Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Setup
2.3. Determination Method and Data Analysis
3. Results and Discussion
3.1. Impact of Fulvic Acid on the Fe2+ Oxidation Efficiency and Rate of Secondary Mineral Synthesis Systems
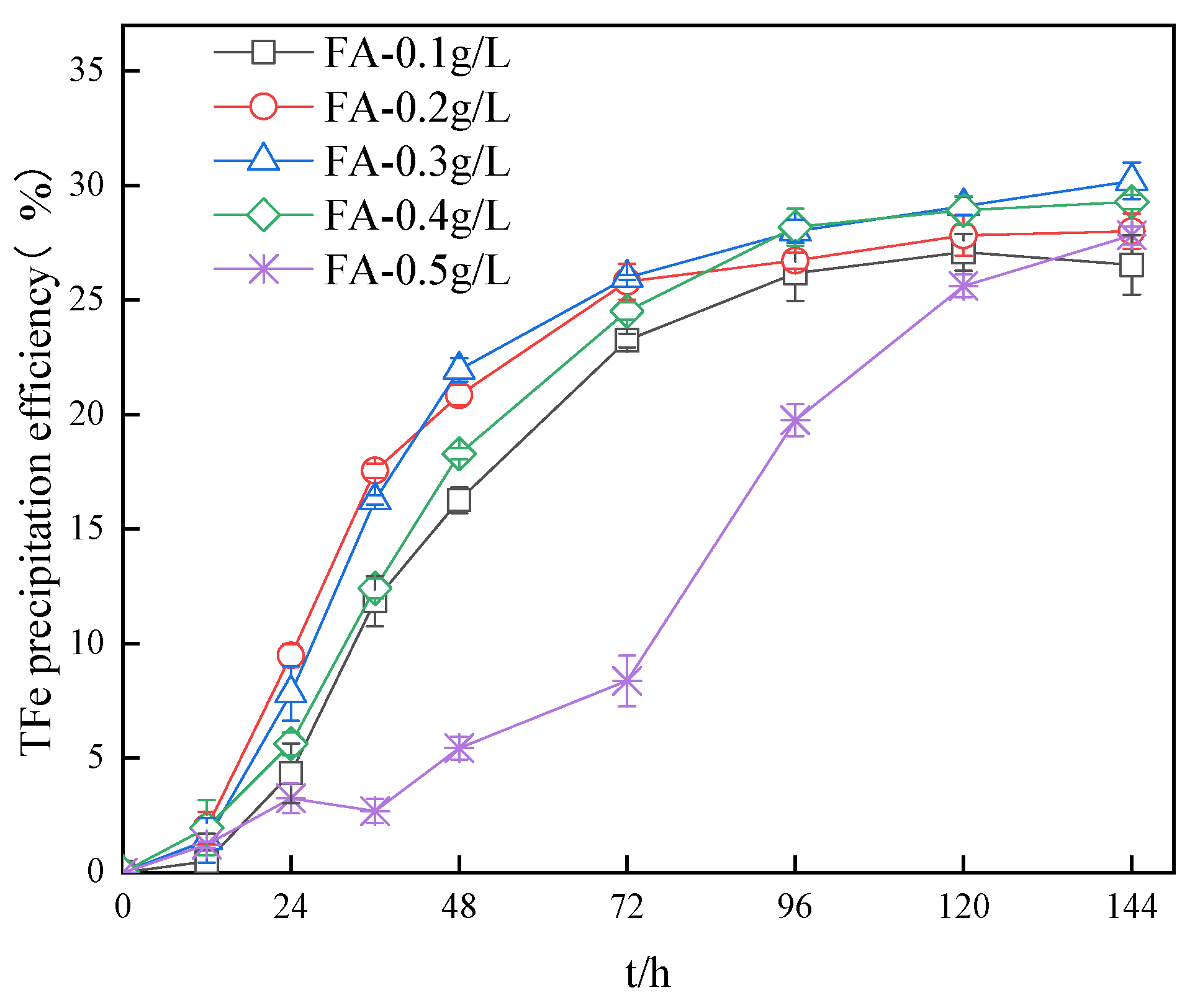
3.2. Impact of Fulvic Acid on the TFe Precipitation Efficiency in Secondary Mineral Synthesis Systems
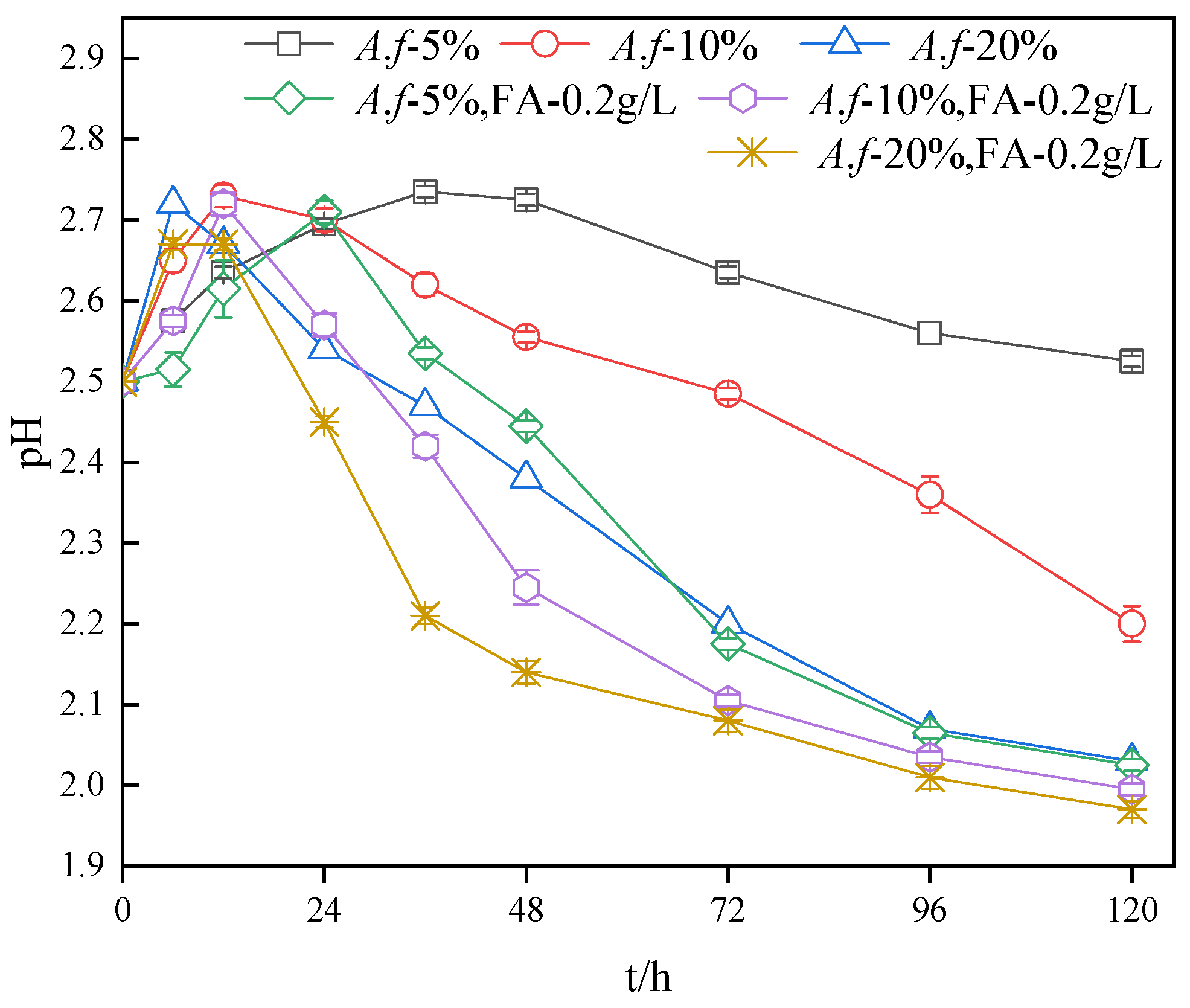
3.3. Impact of A. ferrooxidans Inoculum Amount on the pH in the Secondary Mineral Synthesis System
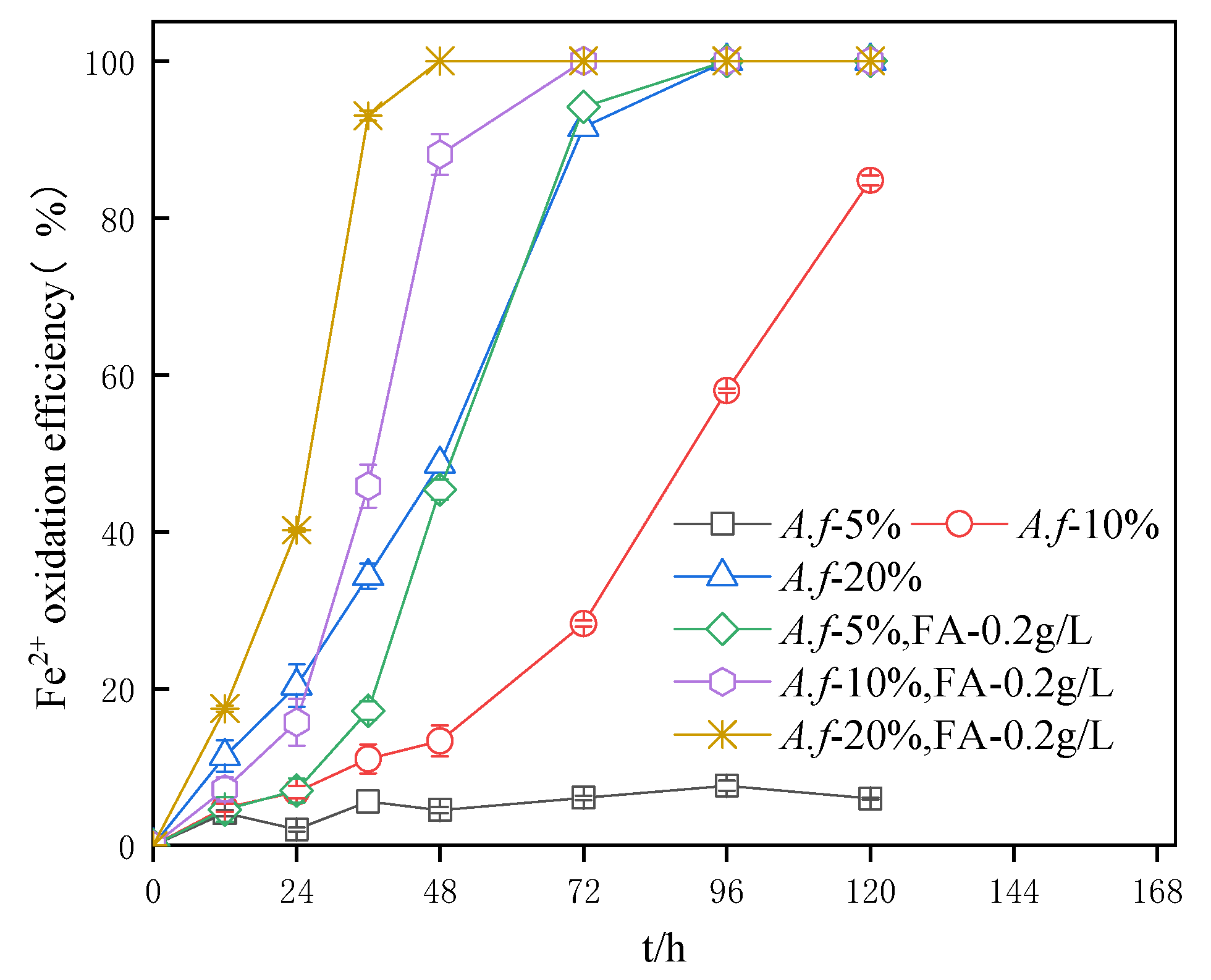
3.4. Impact of A. ferrooxidans Inoculum Amount on the Fe2+ Oxidation Efficiency in Secondary Mineral Synthesis Systems
3.5. Kinetic Analysis under Different Inoculum Amount of A. ferrooxidans
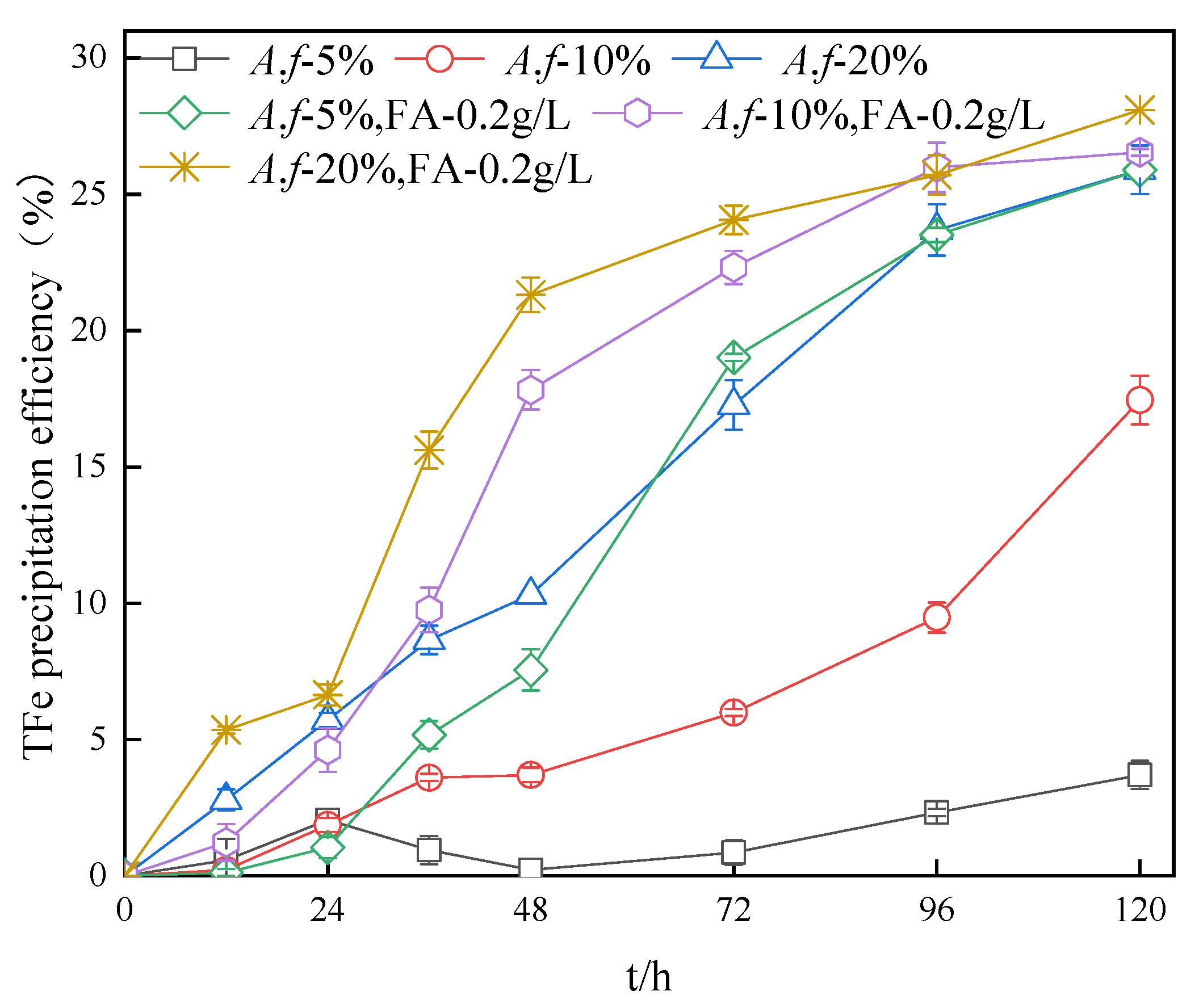
3.6. Impact of A. ferrooxidans Inoculum Amount on the TFe Precipitation Efficiency in Secondary Mineral Synthesis Systems
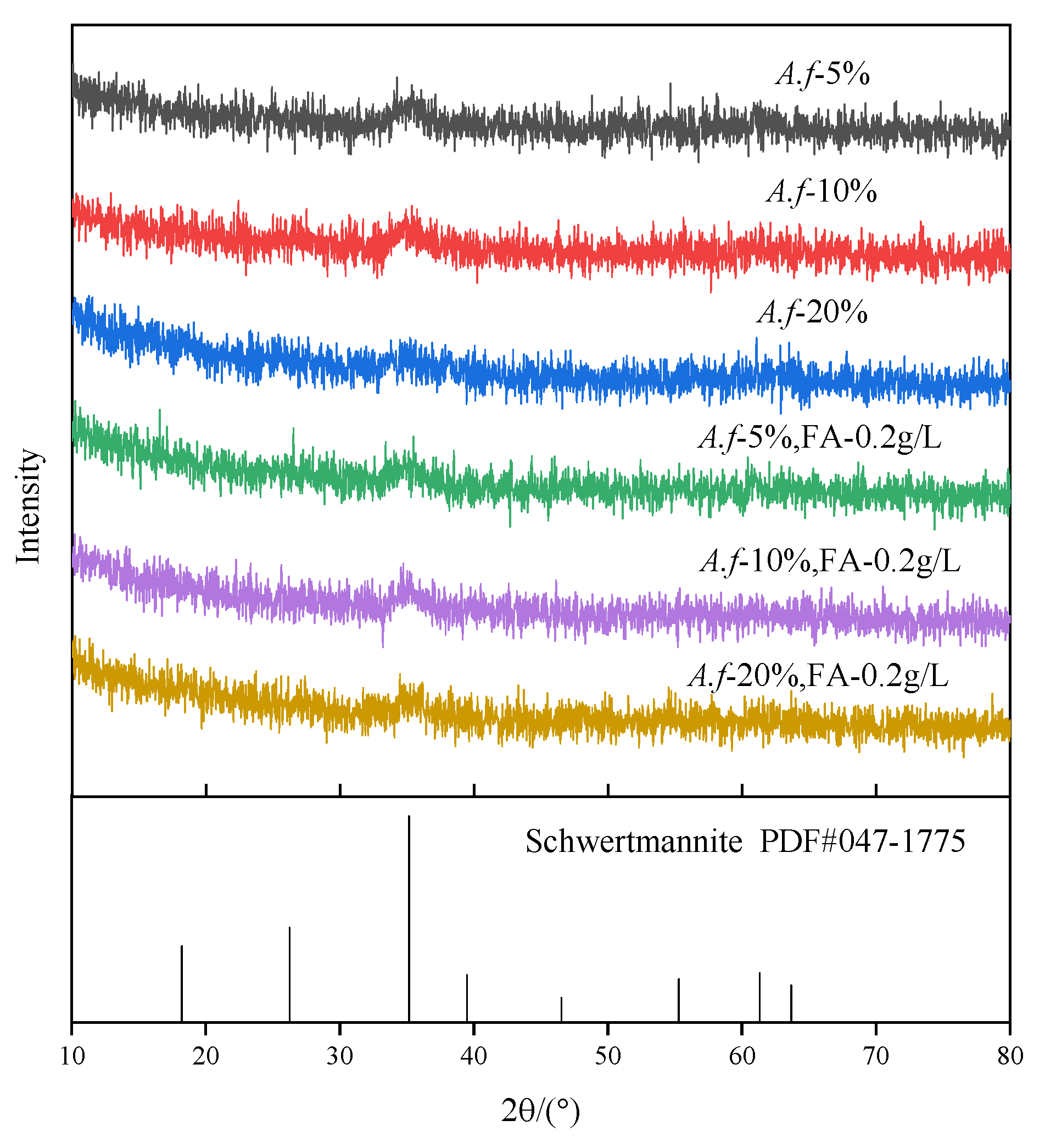
3.7. XRD Analysis
3.8. FTIR Analysis
4. Conclusions
- When the concentration of the fulvic acid was less than 0.2 g/L, the enhancement of the concentration of fulvic acid could promote the activity of A. ferrooxidans. When the concentration of the fulvic acid was greater than 0.2 g/L, the activity of the bacteria gradually decreased as the fulvic acid concentration increased, indicating the manifestation of an inhibitory effect. The low concentration of fulvic acid also had a promoting effect on the precipitation efficiency of TFe in the system, while the high concentration of fulvic acid yielded an inhibiting effect on the precipitation efficiency of TFe.
- The oxidation efficiency of A. ferrooxidans in the system with fulvic acid was higher than that of the system with the same inoculation amount at the same time. Interestingly, a reduced amount of the inoculum of A. ferrooxidans led to a more obvious oxidation effect. The oxidation activity of A. ferrooxidans in the system was in direct accordance with the first-order reaction equation. The increase in the A. ferrooxidans inoculation amount also accelerated the oxidation of Fe2+ and increased the precipitation efficiency of TFe.
- The changes in the concentration of 0.2 g/L fulvic acid and the bacterial load did not affect the mineral phase and the functional groups of the secondary minerals in the system, and pure schwertmannite was obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Chen, B. Secondary Minerals and Their Significance from AMD. Sci. Discov. 2018, 6, 481–488. [Google Scholar]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Tolonen, E.T.; Sarpola, A.; Hu, T.; Rämö, J.; Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 2014, 117, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galán, M.; Baena-Moreno, F.M.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.F.; Zhang, Z. Remediation of acid mine drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Herrera, P.; Uchiyama, H.; Igarashi, T.; Asakura, K.; Ochi, Y.; Iyatomi, N.; Nagae, S. Treatment of acid mine drainage through a ferrite formation process in central Hokkaido, Japan: Evaluation of dissolved silica and aluminium interference in ferrite formation. Miner. Eng. 2007, 20, 1255–1260. [Google Scholar] [CrossRef]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef]
- Lixiang, Z. Bioleaching role in improving sludge in-deep dewatering and removal of sludge-borne metals and its engineering application. J. Nanjing Agric. Univ. 2012, 35, 154–166. [Google Scholar]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extrem. Life Extrem. Cond. 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Torma, A.E.; Olsen, T.M. Kinetics of biodesulfurization of a high-sulfur coal. Appl. Biochem. Biotechnol. 1988, 18, 341–354. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, P.P.; Pradhan, N.; Satapathy, D.; Subudhi, U.; Biswal, S.K.; Mishra, B.K. Effect of native bacteria Sinomonas flava 1C and Acidithiobacillus ferrooxidans on desulphurization of Meghalaya coal and its combustion properties. Fuel 2014, 117, 415–421. [Google Scholar] [CrossRef]
- Shangming, Z.; Huan, H.; Zhongqi, Y.; Guanhua, H.; Yunwei, L.; Xiuxiang, T. Factor screening and condition optimization of desulfurization of coal gangue with Acidithiobacillus ferrooxidans. Chin. J. Environ. Eng. 2015, 9, 4585–4590. [Google Scholar]
- Ahmad, M.; Yousaf, M.; Wang, S.; Cai, W.; Sang, L.; Li, Z.; Zhao, Z.P. Development of rapid CO2 utilizing microbial ecosystem onto the novel & porous FPUF@ nZVI@ TAC@ ASP hybrid for green coal desulphurization. Chem. Eng. J. 2022, 433, 134361. [Google Scholar]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.-Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Zhou, J.; Song, X.; Wang, D. Improvement of sludge dewaterability and removal of sludge-borne metals by bioleaching at optimum pH. J. Hazard. Mater. 2012, 221, 170–177. [Google Scholar] [CrossRef]
- Murugesan, K.; Ravindran, B.; Selvam, A.; Kurade, M.B.; Yu, S.M.; Wong, J.W. Enhanced dewaterability of anaerobically digested sewage sludge using Acidithiobacillus ferrooxidans culture as sludge conditioner. Bioresour. Technol. 2014, 169, 374–379. [Google Scholar] [CrossRef]
- Chen, M.; Lu, G.; Guo, C.; Yang, C.; Wu, J.; Huang, W.; Yee, N.; Dang, Z. Sulfate migration in a river affected by acid mine drainage from the Dabaoshan mining area, South China. Chemosphere 2015, 119, 734–743. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Yang, J.; Cao, Y. Influence of monovalent cations on the efficiency of ferrous ion oxidation, total iron precipitation, and adsorptive removal of Cr (VI) and As (III) in simulated acid mine drainage with inoculation of Acidithiobacillus ferrooxidans. Metals 2018, 8, 596. [Google Scholar] [CrossRef]
- Ulatowska, J.; Stala, Ł.; Polowczyk, I. Comparison of Cr (VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism. Int. J. Mol. Sci. 2021, 22, 8175. [Google Scholar] [CrossRef]
- Min, G.A.N.; Li, M.M.; Jian ZE, N.G.; Liu, X.X.; Zhu, J.Y.; Hu, Y.H.; Qiu, G.Z. Acidithiobacillus ferrooxidans enhanced heavy metals immobilization efficiency in acidic aqueous system through bio-mediated coprecipitation. Trans. Nonferrous Met. Soc. China 2017, 27, 1156–1164. [Google Scholar]
- Eshwar, M.; Srilatha, M.; Rekha, K.B.; Sharma, S.H.K. Characterization of humic substances by functional groups and spectroscopic methods. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1768–1774. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Gorbov, S.N.; Tischenko, S.A.; Aleksikova, A.S.; Tagiverdiev, S.S.; Sherstnev, A.K.; Dubinina, M.N. Accumulation and migration of heavy metals in soils of the Rostov region, south of Russia. J. Soils Sediments 2016, 16, 1203–1213. [Google Scholar] [CrossRef]
- Diallo, M.S.; Glinka, C.J.; Goddard, W.A.; Johnson, J.H. Characterization of Nanoparticles and Colloids in Aquatic Systems 1. Small Angle Neutron Scattering Investigations of Suwannee River Fulvic Acid Aggregates in Aqueous Solutions. J. Nanopart. Res. 2005, 7, 435–448. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Schnitzer, M. Reactions between fulvic acid, a soil humic compound and inorganic soil constituents. Soil Sci. Soc. Am. J. 1969, 33, 75–81. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Soda, S.; He, X.; Hao, S.; You, Y.; Peng, Y. Effect of fulvic acid on bioreactor performance and on microbial populations within the anammox process. Bioresour. Technol. 2020, 318, 124094. [Google Scholar] [CrossRef]
- Vithana, C.L.; Sullivan, L.A.; Burton, E.D.; Bush, R.T. Liberation of acidity and arsenic from schwertmannite: Effect of fulvic acid. Chem. Geol. 2014, 372, 1–11. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, G.; Ye, H.; Yang, C.; Xia, D.; Yi, X.; Reinfelder, J.; Dang, Z. Fulvic acid induced the liberation of chromium from CrO42−-substituted schwertmannite. Chem. Geol. 2017, 475, 52–61. [Google Scholar] [CrossRef]
- Bai, S.; Xu, Z.; Wang, M.; Liao, Y.; Liang, J.; Zheng, C.; Zhou, L. Both initial concentrations of Fe (II) and monovalent cations jointly determine the formation of biogenic iron hydroxysulfate precipitates in acidic sulfate-rich environments. Mater. Sci. Eng. C 2012, 32, 2323–2329. [Google Scholar] [CrossRef]
- Fang, D.; Zhou, L.X. Effect of Sludge Dissolved Organic Matter on Oxidation of Ferrous Iron and Sulfur by Acidithiobacillus Ferrooxidans and Acidithiobacillus Thiooxidans. Water Air Soil Pollut. 2006, 171, 81–94. [Google Scholar] [CrossRef]
- Wu, Y.D.; Li, F.B.; Liu, T.X. Mechanism of extracellular electron transfer among microbe-humus-mineral in soil: A review. Acta Pedol. Sin. 2016, 53, 277–291. [Google Scholar]
- Cheng, Z.; Yang, J.; Li, L.; Chen, Y.; Wang, X. Flocculation inspired combination of layerd double hydroxides and fulvic acid to form a novel composite adsorbent for the simultaneous adsorption of anionic dye and heavy metals. J. Colloid Interface Sci. 2022, 618, 386–398. [Google Scholar] [CrossRef]
- Liu, F.W.; Qiao, X.X.; Xing, K.; Shi, J.; Zhou, L.X.; Dong, Y.; Bi, W.L.; Zhang, J. Effect of nitrate ions on Acidithiobacillus ferrooxidans-mediated bio-oxidation of ferrous ions and pyrite. Curr. Microbiol. 2020, 77, 1070–1080. [Google Scholar] [CrossRef]
- Kanakiya, S.; Adam, L.; Esteban, L.; Rowe, M.C.; Shane, P. Dissolution and secondary mineral precipitation in basalts due to reactions with carbonic acid. J. Geophys. Res. Solid Earth 2017, 122, 4312–4327. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.-H.; Si, Y.-B.; Wang, R.-F. Release and transformation of arsenic from As-bearing iron minerals by Fe-reducing bacteria. Chem. Eng. J. 2016, 295, 29–38. [Google Scholar] [CrossRef]
- Gramp, J.P.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Monovalent cation concentrations determine the types of Fe(III) hydroxysulfate precipitates formed in bioleach solutions. Hydrometallurgy 2008, 94, 29–33. [Google Scholar] [CrossRef]
- Boily, J.F.; Gassman, P.L.; Peretyazhko, T.; Szanyi, J.; Zachara, J.M. FTIR spectral components of schwertmannite. Environ. Sci. Technol. 2010, 44, 1185–1190. [Google Scholar] [CrossRef]
- Chen, Q.; Cohen, D.R.; Andersen, M.S.; Robertson, A.M.; Jones, D.R. Stability and trace element composition of natural schwertmannite precipitated from acid mine drainage. Appl. Geochem. 2022, 143, 105370. [Google Scholar] [CrossRef]

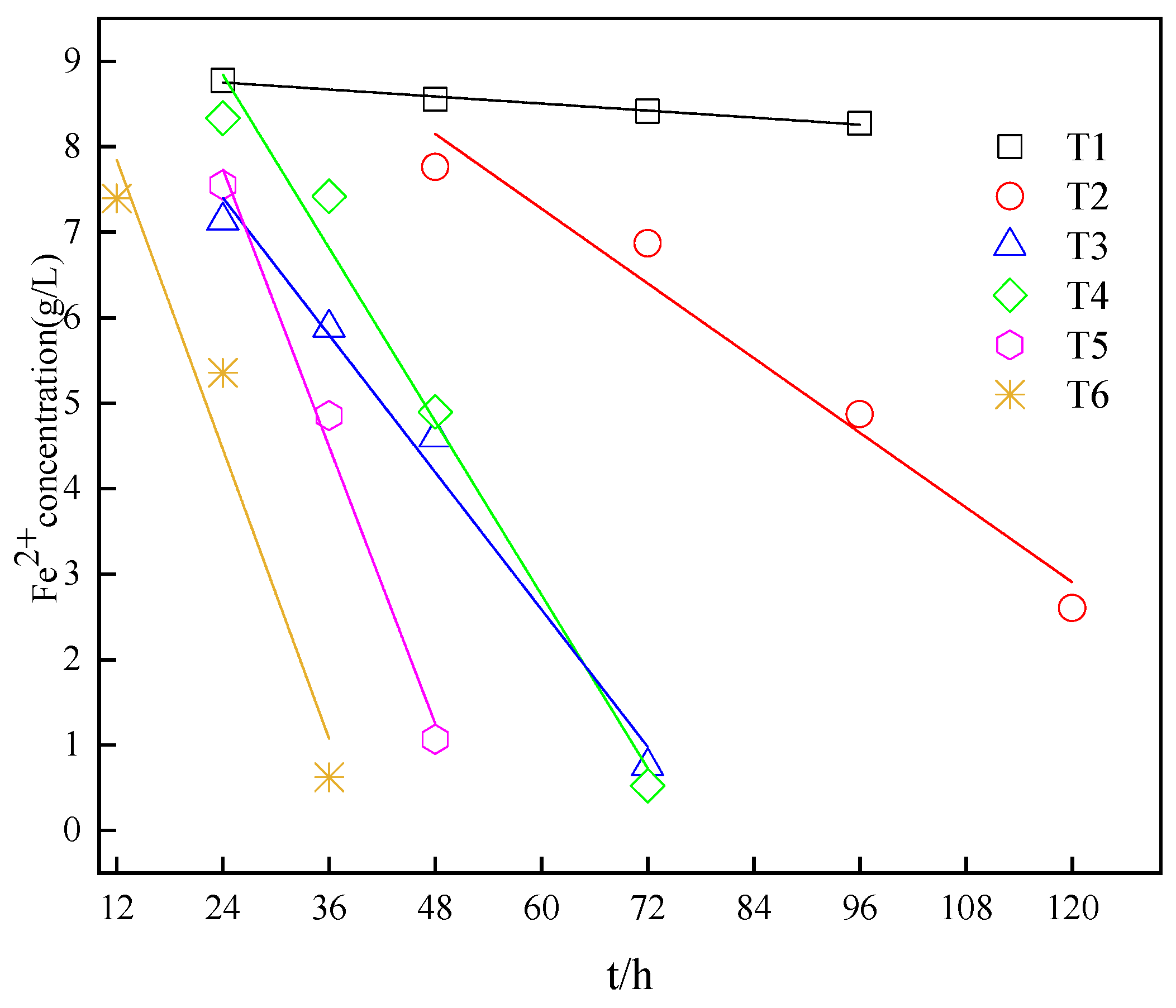

| Initial Conditions | Kinetic Equation | Correlation Coefficient R2 | Rate Constants (g/L·h−1) |
|---|---|---|---|
| 5% A.f | C = −0.007X + 8.916 | 0.977 | 0.007 |
| 10% A.f | C = −0.073X + 11.646 | 0.952 | 0.073 |
| 20% A.f | C = −0.134X + 10.613 | 0.980 | 0.134 |
| 5% A.f, FA—0.2 g/L | C = −0.169X + 12.897 | 0.972 | 0.169 |
| 10% A.f, FA—0.2 g/L | C = −0.270X + 14.218 | 0.981 | 0.270 |
| 20% A.f, FA—0.2 g/L | C = −0.282X + 11.231 | 0.900 | 0.282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Geng, K.; Wang, C.; Wu, X.; Wei, C. Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals. Int. J. Environ. Res. Public Health 2023, 20, 4736. https://doi.org/10.3390/ijerph20064736
Huang H, Geng K, Wang C, Wu X, Wei C. Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals. International Journal of Environmental Research and Public Health. 2023; 20(6):4736. https://doi.org/10.3390/ijerph20064736
Chicago/Turabian StyleHuang, Haitao, Kanghui Geng, Chong Wang, Xianhui Wu, and Caichun Wei. 2023. "Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals" International Journal of Environmental Research and Public Health 20, no. 6: 4736. https://doi.org/10.3390/ijerph20064736
APA StyleHuang, H., Geng, K., Wang, C., Wu, X., & Wei, C. (2023). Impact of Fulvic Acid and Acidithiobacillus ferrooxidan Inoculum Amount on the Formation of Secondary Iron Minerals. International Journal of Environmental Research and Public Health, 20(6), 4736. https://doi.org/10.3390/ijerph20064736






