = 14) and (1b) healthy people (N = 2); and (2) studies on the impact of COVID-19 infection on cognitive functions (N = 46). No studies on people with neurological conditions met our inclusion criteria in this category; thus, all studies in this category referred to healthy people (N = 46; see Figure 2). Finally, for each category, studies were organized into three further subgroups according to the time elapsed between the testing and the beginning of lockdown measures or COVID-19 infection: short-term period (1–4 months), medium-term period (5–8 months), and long-term period (9–12 months; see Table 1 and Table 2).
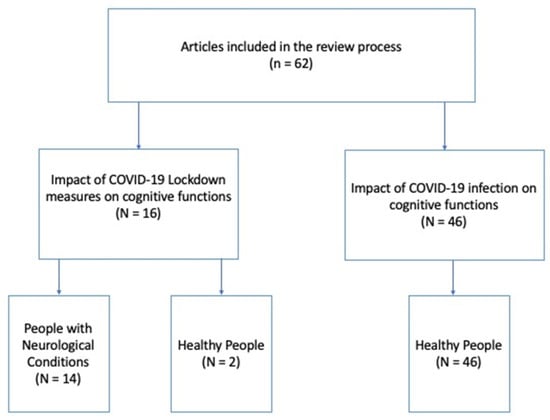
Figure 2.
Articles included in the review process.

Table 1.
Summary of studies reporting the negative effects of COVID-19 lockdown measures on cognitive performance, respectively, in people with neurological conditions and healthy people for the three-time testing periods (short 1–4 months, medium 5–8 months, long 9–12 months).

Table 2.
Summary of studies reporting the negative effects of COVID-19 infection on cognitive performance in healthy people for the three time periods (short 1–4 months, medium 5–8 months, long 9–12 months).
3. Results
The results obtained in this review are shown in Table 1 for cognitive studies related to COVID-19 lockdown measures on people with neurological conditions and healthy people, and in Table 2 for cognitive studies related to COVID-19 cognitive sequelae due to COVID-19 infection in healthy people.
As reported in Table 1, we identified the negative effects of COVID-19 lockdown measures on cognitive functions in 12 out of 16 studies. In particular, during the first four months of COVID-19 lockdown measures (short period), a worsening in cognitive performance was reported in four out of seven studies in different neurological populations [25,42,43,44]. In particular, in most of the patients, a decline in cognitive functions resulted from the MMSE, while in Tsatali et al. [44], a worsening in learning and phonemic fluency in people with MCI and AD was reported. Conversely, Dura-Perez et al. [40], Gareri et al. [41] and Vislapuu et al. [45] did not find significant cognitive differences in people with neurological conditions due to COVID-19 lockdown measures. During the medium and long period of COVID-19 lockdown measures, all groups of neurological patients exhibited a significant decline in functional and cognitive status compared to the pre-COVID period. During the medium period (5 to 8 months), three out of four studies showed adverse effects of COVID-19 lockdown measures on attention [46], and on the overall patients’ cognitive status [48,49], except for Ref. [47]. During the long period (9 to 12 months), three studies reported a decrease in the patients’ overall cognitive status [27,50,51].
Only two studies were performed on healthy people by using standardized tests. The study by Pisano et al. [20], performed in the first four months of the lockdown measures (short period), reported a worsening in working and prospective memory performance in a group of 150 college students; while in the medium period, the only study by Favieri et al. [52] showed impaired executive functioning and motor inhibition in a sample of 90 college students.
As reported in Table 2, the negative effects of COVID-19 infection on cognitive performance in healthy people were identified in 39 out of 46 studies (85%). Five out of seven studies performed in the short period (1–4 months) found a general worsening in cognitive performance [53,54,57,58], specifically, in verbal memory [54,57] and attention tasks [55]. On the contrary, Johnsen et al. [56] and Priftis at al. [59] did not find significative differences in any cognitive domains.
A total of 27 out of 32 articles reported negative effects of COVID-19 infection during the medium period (5–8 months). As in the short period, most of the authors found a significant general cognitive decline [60,61,62,63,64,68,69,70,73,77,78,82,83,84,85], in particular, in memory [62,65,66,74,77,81,85,86,87,88], verbal fluency [62,65,66,71,72,88], executive functions [65,69,72,74,75,81,87,88] and attention tasks [65,72,76,89]. Three studies did not report significant effects on cognitive performance in hospitalized people that resulted positive in the SARS CoV-2 nasopharyngeal test compared to those with no history of the virus [37,38,79]; while, in the Pilotto et al. [83] and Stallmach et al. [84] study, a very low percentage of people with COVID-19 infection showed the presence of cognitive decline.
The seven studies which have investigated the long-term effects of COVID infection (9 to 12 months) found a deterioration of cognitive performance in different cognitive domains, such as in overall cognition [91], memory [90,92,93,94], attention [95,96], executive functions [90,92] and visuospatial abilities [90].
4. Discussion
This review aims to present a comprehensive overview of the literature related to the effects of lockdown measures and COVID-19 infection on cognitive functioning in healthy and neurological populations. Considering the large number of papers published to date on these topics, as far as we know, this is the first review which investigates the effects of the pandemic on cognitive functioning by using standardized cognitive tests. Indeed, most of the studies have included self-reported measures, such as questionnaires. In clinical practice and/or research investigation, choosing an appropriate cognitive functional measure is first of all a critical decision for the necessity to refer to measures with robust reliability [97]. In general, two main measures, self-reported questionnaires and standardized tests, are used to assess cognitive functioning. Self-reported measures are favored among clinicians and researchers because they are relatively easy to administer and they are time and cost-effective [98,99]. However, it is well known that they are more susceptible to social desirability and self-reported bias [100]. The main disadvantage of self-reported questionnaires might also be the possibility of providing invalid answers. While responding to the items, respondents may not answer truthfully, especially on sensitive questions [101]. Conversely, standardized tests overcome some of these limitations. The main benefit of standardized tests is that they are objective measures, more reliable and valid than non-standardized measures [102]. They often provide some type of “standard score” which can help interpret how far participant’s results range from the average [102]. A recent multilevel random-effects meta-analysis revealed no relationship between self-reported and neuropsychological tests of cognitive flexibility, suggesting that self-reported questionnaires should no longer be considered valid proxies for measuring cognitive flexibility [102]. For these reasons, in the present review, we have decided to include only studies on the impact of COVID-19 lockdown measures or COVID-19 infection on cognitive standardized tests.
Surprisingly, our research revealed that only two works have used standardized tests during COVID-19 lockdown measures in healthy subjects compared to neurological populations. Indeed, during the lockdown, most studies have applied standardized tests in people with neurodegenerative diseases (i.e., MCI, PD, AD). Probably because healthy subjects are considered capable of responding autonomously, researchers have preferred to test them by using self-reported questionnaires that are easily administered online. In contrast, researchers were very much concerned with investigating whether or not, due to the adoption of lockdown measures, neurodegenerative populations presented a worsening in their cognitive status; thus, they chose standardized tests as more reliable measures. In general, almost all studies indicated a decrease in the MMSE and MoCA’s score, two measurements widely adopted in clinical practice to detect the presence of cognitive decline in neurodegenerative diseases as an index of disease progression [39,103,104]. We cannot state unequivocally whether or not this worsening was due to the adoption of confinement measures, or to the characteristics of the disease whose symptoms tend to worsen over time in neurodegenerative populations. It could also be argued that, since several studies have reported higher levels of anxiety and depression in these people [22,105,106], their psychological status has, in turn, contributed to an increase in cognitive decline. Indeed, changes in everyday life routines were applied during the pandemic leading to a worsening in the psychological status of different populations [107]. For instance, since people with dementia usually require daily assistance, they could not have rapidly adapted themselves to changing situations as was required by the pandemic [108]. Thus, the lack of social stimulation and pleasurable activities favored the onset of anxiety and depression, which, in turn, cognitively affected the progression of the disease [106]. During the first wave of COVID-19, together with a general cognitive decline, Aragón et al., 2022 [46] reported a worsening in selective attention tasks in four patients with subjective cognitive decline and forty-seven MCI participants. These tasks were appropriately designed by the authors for testing executive attention. The first task was an audio dictation of reverse digits backwards. The second task included another audio with a song fragment in which patients had to count the number of times they heard a designated word and write the answer with a maximum score of 19 [46].
In terms of the two studies on healthy subjects, Pisano et al. [20] showed a decline in working and prospective memory, measured with the PASAT [109] and the MIST [110] test, in a sample of young university students, while Favieri et al., 2022 [52] reported a decline in executive functions, measured with the STROOP test [111], and in motor inhibition in a Go/No-Go task, in ninety college students.
In contrast, all studies on the effects of COVID-19 infection on cognition, measured through standardized tests, have been conducted on healthy individuals. Indeed, the vast majority of research has intentionally excluded individuals with previous neurological and psychiatric disorders, who would have confounded the interpretation of the results [93]. Almost all studies reported the presence of a general cognitive decline [60,61,62,63,64,68,69,70,73,77,78,82,83,84,85] (see Table 2), which is a common sequela of other viral diseases, such as AIDS [112,113] and sepsis ([114,115]. In the literature, this status is often referred to as ‘Long COVID’ [116,117], or ‘brain fog’ with accompanied clinical symptoms, such as low energy, insomnia, problems in concentration and spatial orientation and difficulty in finding the right words [118]. In particular, some studies reported a decrease in short-and-long term memory performance [62,65,66,74,77,81,85,86,87,88], in verbal fluency [62,65,66,71,72,88], in executive functions [65,69,72,74,75,81,87,88] and in selective attention tasks [65,72,76,89]. It is likely the case, as suggested by previous studies, that these cognitive deficits occurred as a consequence of respiratory symptoms severity due to the pandemic [119,120]. Indeed, cognitive deficits in people who were intubated and/or required a lengthy hospital stay are expected due to the lack of oxygen to the brain [118]. Respiratory viruses manage to bypass the blood–brain barrier using either infected blood cells, such as “Trojan Horses”, or by exploiting the axonal route, crossing neurons one by one [121]. Similarly, in milder cases who have not been hospitalized, it is possible that the lowest cognitive implications were due to less severe hypoxia [118]. Indeed, several studies have suggested that COVID-19 infection may cause alterations in white and grey matter volume of the hippocampus, which plays a central role in learning and memory [122,123,124]. Accordingly, the effects on the hippocampus are due to the hypoxic and hypoxemic conditions of COVID-19 patients, which exert a negative effect on hippocampal neurogenesis [125]. As previously reported, other impaired cognitive domains, reported in healthy people due to COVID-19 infection, were present in selective attention and executive functions tasks [55,57,62,65,71,81,88]. Interestingly, a recent report on a single case neuroimaging study with anosmia, due to COVID-19, revealed reduced metabolic activity in the orbitofrontal cortex, suggesting impaired neural function in this region [126]. It is well-known that the orbitofrontal cortex is responsible not only for the detection of common odors [127], but also for executive functions and attentional processing [128,129,130]. Thus, although future studies should elucidate this issue, the hypothesis might be advanced that, together with the lack of oxygen to the brain due to respiratory symptoms, executive functions and attentional deficits also arise as a consequence of abnormal activity in the orbitofrontal cortex.
It is worth considering that the studies reported in our review on neurological populations revealed the presence of cognitive decline regardless of the time elapsed between the beginning of the confinement measures and the administration of standardized tests. Indeed, the presence of a worsening in cognitive performance in these populations was present independently of the time period in which the tests were performed (short 1–4 months, medium 5–8 months, long 9–12 months; see Table 1). Similarly, studies in healthy subjects revealed the presence of cognitive deficits in the three time periods following COVID-19 infection, albeit most studies tested participants between five to eight months after the infection. As far as we know, this is the first review which investigates the impact of confinement measures and COVID-19 infection in neurological and healthy populations by including only standardized cognitive tests. The pandemic has been an unexpected, dramatic event that spread panic among civilians and insecurity at all socio-political and economic levels, suddenly disrupting everyday life. Thus, it was expected that it would immediately impact the population as a whole with severe psychological and cognitive implications. Indeed, our findings are in line with previous literature on COVID-19 which report the presence of cognitive decline in the short [20,42,53], medium [49,52,61], and long-term periods [50,92].
5. Conclusions
In conclusion, our review is the first to highlight the importance of considering standardized tests as reliable measures to quantify the presence of cognitive deficits due to COVID-19. Indeed, we strongly believe that these tests guarantee a valid, objective measure of the cognitive status tested in various populations. By administrating the same test over time, clinicians and researchers have the main advantage to show significant changes referring to the same normative data. In addition, patients’ test scores can also be easily compared to each other to identify the presence of cognitive difficulties in a particular area, thus, allowing clinicians for the planning of rehabilitation treatments focused on the impaired cognitive domain. This choice could be of great help to many patients who still, nowadays, experience post-COVID-19 symptoms.
Author Contributions
Conceptualization, P.M. and A.M.; methodology, A.M. and C.I.; data curation, A.M., F.P., C.I. and P.M.; writing—original draft preparation, A.M. and F.P.; writing—review and editing, P.M.; supervision, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, V.C.C.; Ip, J.D.; Chu, A.W.H.; Tam, A.R.; Chan, W.M.; Abdullah, S.M.U.; Chan, B.P.C.; Wong, S.C.; Kwan, M.Y.W.; Chua, G.T.; et al. Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Subvariant BA.2 in a Single-Source Community Outbreak. Clin. Infect. Dis. 2022, 75, e44–e49. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J. Infect. Public Health 2022, 15, 228–240. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Benros, M.E.; Klein, R.S.; Vinkers, C.H. How COVID-19 shaped mental health: From infection to pandemic effects. Nat. Med. 2022, 28, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; et al. Physical Distancing, Face Masks, and Eye Protection to Prevent Person-to-Person Transmission of SARS-CoV-2 and COVID-19: A Systematic Review and Meta-Analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Escandón, K.; Rasmussen, A.L.; Bogoch, I.I.; Murray, E.J.; Escandón, K.; Popescu, S.V.; Kindrachuk, J. COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. BMC Infect. Dis. 2021, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Nainu, F.; Frediansyah, A.; Yatoo, M.I.; Mohapatra, R.K.; Chakraborty, S.; Zhou, H.; Islam, R.; Mamada, S.S.; Kusuma, H.I.; et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health 2023, 16, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Haug, N.; Geyrhofer, L.; Londei, A.; Dervic, E.; Desvars-Larrive, A.; Loreto, V.; Pinior, B.; Thurner, S.; Klimek, P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2020, 4, 1303–1312. [Google Scholar] [CrossRef]
- Qiu, J.; Shen, B.; Zhao, M.; Wang, Z.; Xie, B.; Xu, Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen. Psychiatry 2020, 33, e100213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Wang, J.; Li, M.; Wang, S.; He, X.; Zhou, C. Evolution and control of the COVID-19 pandemic: A global perspective. Cities 2022, 130, 103907. [Google Scholar] [CrossRef]
- Dzúrová, D.; Květoň, V. How health capabilities and government restrictions affect the COVID-19 pandemic: Cross-country differences in Europe. Appl. Geogr. 2021, 135, 102551. [Google Scholar] [CrossRef]
- Vagnini, D.; Hou, W.K.; Hougen, C.; Cano, A.; Bonanomi, A.; Facchin, F.; Molgora, S.; Pagnini, F.; Saita, E. The impact of COVID-19 perceived threat and restrictive measures on mental health in Italy, Spain, New York, and Hong Kong: An international multisite study. Front. Psychol. 2022, 13, 1002936. [Google Scholar] [CrossRef]
- Ammar, A.; Mueller, P.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Brach, M.; Schmicker, M.; Bentlage, E.; et al. Psychological consequences of COVID-19 home confinement: The ECLB-COVID19 multicenter study. PLoS ONE 2020, 15, e0240204. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Campion, J.; Horowitz, T.; Barthelemy, F.; Cammilleri, S.; Ceccaldi, M. The impact of COVID-19 lockdown on brain metabolism. Hum. Brain Mapp. 2021, 43, 593–597. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Benzeval, M.; Hainey, K.; Leyland, A.H.; Katikireddi, S.V. Psychological distress among people with probable COVID-19 infection: Analysis of the UK Household Longitudinal Study. BJPsych Open 2021, 7, e104. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.E.V.; Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. Neurological Manifestations in COVID-19 Infection: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2021, 48, 66–76. [Google Scholar] [CrossRef]
- Vahia, I.V.; Jeste, D.V.; Reynolds, C.F. Older Adults and the Mental Health Effects of COVID-19. JAMA 2020, 324, 2253. [Google Scholar] [CrossRef]
- Grolli, R.E.; Mingoti, M.E.D.; Bertollo, A.G.; Luzardo, A.R.; Quevedo, J.; Réus, G.Z.; Ignácio, Z.M. Impact of COVID-19 in the Mental Health in Elderly: Psychological and Biological Updates. Mol. Neurobiol. 2021, 58, 1905–1916. [Google Scholar] [CrossRef]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; McIntyre, R.S.; Choo, F.N.; Tran, B.; Ho, R.; Sharma, V.K.; et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 2020, 87, 40–48. [Google Scholar] [CrossRef]
- Wathelet, M.; Duhem, S.; Vaiva, G.; Baubet, T.; Habran, E.; Veerapa, E.; Debien, C.; Molenda, S.; Horn, M.; Grandgenèvre, P.; et al. Factors associated with mental health disorders among College students in France confined during the COVID-19 pandemic. JAMA Netw. Open 2020, 3, e2025591. [Google Scholar] [CrossRef]
- Pisano, F.; Torromino, G.; Brachi, D.; Quadrini, A.; Incoccia, C.; Marangolo, P. A Standardized Prospective Memory Evaluation of the Effects of COVID-19 Confinement on Young Students. J. Clin. Med. 2021, 10, 3919. [Google Scholar] [CrossRef]
- Pisano, F.; Giachero, A.; Rugiero, C.; Calati, M.; Marangolo, P. Does COVID-19 Impact Less on Post-stroke Aphasia? This Is Not the Case. Front. Psychol. 2020, 11, 564717. [Google Scholar] [CrossRef] [PubMed]
- El Haj, M.; Altintas, E.; Chapelet, G.; Kapogiannis, D.; Gallouj, K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020, 291, 113294. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, E.; Artusi, C.A.; Rosano, C.; Boschetto, C.; Imbalzano, G.; Romagnolo, A.; Bozzali, M.; Rizzone, M.G.; Zibetti, M.; Lopiano, L. Anxiety, depression, and worries in advanced Parkinson disease during COVID-19 pandemic. Neurol. Sci. 2022, 43, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.; Capuano, R.; Bisecco, A.; D’Ambrosio, A.; Buonanno, D.; Tedeschi, G.; Santangelo, G.; Gallo, A. The psychological impact of Covid-19 pandemic on people with Multiple Sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2022, 61, 103774. [Google Scholar] [CrossRef] [PubMed]
- Baschi, R.; Luca, A.; Nicoletti, A.; Caccamo, M.; Cicero, C.E.; D’Agate, C.; Di Giorgi, L.; La Bianca, G.; Castro, T.L.; Zappia, M.; et al. Changes in Motor, Cognitive, and Behavioral Symptoms in Parkinson’s Disease and Mild Cognitive Impairment During the COVID-19 Lockdown. Front. Psychiatry 2020, 11, 590134. [Google Scholar] [CrossRef] [PubMed]
- Pisano, F.; Manfredini, A.; Brachi, D.; Landi, L.; Sorrentino, L.; Bottone, M.; Incoccia, C.; Marangolo, P. How Has COVID-19 Impacted Our Language Use? Int. J. Environ. Res. Public Health 2022, 19, 13836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-C.; Liu, S.; Gan, J.; Ma, L.; Du, X.; Zhu, H.; Han, J.; Xu, J.; Wu, H.; Fei, M.; et al. The Impact of the COVID-19 Pandemic and Lockdown on Mild Cognitive Impairment, Alzheimer’s Disease and Dementia with Lewy Bodies in China: A 1-Year Follow-Up Study. Front. Psychiatry 2021, 12, 711658. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.; Gerardo, B.; Silva, A.R.; Pinto, P.; Barbosa, R.; Soares, S.; Baptista, B.; Paquete, C.; Cabral-Pinto, M.; Vilar, M.M.; et al. Effects of restraining measures due to COVID-19: Pre- and post-lockdown cognitive status and mental health. Curr. Psychol. 2021, 41, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2021, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Charest, O.; Lui, L.M.; Eskander, S.; Ceban, F.; Ho, R.; Di Vincenzo, J.D.; Rosenblat, J.D.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 2021, 144, 129–137. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Alwan, N.A.; Johnson, L. Defining long COVID: Going back to the start. Med 2021, 2, 501–504. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Long COVID, or Post-COVID Syndrome, and the Global Impact on Health Care. Med. Sci. Monit. 2021, 27, e933446-1–e933446-2. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Boesl, F.; Audebert, H.; Endres, M.; Prüss, H.; Franke, C. A Neurological Outpatient Clinic for Patients with Post-COVID-19 Syndrome—A Report on the Clinical Presentations of the First 100 Patients. Front. Neurol. 2021, 12, 738405. [Google Scholar] [CrossRef]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef]
- Holdsworth, D.A.; Chamley, R.; Barker-Davies, R.; O’Sullivan, O.; Ladlow, P.; Mitchell, J.L.; Dewson, D.; Mills, D.; May, S.L.J.; Cranley, M.; et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS ONE 2022, 17, e0267392. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Dura-Perez, E.; Goodman-Casanova, J.M.; Vega-Nuñez, A.; Guerrero-Pertiñez, G.; Varela-Moreno, E.; Garolera, M.; Quintana, M.; Cuesta-Vargas, A.I.; Barnestein-Fonseca, P.; Sánchez-Lafuente, C.G.; et al. The Impact of COVID-19 Confinement on Cognition and Mental Health and Technology Use Among Socially Vulnerable Older People: Retrospective Cohort Study. J. Med. Internet Res. 2022, 24, e30598. [Google Scholar] [CrossRef]
- Gareri, P.; Fumagalli, S.; Malara, A.; Mossello, E.; Trevisan, C.; Volpato, S.; Coin, A.; Calsolaro, V.; Bellelli, G.; Del Signore, S.; et al. Management of Older Outpatients during the COVID-19 Pandemic: The GeroCovid Ambulatory Study. Gerontology 2021, 68, 412–417. [Google Scholar] [CrossRef]
- Paolini, S.; Devita, M.; Epifania, O.M.; Anselmi, P.; Sergi, G.; Mapelli, D.; Coin, A. Perception of stress and cognitive efficiency in older adults with mild and moderate dementia during the COVID-19-related lockdown. J. Psychosom. Res. 2021, 149, 110584. [Google Scholar] [CrossRef]
- Tondo, G.; Sarasso, B.; Serra, P.; Tesser, F.; Comi, C. The Impact of the COVID-19 Pandemic on the Cognition of People with Dementia. Int. J. Environ. Res. Public Health 2021, 18, 4285. [Google Scholar] [CrossRef]
- Tsatali, M.; Moraitou, D.; Poptsi, E.; Sia, E.; Agogiatou, C.; Gialaouzidis, M.; Tabakis, I.-M.; Avdikou, K.; Bakoglidou, E.; Batsila, G.; et al. Are There Any Cognitive and Behavioral Changes Potentially Related to Quarantine Due to the COVID-19 Pandemic in People with Mild Cognitive Impairment and AD Dementia? A Longitudinal Study. Brain Sci. 2021, 11, 1165. [Google Scholar] [CrossRef]
- Vislapuu, M.; Angeles, R.C.; Berge, L.I.; Kjerstad, E.; Gedde, M.H.; Husebo, B.S. The consequences of COVID-19 lockdown for formal and informal resource utilization among home-dwelling people with dementia: Results from the prospective PAN.DEM study. BMC Health Serv. Res. 2021, 21, 1003. [Google Scholar] [CrossRef]
- Aragón, I.; Flores, I.; Dorman, G.; Rojas, G.; Sanjurjo, N.S.; O’Neill, S. Quality of life, mood, and cognitive performance in older adults with cognitive impairment during the first wave of COVID 19 in Argentina. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- Custodio, N.; Castro-Suárez, S.; Montesinos, R.; Failoc-Rojas, V.E.; del Castillo, R.C.; Herrera-Perez, E. Neuropsychiatric Symptoms in Patients with Alzheimer’s Disease During SARS-COV-2 Pandemic in Peru. Am. J. Alzheimer’s Dis. Other Dement. 2021, 36, 153331752110390. [Google Scholar] [CrossRef]
- Pereiro, A.; Dosil-Díaz, C.; Mouriz-Corbelle, R.; Pereira-Rodríguez, S.; Nieto-Vieites, A.; Pinazo-Hernandis, S.; Pinazo-Clapés, C.; Facal, D. Impact of the COVID-19 Lockdown on a Long-Term Care Facility: The Role of Social Contact. Brain Sci. 2021, 11, 986. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Vlotinou, P.; Terzoudi, A.; Heliopoulos, I.; Vadikolias, K. Cognitive, Functional, and Emotional Changes During the COVID-19 Pandemic in Greek Patients with Neurocognitive Disorders. J. Alzheimer’s Dis. 2022, 88, 537–547. [Google Scholar] [CrossRef]
- Gan, J.; Liu, S.; Wu, H.; Chen, Z.; Fei, M.; Xu, J.; Dou, Y.; Wang, X.; Ji, Y. The Impact of the COVID-19 Pandemic on Alzheimer’s Disease and Other Dementias. Front. Psychiatry 2021, 12, 703481. [Google Scholar] [CrossRef]
- Vernuccio, L.; Sarà, D.; Inzerillo, F.; Catanese, G.; Catania, A.; Vesco, M.; Cacioppo, F.; Dominguez, L.J.; Veronese, N.; Barbagallo, M. Effect of COVID-19 quarantine on cognitive, functional and neuropsychiatric symptoms in patients with mild cognitive impairment and dementia. Aging Clin. Exp. Res. 2022, 34, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Agostini, F.; Giovannoli, J.; Di Pace, E.; Langher, V.; Tambelli, R.; Pazzaglia, M.; Giannini, A.M.; Casagrande, M. The Cognitive Consequences of the COVID-19 Pandemic on Members of the General Population in Italy: A Preliminary Study on Executive Inhibition. J. Clin. Med. 2021, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, M.; Raggi, A.; Pilotto, A.; Cristillo, V.; Guastafierro, E.; Toppo, C.; Magnani, F.G.; Sattin, D.; Mariniello, A.; Silvaggi, F.; et al. Neurological and Mental Health Symptoms Associated with Post-COVID-19 Disability in a Sample of Patients Discharged from a COVID-19 Ward: A Secondary Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4242. [Google Scholar] [CrossRef] [PubMed]
- Cian, V.; De Laurenzis, A.; Siri, C.; Gusmeroli, A.; Canesi, M. Cognitive and Neuropsychiatric Features of COVID-19 Patients After Hospital Dismission: An Italian Sample. Front. Psychol. 2022, 13, 908363. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.D.C.; van Duinkerken, E.; Tolentino, J.C.; Schmidt, S.L. Attention profile of physically recovered COVID-19 inpatients on the day of discharge. J. Psychiatr. Res. 2022, 150, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jørgensen, B.J.; Heebøll, H.; Andersen, M.B.; et al. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021, 7, 00205-2021. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.; Balanzá-Martínez, V.; Luperdi, S.C.; Estrada, I.; Latorre, A.; González-Jiménez, P.; Feced, L.; Bouzas, L.; Yépez, K.; Ferrando, A.; et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021, 290, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Pistarini, C.; Fiabane, E.; Houdayer, E.; Vassallo, C.; Manera, M.R.; Alemanno, F. Cognitive and Emotional Disturbances Due to COVID-19: An Exploratory Study in the Rehabilitation Setting. Front. Neurol. 2021, 12, 643646. [Google Scholar] [CrossRef] [PubMed]
- Priftis, K.; Velardo, V.; Vascello, M.G.F.; Villella, S.; Galeri, S.; Spada, M.S.; Algeri, L. Limited evidence for neuropsychological dysfunction in patients initially affected by severe COVID-19. Neurol. Sci. 2022, 43, 6661–6663. [Google Scholar] [CrossRef] [PubMed]
- Thornberg, U.B.; Andersson, A.; Lindh, M.; Hellgren, L.; Divanoglou, A.; Levi, R. Neurocognitive deficits in COVID-19 patients five months after discharge from hospital. Neuropsychol. Rehabil. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.; Oliveira, S.; Moreira, A.; Pereira, M.; Carneiro, V.; Serio, A.; Freitas, L.; Isidro, H.; Souza, L. Neuropsychological manifestations of long COVID in hospitalized and non-hospitalized Brazilian Patients. Neurorehabilitation 2022, 50, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; García, M.d.C.E.; Belvís, R.; Morollón, N.; Igual, J.V.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef] [PubMed]
- Costas-Carrera, A.; Sánchez-Rodríguez, M.M.; Cañizares, S.; Ojeda, A.; Martín-Villalba, I.; Primé-Tous, M.; Rodríguez-Rey, M.A.; Segú, X.; Valdesoiro-Pulido, F.; Borras, R.; et al. Neuropsychological functioning in post-ICU patients after severe COVID-19 infection: The role of cognitive reserve. Brain Behav. Immun. Health 2022, 21, 100425. [Google Scholar] [CrossRef] [PubMed]
- Cristillo, V.; Pilotto, A.; Piccinelli, S.C.; Bonzi, G.; Canale, A.; Gipponi, S.; Bezzi, M.; Leonardi, M.; Padovani, A.; Libri, I.; et al. Premorbid vulnerability and disease severity impact on Long-COVID cognitive impairment. Aging Clin. Exp. Res. 2022, 34, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, L.; Calandri, I.; Corvalán, N.; Carello, M.A.; Keller, G.; Martínez, C.; Arruabarrena, M.; Allegri, R. Cognitive consequences of COVID-19: Results of a cohort study from South America. Arq. Neuro-Psiquiatr. 2022, 80, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Dondaine, T.; Ruthmann, F.; Vuotto, F.; Carton, L.; Gelé, P.; Faure, K.; Deplanque, D.; Bordet, R. Long-term cognitive impairments following COVID-19: A possible impact of hypoxia. J. Neurol. 2022, 269, 3982–3989. [Google Scholar] [CrossRef] [PubMed]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychologic Profiles and Cerebral Glucose Metabolism in Neurocognitive Long COVID Syndrome. J. Nucl. Med. 2021, 63, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Duindam, H.B.; Kessels, R.P.; van den Borst, B.; Pickkers, P.; Abdo, W.F. Long-term cognitive performance and its relation to anti-inflammatory therapy in a cohort of survivors of severe COVID-19. Brain Behav. Immun. Health 2022, 25, 100513. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; Monforte, A.D.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- García-Grimshaw, M.; Chirino-Pérez, A.; Flores-Silva, F.D.; Valdés-Ferrer, S.I.; Vargas-Martínez, M.D.L.; Jiménez-Ávila, A.I.; Chávez-Martínez, O.A.; Ramos-Galicia, E.M.; Marché-Fernández, O.A.; Ramírez-Carrillo, M.F.; et al. Critical role of acute hypoxemia on the cognitive impairment after severe COVID-19 pneumonia: A multivariate causality model analysis. Neurol. Sci. 2022, 43, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- García-Molina, A.; García-Carmona, S.; Espiña-Bou, M.; Rodríguez-Rajo, P.; Sánchez-Carrión, R.; Enseñat-Cantallops, A. Neuropsychological Rehabilitation for Post-COVID-19 Syndrome: Results of a Clinical Program and Six-Month Follow Up. Neurologia 2022. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, C.; Calabria, M.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Lleó, A.; Alcolea, D.; Belvís, R.; Morollón, N.; et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022, 12, e2508. [Google Scholar] [CrossRef] [PubMed]
- Hadad, R.; Khoury, J.; Stanger, C.; Fisher, T.; Schneer, S.; Ben-Hayun, R.; Possin, K.; Valcour, V.; Aharon-Peretz, J.; Adir, Y. Cognitive dysfunction following COVID-19 infection. J. Neurovirol. 2022, 28, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Chatfield, D.A.; Mphil, A.M.; Jolly, A.; Trender, W.; Hellyer, P.J.; del Giovane, M.; Newcombe, V.F.J.; Outtrim, J.G.; Warne, B.; et al. Multivariate Profile and Acute-Phase Correlates of Cognitive Deficits in a COVID-19 Hospitalised Cohort. EClinicalMedicine 2022, 47, 101417. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Miller, A.K.; Reiter, K.; Bonner-Jackson, A. Neurocognitive Profiles in Patients with Persisting Cognitive Symptoms Associated With COVID-19. Arch. Clin. Neuropsychol. 2022, 37, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, S.J.; Winters, M.F.; Pizzagalli, D.A.; Olmstead, M.C. Post-acute sequelae of COVID-19: Evidence of mood & cognitive impairment. Brain Behav. Immun. Health 2021, 17, 100347. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Stoll, K.; Obrig, H.; Baum, P.; Deterding, L.; Bernsdorff, N.; Hermsdorf, F.; Kunis, I.; Bräsecke, A.; Herzig, S.; et al. Neuropsychiatric phenotype of post COVID-19 syndrome in non-hospitalized patients. Front. Neurol. 2022, 13, 98835. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Ferrando, S.J.; Dornbush, R.; Shahar, S.; Smiley, A.; Klepacz, L. Screening for brain fog: Is the montreal cognitive assessment an effective screening tool for neurocognitive complaints post-COVID-19? Gen. Hosp. Psychiatry 2022, 78, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Stampatori, C.; Righetti, F.; Sala, E.; Tomasi, C.; De Palma, G. Neurological and cognitive sequelae of Covid-19: A four month follow-up. J. Neurol. 2021, 268, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Piva, S.; Stampatori, C.; Righetti, F.; Mega, I.; Peli, E.; Sala, E.; Tomasi, C.; Indelicato, A.M.; Latronico, N.; et al. Neurologic and cognitive sequelae after SARS-CoV2 infection: Different impairment for ICU patients. J. Neurol. Sci. 2021, 432, 120061. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.; Johnsen, S.; Sattler, S.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Maestri, R.; Dezi, S.; Spampinato, D.; Saltuari, L.; Alibardi, A.; Engl, M.; Kofler, M.; et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur. J. Neurol. 2022, 29, 1652–1662. [Google Scholar] [CrossRef]
- Pilotto, A.; Cristillo, V.; Piccinelli, S.C.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-term neurological manifestations of COVID-19: Prevalence and predictive factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Stallmach, A.; Kesselmeier, M.; Bauer, M.; Gramlich, J.; Finke, K.; Fischer, A.; Fleischmann-Struzek, C.; Heutelbeck, A.; Katzer, K.; Mutschke, S.; et al. Comparison of fatigue, cognitive dysfunction and psychological disorders in post-COVID patients and patients after sepsis: Is there a specific constellation? Infection 2022, 50, 661–669. [Google Scholar] [CrossRef]
- Vannorsdall, T.D.; Brigham, E.; Fawzy, A.; Raju, S.; Gorgone, A.; Pletnikova, A.; Lyketsos, C.G.; Parker, A.M.; Oh, E.S. Cognitive Dysfunction, Psychiatric Distress, and Functional Decline After COVID-19. J. Acad. Consult. Liaison Psychiatry 2021, 63, 133–143. [Google Scholar] [CrossRef]
- Voruz, P.; Cionca, A.; de Alcântara, I.J.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Thomasson, M.; Lalive, P.H.; Lövblad, K.-O.; Braillard, O.; et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: Is anosognosia a key determinant? Brain Commun. 2022, 4, fcac057. [Google Scholar] [CrossRef]
- Voruz, P.; de Alcântara, I.J.; Nuber-Champier, A.A.; Cionca, A.A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.-O.; Braillard, O.O.; Nehme, M.; et al. Frequency of Abnormally Low Neuropsychological Scores in Post-COVID-19 Syndrome: The Geneva COVID-COG Cohort. Arch. Clin. Neuropsychol. 2023, 38, 1–11. [Google Scholar] [CrossRef]
- Whiteside, D.M.; Basso, M.R.; Naini, S.M.; Porter, J.; Holker, E.; Waldron, E.J.; Melnik, T.E.; Niskanen, N.; Taylor, S.E. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection Part 1: Cognitive functioning. Clin. Neuropsychol. 2022, 36, 806–828. [Google Scholar] [CrossRef]
- Zhao, S.; Shibata, K.; Hellyer, P.J.; Trender, W.; Manohar, S.; Hampshire, A.; Husain, M. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 2022, 4, fcab295. [Google Scholar] [CrossRef]
- Andriuta, D.; Si-Ahmed, C.; Roussel, M.; Constans, J.-M.; Makki, M.; Aarabi, A.; Basille, D.; Andrejak, C.; Godefroy, O. Clinical and Imaging Determinants of Neurocognitive Disorders in Post-Acute COVID-19 Patients with Cognitive Complaints. J. Alzheimer’s Dis. 2022, 87, 1239–1250. [Google Scholar] [CrossRef]
- Cristillo, V.; Pilotto, A.; Piccinelli, S.C.; Gipponi, S.; Leonardi, M.; Bezzi, M.; Padovani, A. Predictors of “brain fog” 1 year after COVID-19 disease. Neurol. Sci. 2022, 43, 5795–5797. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Yus, M.; Gómez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matías-Guiu, J.; et al. Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J. Psychiatr. Res. 2022, 150, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 2022, awac384. [Google Scholar] [CrossRef]
- Fiorentino, J.; Payne, M.; Cancian, E.; Plonka, A.; Dumas, L.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; Guevara, N.; et al. Correlations between Persistent Olfactory and Semantic Memory Disorders after SARS-CoV-2 Infection. Brain Sci. 2022, 12, 714. [Google Scholar] [CrossRef]
- Jennings, G.; Monaghan, A.; Xue, F.; Duggan, E.; Romero-Ortuño, R. Comprehensive Clinical Characterisation of Brain Fog in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 3440. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Mora, M.; Villaseñor-Mora, C.; Cardona-Torres, L.M.; Martínez-Nolasco, J.J.; Barranco-Gutiérrez, A.I.; Padilla-Medina, J.A.; Bravo-Sánchez, M.G. COVID-19 Long-Term Effects: Is There an Impact on the Simple Reaction Time and Alternative-Forced Choice on Recovered Patients? Brain Sci. 2022, 12, 1258. [Google Scholar] [CrossRef]
- Stratford, P.; Kennedy, D.; Pagura, S.M.C.; Gollish, J.D. The relationship between self-report and performance-related measures: Questioning the content validity of timed tests. Arthritis Rheum. 2003, 49, 535–540. [Google Scholar] [CrossRef]
- Dennis, J.P.; Wal, J.S.V. The Cognitive Flexibility Inventory: Instrument Development and Estimates of Reliability and Validity. Cogn. Ther. Res. 2009, 34, 241–253. [Google Scholar] [CrossRef]
- Johnco, C.; Wuthrich, V.M.; Rapee, R.M. Reliability and validity of two self-report measures of cognitive flexibility. Psychol. Assess. 2014, 26, 1381–1387. [Google Scholar] [CrossRef]
- Holtgraves, T. Social Desirability and Self-Reports: Testing Models of Socially Desirable Responding. Pers. Soc. Psychol. Bull. 2004, 30, 161–172. [Google Scholar] [CrossRef]
- Sullman, M.J.; Taylor, J.E. Social desirability and self-reported driving behaviours: Should we be worried? Transp. Res. Part F Traffic Psychol. Behav. 2010, 13, 215–221. [Google Scholar] [CrossRef]
- Howlett, C.A.; Wewege, M.A.; Berryman, C.; Oldach, A.; Jennings, E.; Moore, E.; Karran, E.L.; Szeto, K.; Pronk, L.; Miles, S.; et al. Same room—Different windows? A systematic review and meta-analysis of the relationship between self-report and neuropsychological tests of cognitive flexibility in healthy adults. Clin. Psychol. Rev. 2021, 88, 102061. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.S.A.; Hagemann, P.D.M.S.; Coelho, D.D.S.; Dos Santos, F.H.; Bertolucci, P.H.F. Can MoCA and MMSE Be Interchangeable Cognitive Screening Tools? A Systematic Review. Gerontologist 2018, 59, e743–e763. [Google Scholar] [CrossRef]
- Shalash, A.; Roushdy, T.; Essam, M.; Fathy, M.; Dawood, N.L.; Abushady, E.M.; Elrassas, H.; Helmi, A.; Hamid, E. Mental Health, Physical Activity, and Quality of Life in Parkinson’s Disease During COVID-19 Pandemic. Mov. Disord. 2020, 35, 1097–1099. [Google Scholar] [CrossRef]
- Suárez-González, A.; Rajagopalan, J.; Livingston, G.; Alladi, S. The effect of COVID-19 isolation measures on the cognition and mental health of people living with dementia: A rapid systematic review of one year of quantitative evidence. Eclinicalmedicine 2021, 39, 101047. [Google Scholar] [CrossRef]
- Barguilla, A.; Fernández-Lebrero, A.; Estragués-Gázquez, I.; García-Escobar, G.; Navalpotro-Gómez, I.; Manero, R.M.; Puente-Periz, V.; Roquer, J.; Puig-Pijoan, A. Effects of COVID-19 Pandemic Confinement in Patients with Cognitive Impairment. Front. Neurol. 2020, 11, 589901. [Google Scholar] [CrossRef]
- Liu, K.Y.; Howard, R.; Banerjee, S.; Comas-Herrera, A.; Goddard, J.; Knapp, M.; Livingston, G.; Manthorpe, J.; O’Brien, J.T.; Paterson, R.W.; et al. Dementia wellbeing and COVID-19: Review and expert consensus on current research and knowledge gaps. Int. J. Geriatr. Psychiatry 2021, 36, 1597–1639. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, E.; Serino, A.; Benassi, M.; Bolzani, R. Paced Auditory Serial Addition Task (PASAT) Standardizzazione Di Tre Test Di Memoria Di Lavoro. G. Ital. Di Psicol. 2006, 33, 607–624. [Google Scholar]
- Raskin, S.A. Memory for Intentions Screening Test: Psychometric Properties and Clinical Evidence. Brain Impair. 2009, 10, 23–33. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Rodríguez, A.; Aguilar-Navarro, S.; Avila-Funes, J.A. Deterioro cognitivo en adultos mayores con VIH/sida y síndrome de fragilidad. Gac. Med. Mex. 2017, 153, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, X.; Gao, Y.; Turner, D.; Qian, F.; Lu, H.; Vermund, S.H.; Zhang, Y.; Qian, H.-Z. Association of HIV infection and cognitive impairment in older adults: A meta-analysis. Ageing Res. Rev. 2021, 68, 101310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, M.; Yang, J. Current Understanding of Long-Term Cognitive Impairment After Sepsis. Front. Immunol. 2022, 13, 855006. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef]
- Baig, A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 2020, 26, 499–501. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2020, 268, 113426. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. Eclinicalmedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef]
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The Effect of the COVID-19 Pandemic on People with Parkinson’s Disease. J. Park. Dis. 2020, 10, 1365–1377. [Google Scholar] [CrossRef]
- Mishra, R.; Banerjea, A.C. Neurological Damage by Coronaviruses: A Catastrophe in the Queue! Front. Immunol. 2020, 11, 565521. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-based 3-month Follow-up Study. Eclinicalmedicine 2020, 25, 100484. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef]
- Soung, A.L.; Vanderheiden, A.; Nordvig, A.S.; Sissoko, C.A.; Canoll, P.; Mariani, M.B.; Jiang, X.; Bricker, T.; Rosoklija, G.B.; Arango, V.; et al. COVID-19 induces CNS cytokine expression and loss of hippocampal neurogenesis. Brain 2022, 145, 4193–4201. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Galougahi, M.; Yousefi-Koma, A.; Bakhshayeshkaram, M.; Raad, N.; Haseli, S. 18FDG PET/CT Scan Reveals Hypoactive Orbitofrontal Cortex in Anosmia of COVID-19. Acad. Radiol. 2020, 27, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Micarelli, A.; Pagani, M.; Chiaravalloti, A.; Bruno, E.; Pavone, I.; Candidi, M.; Danieli, R.; Schillaci, O.; Alessandrini, M. Cortical Metabolic Arrangement During Olfactory Processing. Medicine 2014, 93, e103. [Google Scholar] [CrossRef] [PubMed]
- Kuusinen, V.; Cesnaite, E.; Peräkylä, J.; Ogawa, K.H.; Hartikainen, K.M. Orbitofrontal Lesion Alters Brain Dynamics of Emotion-Attention and Emotion-Cognitive Control Interaction in Humans. Front. Hum. Neurosci. 2018, 12, 437. [Google Scholar] [CrossRef]
- Bryden, D.W.; Roesch, M.R. Executive Control Signals in Orbitofrontal Cortex during Response Inhibition. J. Neurosci. 2015, 35, 3903–3914. [Google Scholar] [CrossRef]
- Rolls, E.T.; Grabenhorst, F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog. Neurobiol. 2008, 86, 216–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).