Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass and Inoculum Collection
2.2. Biomass Pretreatment and Solid–Liquid Separation
2.3. Biochemical Methane Potential (BMP) Tests
2.4. Analytical Methods
2.5. Mass Balance and Stoichiometric Calculations
2.6. Data Analysis
3. Results and Discussion
3.1. Compositions of Raw and Processed Biomass
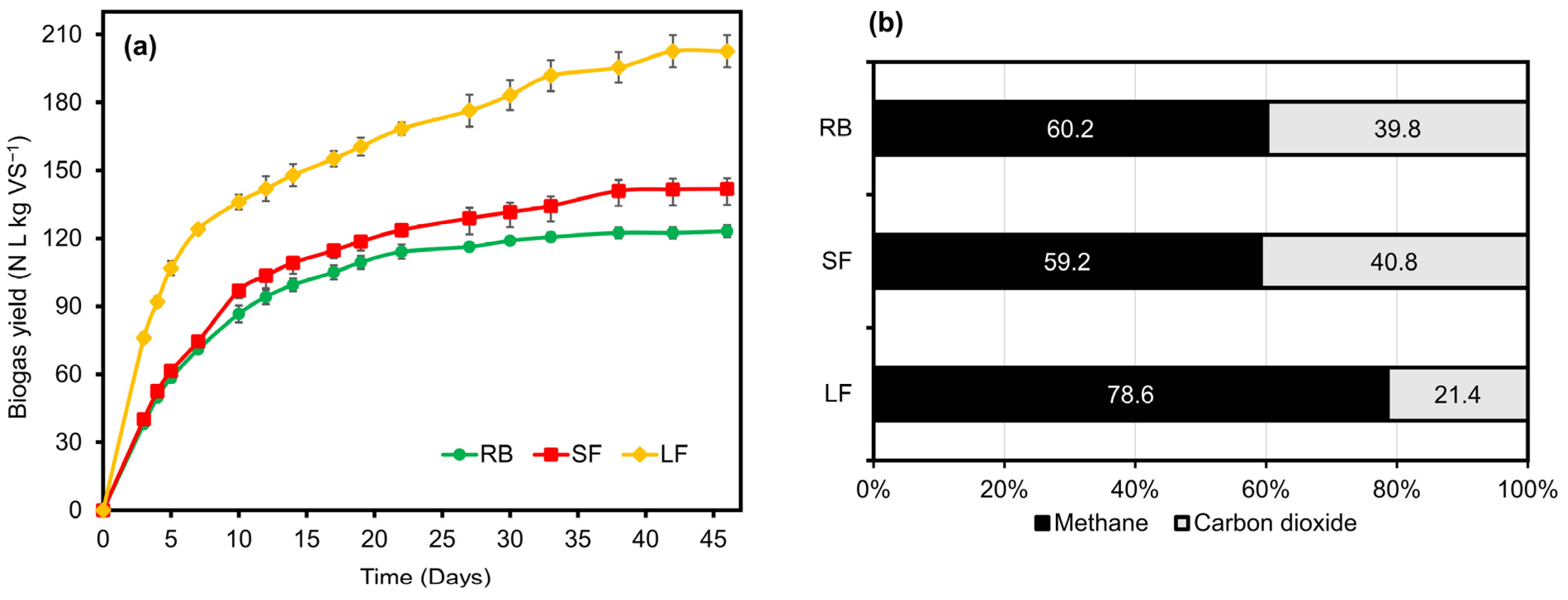
3.2. Production of Biogas and Methane from Biomass
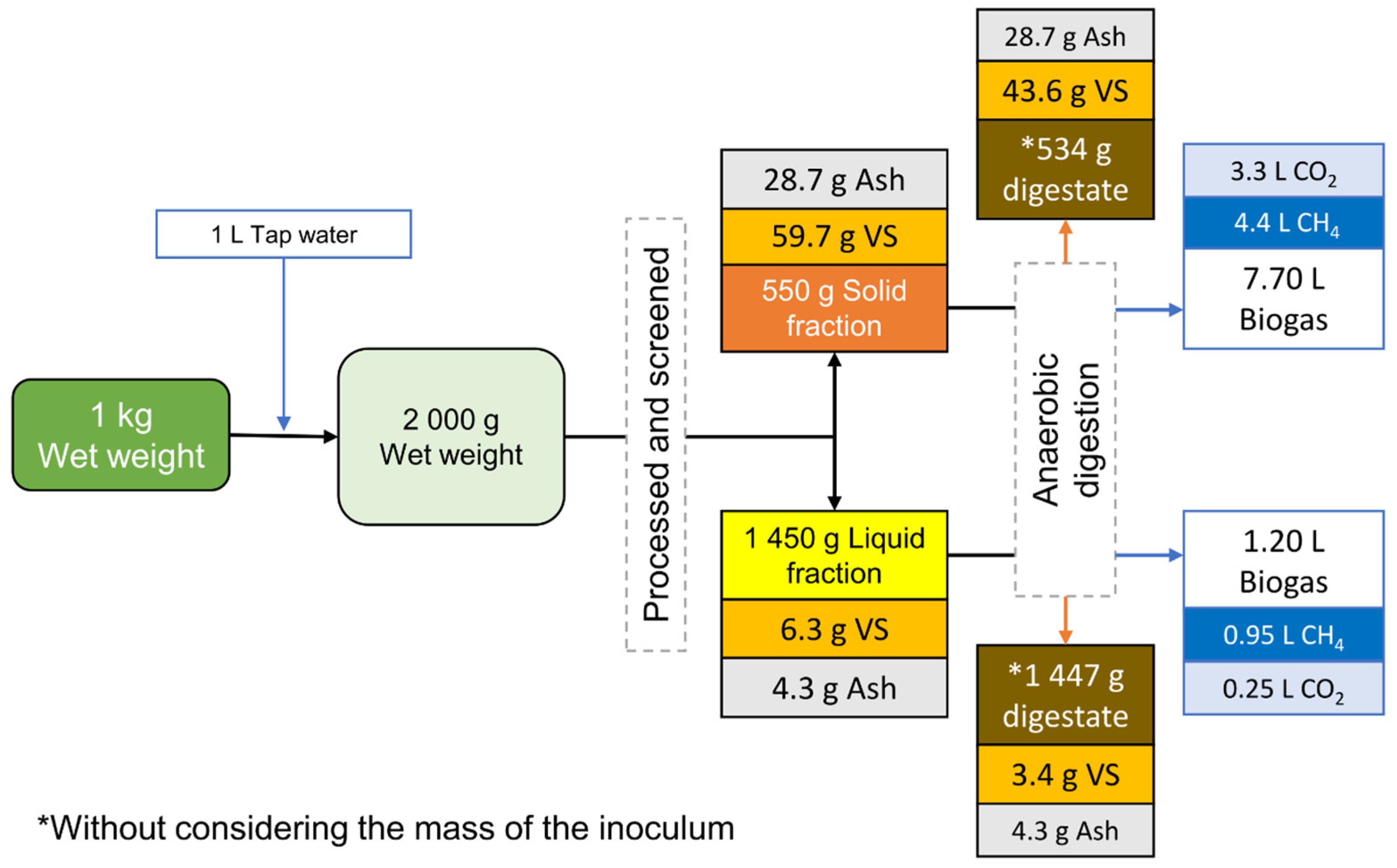
3.3. Balance and Distribution of the Components of the Solid–Liquid Separation Process
3.4. Efficiency of Anaerobic Digestion and Energy Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The Great Atlantic Sargassum Belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element Concentrations in Pelagic Sargassum along the Mexican Caribbean Coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal Changes in the Composition and Biomass of Beached Pelagic Sargassum Species in the Mexican Caribbean. Aquat. Bot. 2020, 167, 103275. [Google Scholar] [CrossRef]
- Gower, J.; Young, E.; King, S. Satellite Images Suggest a New Sargassum Source Region in 2011. Remote Sens. 2013, 4, 764–773. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass Composition of the Golden Tide Pelagic Seaweeds Sargassum Fluitans and S. Natans (Morphotypes I and VIII) to Inform Valorisation Pathways. Sci. Total 2021, 762, 143134. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Hernández Arana, H.A.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe Impacts of Brown Tides Caused by Sargassum Spp. on near-Shore Caribbean Seagrass Communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Melchor-Martínez, E.M.; Carrillo-Nieves, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Seasonal Characterization and Quantification of Biomolecules from Sargassum Collected from Mexican Caribbean Coast—A Preliminary Study as a Step Forward to Blue Economy. J. Environ. Manag. 2021, 298, 113507. [Google Scholar] [CrossRef]
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of Pelagic Sargassum Biomass into Sustainable Applications: Current Trends and Challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef]
- Milledge, J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Advances in the Pretreatment of Brown Macroalgae for Biogas Production. Fuel Process. Technol. 2019, 195, 106151. [Google Scholar] [CrossRef]
- Song, M.; Pham, H.D.; Seon, J.; Woo, H.C. Overview of Anaerobic Digestion Process for Biofuels Production from Marine Macroalgae: A Developmental Perspective on Brown Algae. Korean J. Chem. Eng. 2015, 32, 567–575. [Google Scholar] [CrossRef]
- Milledge, J.J.; Maneein, S.; Arribas López, E.; Bartlett, D. Sargassum Inundations in Turks and Caicos: Methane Potential and Proximate, Ultimate, Lipid, Amino Acid, Metal and Metalloid Analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef]
- Tapia-Tussell, R.; Avila-Arias, J.; Domínguez Maldonado, J.; Valero, D.; Olguin-Maciel, E.; Pérez-Brito, D.; Alzate-Gaviria, L. Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes Hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential. Energies 2018, 11, 494. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of Hydrothermal Pretreatment on the Anaerobic Digestion of Pelagic Sargassum for Biogas and Fertiliser Recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for Energy and Fertiliser Production in the Caribbean: A Case Study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany Macroalgae Screening: Composition and Methane Potential for Potential Alternative Sources of Energy and Products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Kaparaju, P.L.N.; Rintala, J.A. Effects of Solid–Liquid Separation on Recovering Residual Methane and Nitrogen from Digested Dairy Cow Manure. Bioresour. Technol. 2008, 99, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Rico, J.L.; García, H.; García, P.A. Solid—Liquid Separation of Dairy Manure: Distribution of Components and Methane Production. Biomass Bioenergy 2012, 39, 370–377. [Google Scholar] [CrossRef]
- Sutaryo, S.; Ward, A.J.; Møller, H.B. Anaerobic Digestion of Acidified Slurry Fractions Derived from Different Solid–Liquid Separation Methods. Bioresour. Technol. 2013, 130, 495–501. [Google Scholar] [CrossRef]
- Rico, J.L.; García, H.; Rico, C.; Tejero, I. Characterisation of Solid and Liquid Fractions of Dairy Manure with Regard to Their Component Distribution and Methane Production. Bioresour. Technol. 2007, 98, 971–979. [Google Scholar] [CrossRef]
- Buitrón, G.; Hernández-Juárez, A.; Hernández-Ramírez, M.D.; Sánchez, A. Biochemical Methane Potential from Lignocellulosic Wastes Hydrothermally Pretreated. Ind. Crops Prod. 2019, 139, 111555. [Google Scholar] [CrossRef]
- Alvarado-Lassman, A.; Méndez-Contreras, J.M.; Martínez-Sibaja, A.; Rosas-Mendoza, E.S.; Vallejo-Cantú, N.A. Biogas Production from the Mechanically Pretreated, Liquid Fraction of Sorted Organic Municipal Solid Wastes. Environ. Technol. 2017, 38, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Meneses, L.; Ferreira, J.A.; Bonturi, N.; Orupõld, K.; Kikas, T. Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates. Energies 2019, 12, 3683. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Evaluation of Biogas Production from Seaweed in Batch Tests and in UASB Reactors Combined with the Removal of Heavy Metals. J. Environ. Manag. 2010, 91, 1573–1579. [Google Scholar] [CrossRef]
- Govindarajan, A.F.; Cooney, L.; Whittaker, K.; Bloch, D.; Burdorf, R.M.; Canning, S.; Carter, C.; Cellan, S.M.; Eriksson, F.A.A.; Freyer, H.; et al. The Distribution and Mitochondrial Genotype of the Hydroid Aglaophenia Latecarinata Is Correlated with Its Pelagic Sargassum Substrate Type in the Tropical and Subtropical Western Atlantic Ocean. PeerJ 2019, 7, e7814. [Google Scholar] [CrossRef]
- Holliger, C.; Astals, S.; de Laclos, H.F.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a Standardization of Biomethane Potential Tests: A Commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005.
- Fu, C.; Chun Wai, H.; Yong, W.; Abas, F.; Tan, T.; Tan, C. Extraction of Phenolic Antioxidants from Four Selected Seaweeds Obtained from Sabah. Int. Food Res. J. 2016, 23, 2363–2369. [Google Scholar]
- Tedesco, S.; Daniels, S. Evaluation of Inoculum Acclimatation and Biochemical Seasonal Variation for the Production of Renewable Gaseous Fuel from Biorefined Laminaria Sp. Waste Streams. Renew. Energy 2019, 139, 1–8. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Theoretical Analysis of Biogas Potential Prediction from Agricultural Waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Li, J.; Fu, Y.; Zhu, W. Methane Production Performances of Different Compositions in Lignocellulosic Biomass through Anaerobic Digestion. Energy 2019, 189, 116190. [Google Scholar] [CrossRef]
- Barbot, Y.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, X.; Chen, J.; Fang, K.; Feng, L.; Yan, Y.; Zhou, Q. Enhancing Anaerobic Digestion of Waste Activated Sludge by Pretreatment: Effect of Volatile to Total Solids. Environ. Technol. 2016, 37, 1520–1529. [Google Scholar] [CrossRef]
- McKennedy, J.; Sherlock, O. Anaerobic Digestion of Marine Macroalgae: A Review. Renew. Sustain. Energy Rev. 2015, 52, 1781–1790. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.; Maneein, S.; Harvey, P. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef]
- Habig, C.; Debusk, T.; Ryther, J. The Effect of Nitrogen Content on Methane Production by the Marine Algae Gracilaria Tikvahiae and Ulva Sp. Biomass 1984, 4, 239–251. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and Opportunities of Lignocellulosic Biomass for Anaerobic Digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.V.; Harvey, P.J. The Inhibition of Anaerobic Digestion by Model Phenolic Compounds Representative of Those from Sargassum Muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Zhang, Y.; Alam, M.A.; Kong, X.; Wang, Z.; Li, L.; Sun, Y.; Yuan, Z. Effect of Salinity on the Microbial Community and Performance on Anaerobic Digestion of Marine Macroalgae: Effect of Salinity on Anaerobic Digestion of Marine Macroalgae. J. Chem. Technol. Biotechnol. 2017, 92, 2392–2399. [Google Scholar] [CrossRef]
- Membere, E.; Sallis, P. Effect of Temperature on Kinetics of Biogas Production from Macroalgae. Bioresour. Technol. 2018, 263, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What Is the Gross Energy Yield of Third Generation Gaseous Biofuel Sourced from Seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Benyounis, K.Y.; Quilty, B.; Stokes, J.; Olabi, A.G. Influence of Mechanical Pretreatment and Organic Concentration of Irish Brown Seaweed for Methane Production. Energy 2017, 118, 1079–1089. [Google Scholar] [CrossRef]

| RB | Processed Biomass | ||
|---|---|---|---|
| LF | SF | ||
| Moisture (%) | 80.3 ± 0.95 b | 99.3 ± 0.03 a | 83.9 ± 0.22 b |
| TS (%) | 19.7 ± 0.95 b | 0.70 ± 0.08 a | 16.1 ± 0.22 b |
| SV (%) | 13.2 ± 0.8 b | 0.42 ± 0.04 a | 10.8 ± 0.20 b |
| Ash (%) | 6.4 ± 0.12 b | 0.28 ± 0.03 a | 5.2 ± 0.07 b |
| VS/TS | 0.67 ± 0.01 b | 0.59 ± 0.01 a | 0.67 ± 0.01 b |
| TCOD (g L−1) | NA | 10.4 ± 0.53 | NA |
| SCOD (g L−1) | NA | 6.26 ± 2.01 | NA |
| EC (mS cm−1) | NA | 1.77 | NA |
| TDS (mg L−1) | NA | 1132.8 | NA |
| pH @ 27 °C | NA | 7.10 | NA |
| RB | Processed Biomass | ||
|---|---|---|---|
| LF | SF | ||
| C (%) | 35.5 | 27.3 | 29.8 |
| H (%) | 3.83 | 3.50 | 2.67 |
| O (%) | 25.6 | 25.6 | 32.9 |
| N (%) | 1.82 | 2.99 | 1.60 |
| S (%) | 0.34 | 0.12 | 0.36 |
| C: N Ratio | 19.5 | 9.0 | 18.6 |
| Empirical formula a | C3H3.8O1.6N0.12 | C2.3H3.4O1.6N0.21 | C2.5H2.6O2.0N0.11 |
| RB | Processed Biomass | ||
|---|---|---|---|
| LF | SF | ||
| Insoluble fiber (%) | 32.0 ± 0.9 | 42.5 ± 0.2 | 23.87 ± 1.1 |
| Cellulose (%) | 14.8 ± 0.7 | 4.5 ± 0.1 | 11.7 ± 0.7 |
| Hemicellulose (%) | 0.47 ± 0.3 | 16.64 ± 0.1 | 1.62 ± 0.9 |
| Lignin (%) | 16.8 ± 0.9 | 21.4 ± 0.6 | 10.52 ± 0.9 |
| Total phenols a | 14.4 ± 0.8 | 0.53 ± 0.04 b | 4.05 ± 0.5 |
| RB | Processed Biomass | ||
|---|---|---|---|
| LF | SF | ||
| Na (mg kg−1) | 7900.0 | 4900.0 b | 4400.0 |
| K (mg kg−1) | 7300.0 | 70.0 b | 1800.0 |
| Ca (mg kg−1) | 93,000.0 | 900.0 b | 150,600.0 |
| Mg (mg kg−1) | 8100.0 | 170.0 b | 11,800.0 |
| Total minerals | 116,300.0 a | 6040.0 b | 168,600.0 a |
| First-Order | Modified Gompertz | ||||||
|---|---|---|---|---|---|---|---|
| BMPmax (L CH4 kg VS−1) | K (Day−1) | R2 | BMPmax (L CH4 kg VS−1) | Rmax (L CH4 kg VS−1 Day−1) | λ (Day) | R2 | |
| RB | 71.30 | 0.115 | 0.999 | 71.59 | 4.384 | −1.609 | 0.982 |
| SF | 82.75 | 0.104 | 0.994 | 83.22 | 4.332 | −2.432 | 0.970 |
| LF | 158.66 | 0.110 | 0.942 | 158.57 | 6.853 | −5.165 | 0.921 |
| Raw Biomass (g kg−1) | Solid Fraction (g kg−1) | Distribution (%) | Liquid Fraction (g kg−1) | Distribution (%) | |
|---|---|---|---|---|---|
| TS | 196.5 | 88.65 | 89.2 | 10.69 | 10.8 |
| VS | 132.0 | 59.73 | 90.5 | 6.26 | 9.5 |
| Ash | 64.0 | 28.70 | 87.04 | 4.30 | 12.96 |
| Carbon | 69.8 | 26.42 | 90.1 | 2.92 | 9.9 |
| Nitrogen | 3.60 | 1.42 | 81.6 | 0.32 | 18.4 |
| Cellulose | 29.08 | 10.4 | 95.6 | 0.5 | 4.4 |
| Hemicellulose | 0.92 | 1.44 | 44.67 | 1.78 | 55.33 |
| Lignin | 33.01 | 9.33 | 80.30 | 2.30 | 19.72 |
| Phenols | 2.83 | 0.35 | 30.5 | 0.81 | 69.5 |
| Methane a | 8.84 | 4.40 | 82.2 | 0.95 | 17.8 |
| Mass | 1 kg | 0.55 kg | 26.6 | 1.45 kg | 73.4 |
| Biomethane Potential (L CH4 kg−1 VS) | Efficiencies (%) | Energy Recovery (Wh g−1 VS) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Theoretical | BI | VS Removal | TCOD Removal | SCOD Removal | Ep | Ec | Net Ep | |
| RB | 71.7 ± 2.6 b | 501.51 | 14.3 | 15.1 | NA | NA | 0.69 | 0 | 0.69 |
| SF | 83.45 ± 4.6 b | 336.60 | 24.8 | 27.0 | NA | NA | 0.76 | 0.12 | 0.64 |
| LF | 159.7 ± 7.1 a | 409.78 | 39.0 | 45.5 | 84.0 | 90.0 | 1.53 | 1.16 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado-Hernández, E.; Ortiz-Ceballos, Á.I.; Martínez-Hernández, S.; Rosas-Mendoza, E.S.; Dorantes-Acosta, A.E.; Alvarado-Vallejo, A.; Alvarado-Lassman, A. Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution. Int. J. Environ. Res. Public Health 2023, 20, 219. https://doi.org/10.3390/ijerph20010219
Salgado-Hernández E, Ortiz-Ceballos ÁI, Martínez-Hernández S, Rosas-Mendoza ES, Dorantes-Acosta AE, Alvarado-Vallejo A, Alvarado-Lassman A. Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution. International Journal of Environmental Research and Public Health. 2023; 20(1):219. https://doi.org/10.3390/ijerph20010219
Chicago/Turabian StyleSalgado-Hernández, Enrique, Ángel Isauro Ortiz-Ceballos, Sergio Martínez-Hernández, Erik Samuel Rosas-Mendoza, Ana Elena Dorantes-Acosta, Andrea Alvarado-Vallejo, and Alejandro Alvarado-Lassman. 2023. "Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution" International Journal of Environmental Research and Public Health 20, no. 1: 219. https://doi.org/10.3390/ijerph20010219
APA StyleSalgado-Hernández, E., Ortiz-Ceballos, Á. I., Martínez-Hernández, S., Rosas-Mendoza, E. S., Dorantes-Acosta, A. E., Alvarado-Vallejo, A., & Alvarado-Lassman, A. (2023). Methane Production of Sargassum spp. Biomass from the Mexican Caribbean: Solid–Liquid Separation and Component Distribution. International Journal of Environmental Research and Public Health, 20(1), 219. https://doi.org/10.3390/ijerph20010219







