Three Experimental Common High-Risk Procedures: Emission Characteristics Identification and Source Intensity Estimation in Biosafety Laboratory
Abstract
1. Introduction
2. Method
2.1. Experimental Method
2.1.1. Measurement Instruments
2.1.2. Material
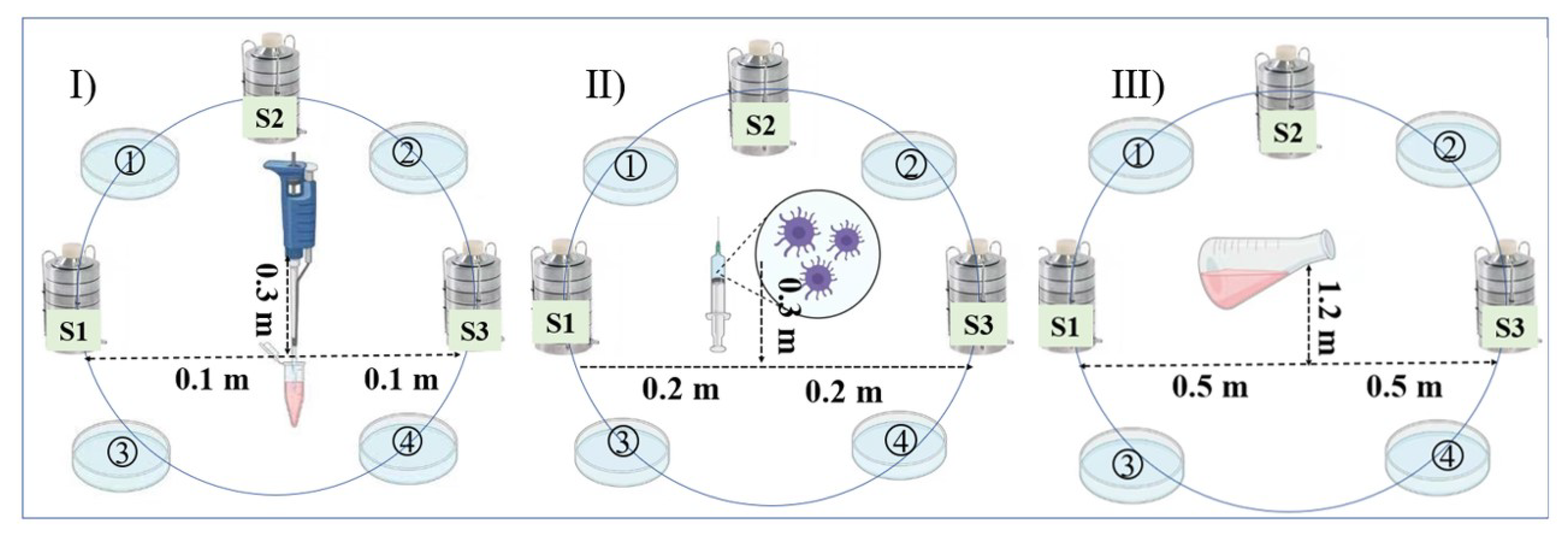
2.1.3. Experimental Design
2.2. Mathematical Method
2.2.1. Source Intensity
2.2.2. Data Statistics
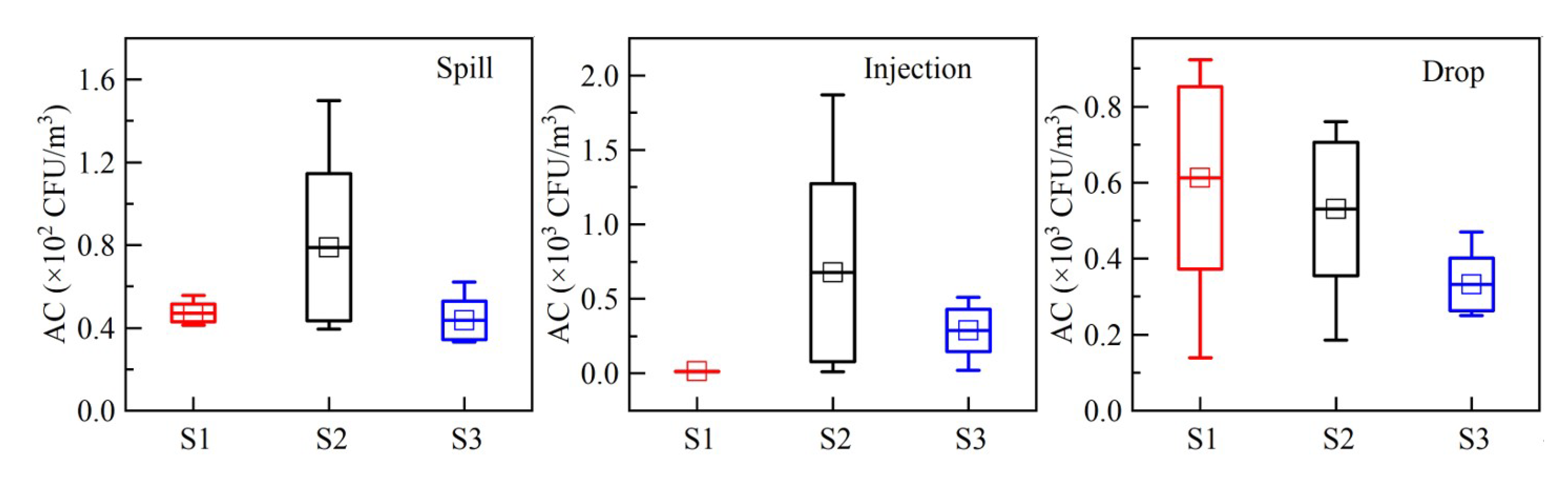
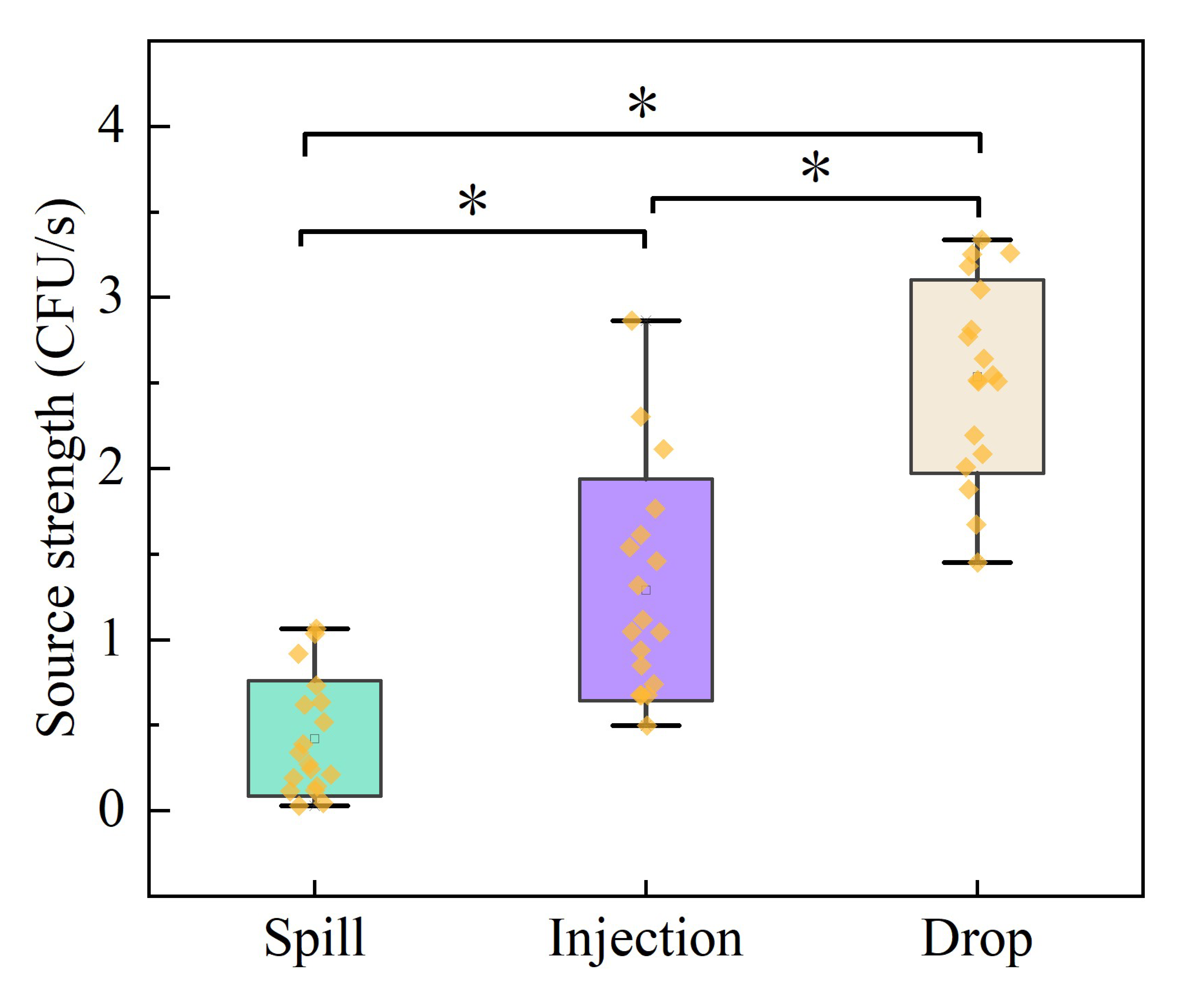
3. Results
3.1. Aerosol Emission Level
3.2. Bioaerosol and Particle Size Segregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, J.; Koritnik, T.; Mioč, V.; Trkov, M.; Bolješič, M.; Berginc, N.; Prosenc, K.; Kotar, T.; Paragi, M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021, 755, 143226. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Naeem, W.; Zeb, H.; Rashid, M.I. Laboratory biosafety measures of SARS-CoV-2 at containment level 2 with particular reference to its more infective variants. Biosaf. Health 2022, 4, 11–14. [Google Scholar] [CrossRef]
- Nava-Frías, M.; Searcy-Pavía, R.E.; Juárez-Contreras, C.A.; Valencia-Bautista, A. Chikungunya fever: Current status in Mexico. Boletín Médico Hosp. Infant. México 2016, 73, 67–74. [Google Scholar]
- Pike, R.M. Laboratory-associated infections: Incidence, fatalities, causes, and prevention. Annu. Rev. Microbiol. 1979, 33, 41–66. [Google Scholar] [CrossRef]
- Shang, Y.; Tao, Y.; Dong, J.; He, F.; Tu, J. Deposition features of inhaled viral droplets may lead to rapid secondary transmission of COVID-19. J. Aerosol Sci. 2021, 154, 105745. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Eames, I.; Chan, P.; Ridgway, G. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006, 64, 100–114. [Google Scholar] [CrossRef]
- Zhiming, Y. Current status and future challenges of high-level biosafety laboratories in China. J. Biosaf. Biosecurity 2019, 1, 123–127. [Google Scholar] [CrossRef]
- Chen, L.W.A.; Zhang, M.; Liu, T.; Fortier, K.; Chow, J.C.; Alonzo, F.; Kolberg, R.; Cao, J.; Lin, G.; Patel, T.Y.; et al. Evaluation of epifluorescence methods for quantifying bioaerosols in fine and coarse particulate air pollution. Atmos. Environ. 2019, 213, 620–628. [Google Scholar] [CrossRef]
- Barbosa, B.P.P.; Brum, N.C.L. Sensitivity tests of biological safety cabinets’ contaminant contention to variations on indoor flow parameters in biosafety level laboratories. Build. Environ. 2017, 124, 1–13. [Google Scholar] [CrossRef]
- Voutilainen, A.; Kaipio, J. Dynamical estimation of aerosol size distributions. J. Aerosol Sci. 2000, 31, S769–S770. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Xu, Z.; Song, D.; Cao, G.; Liang, L. Aerosol containment by airflow in biosafety laboratories. J. Biosaf. Biosecurity 2019, 1, 63–67. [Google Scholar] [CrossRef]
- Ali, W.; Yang, Y.F.; Gong, L.; Yan, C.; Cui, B.B. Emission characteristics and quantitative health risk assessment of bioaerosols in an indoor toilet after flushing under various ventilation scenarios. Build. Environ. 2022, 207, 108463. [Google Scholar] [CrossRef]
- Chang, C.W.; Chou, F.C.; Hung, P.Y. Evaluation of bioaerosol sampling techniques for Legionella pneumophila coupled with culture assay and quantitative PCR. J. Aerosol Sci. 2010, 41, 1055–1065. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, W.; Hu, L.; Rong, R.; Li, J.; Ding, W.; Li, N. Experimental and numerical study of potential infection risks from exposure to bioaerosols in one BSL-3 laboratory. Build. Environ. 2020, 179, 106991. [Google Scholar] [CrossRef]
- Long, Y.; Chang, F.; Yang, F.; Hou, Y.; Mo, Z.; Diao, Q. Biosafety risk assessment and risk control of clinical laboratory in designated hospitals for treating COVID-19 in Chongqing, China. Am. J. Infect. Control 2022, 50, 999–1005. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, W.; Li, N.; Wang, J.; Hu, L.; Li, J.; Yin, Z.; Zhang, K.; Dong, X. Assessment of the risk of infectious aerosols leaking to the environment from BSL-3 laboratory HEPA air filtration systems using model bacterial aerosols. Particuology 2014, 13, 82–87. [Google Scholar] [CrossRef]
- Li, N.; Hu, L.; Jin, A.; Li, J. Biosafety laboratory risk assessment. J. Biosaf. Biosecurity 2019, 1, 90–92. [Google Scholar] [CrossRef]
- Rocha-Melogno, L.; Crank, K.C.; Ginn, O.; Bergin, M.H.; Brown, J.; Gray, G.C.; Hamilton, K.A.; Bibby, K.; Deshusses, M.A. Quantitative microbial risk assessment of outdoor aerosolized pathogens in cities with poor sanitation. Sci. Total Environ. 2022, 827, 154233. [Google Scholar] [CrossRef]
- Van Soolingen, D.; Wisselink, H.; Lumb, R.; Anthony, R.; van der Zanden, A.; Gilpin, C. Practical biosafety in the tuberculosis laboratory: Containment at the source is what truly counts. Int. J. Tuberc. Lung Dis. 2014, 18, 885–889. [Google Scholar] [CrossRef]
- Afshari, A.; Matson, U.; Ekberg, L.E. Characterization of indoor sources of fine and ultrafine particles: A study conducted in a full-scale chamber. Indoor Air 2005, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Lobera, M.P.; Balas, F.; Santamaria, J. Development of a self-cleaning dispersion and exposure chamber: Application to the monitoring of simulated accidents involving the generation of airborne nanoparticles. J. Hazard. Mater. 2014, 280, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, A.W.; Jensen, K.A.; Janfelt, C.; Lauritsen, F.R.; Clausen, P.A.; Wolkoff, P. Release of VOCs and particles during use of nanofilm spray products. Environ. Sci. Technol. 2009, 43, 7824–7830. [Google Scholar] [CrossRef] [PubMed]
- Lytsy, B.; Hambraeus, A.; Ljungqvist, B.; Ransjö, U.; Reinmüller, B. Source strength as a measurement to define the ability of clean air suits to reduce airborne contamination in operating rooms. J. Hosp. Infect. 2022, 119, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Cao, F.; Lin, Y.C.; Haque, M.M.; Wu, X.; Zhang, Y.; Zhang, C.; Xie, F.; Zhang, Y.L. Extremely high abundance of polycyclic aromatic hydrocarbons in aerosols from a typical coal-combustion rural site in China: Size distribution, source identification and cancer risk assessment. Atmos. Res. 2021, 248, 105192. [Google Scholar] [CrossRef]
- Mei, X.; Gong, G. Estimating distributions of airborne contaminants released by sources with dynamic strength and dynamic location by a probabilistic model. Build. Environ. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Raman, R.S.; Ramachandran, S.; Kedia, S. A methodology to estimate source-specific aerosol radiative forcing. J. Aerosol Sci. 2011, 42, 305–320. [Google Scholar] [CrossRef]
- Braniš, M.; Řezáčová, P.; Lazaridis, M. The effect of source type and source strength on inhaled mass of particulate matter during episodic indoor activities. Indoor Built Environ. 2014, 23, 1106–1116. [Google Scholar] [CrossRef]
- Huijie, L.; Mingzhou, Y.; Zhaoqin, Y.; Ying, J.; Miaogen, C. Study on the evolution of nanoparticle size distribution due to continuous injection using the sectional method. Int. J. Numer. Methods Heat Fluid Flow 2014, 24, 1803–1812. [Google Scholar] [CrossRef]
- Ye, W.; Zhou, B.; Tu, Z.; Xiao, X.; Yan, J.; Wu, T.; Wu, F.; Zheng, C.; Tittel, F.K. Leakage source location based on Gaussian plume diffusion model using a near-infrared sensor. Infrared Phys. Technol. 2020, 109, 103411. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Zuo, M.; Zhang, C.; Jia, N.; Liu, X.; Yang, S. A new prediction model of CO2 diffusion coefficient in crude oil under reservoir conditions based on BP neural network. Energy 2022, 239, 122286. [Google Scholar] [CrossRef]
- Shafer, M.M.; Overdier, J.T.; Schauer, J.J. An improved method for sampling and analytical measurement of aerosol platinum in ambient air and workplace environments. Sci. Total Environ. 2022, 814, 152657. [Google Scholar] [CrossRef] [PubMed]
- Alalawi, S.; Issa, S.T.; Takshe, A.A.; ElBarazi, I. A Review of the Environmental Implications of the COVID-19 Pandemic in the United Arab Emirates. Environ. Challenges 2022, 100561. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakis, N.; Paschali, D.; Lekaditis, V.; Pantidou, A.; Eleftheriadis, K.; Lazaridis, M. Indoor air quality—bioaerosol measurements in domestic and office premises. J. Aerosol Sci. 2005, 36, 751–761. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Zheng, H.; Wang, X. Concentration and size distribution of culturable airborne microorganisms in outdoor environments in Beijing, China. Aerosol Sci. Technol. 2008, 42, 325–334. [Google Scholar] [CrossRef]
- Yamamoto, N.; Schmechel, D.; Chen, B.T.; Lindsley, W.G.; Peccia, J. Comparison of quantitative airborne fungi measurements by active and passive sampling methods. J. Aerosol Sci. 2011, 42, 499–507. [Google Scholar] [CrossRef]
- Salas-Villalobos, U.A.; Santacruz, A.; Castillo-Reyna, J.; Aguilar, O. An in-situ approach based in mineral oil to decrease end-product inhibition in prodigiosin production by Serratia marcescens. Food Bioprod. Process. 2022, 135, 217–226. [Google Scholar] [CrossRef]
- Mendes, J.C.; Casado, A. Serratia marcescens outbreak in a COVID-19 intensive care unit–Are there any factors specific to COVID-19 units that facilitate bacterial cross-contamination between COVID-19 patients? Am. J. Infect. Control 2022, 50, 223–225. [Google Scholar] [CrossRef]
- Mainelis, G. Bioaerosol sampling: Classical approaches, advances, and perspectives. Aerosol Sci. Technol. 2020, 54, 496–519. [Google Scholar] [CrossRef]
- Zavieh, F.S.; Mohammadi, M.J.; Vosoughi, M.; Abazari, M.; Raesee, E.; Fazlzadeh, M.; Geravandi, S.; Behzad, A. Assessment of types of bacterial bio-aerosols and concentrations in the indoor air of gyms. Environ. Geochem. Health 2021, 43, 2165–2173. [Google Scholar] [CrossRef]
- Memarzadeh, F.; DiBerardinis, L. Standard ANSI Z9. 14: Testing and performance verification methodologies for ventilation systems for Biological Safety Level 3 (BSL-3) and animal Biological Safety Level 3 (ABSL-3) facilities. J. Chem. Health Saf. 2012, 19, 11–21. [Google Scholar] [CrossRef]
- Pütz, M.; Pollack, M.; Hasse, C.; Oevermann, M. A Gauss/anti-Gauss quadrature method of moments applied to population balance equations with turbulence-induced nonlinear phase-space diffusion. J. Comput. Phys. 2022, 466, 111363. [Google Scholar] [CrossRef]
- Dubovik, O.; Lapyonok, T.; Kaufman, Y.; Chin, M.; Ginoux, P.; Kahn, R.; Sinyuk, A. Retrieving global aerosol sources from satellites using inverse modeling. Atmos. Chem. Phys. 2008, 8, 209–250. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, F.; Li, S.; Zhao, X. Advance in environmental risk assessment of high level biosafety laboratory. Procedia Environ. Sci. 2012, 13, 1458–1461. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Xia, H.; Shi, Y.; Ma, H.; Yuan, Z. Networking for training Level 3/4 biosafety laboratory staff. J. Biosaf. Biosecurity 2019, 1, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.C.; Cook, C.E. Characterization of Infectious Aerosols in Health Care Facilities: An Aid to Effective Engineering Controls and Preventive Strategies. Am. J. Infect. Control 1998, 26, 453–464. [Google Scholar] [CrossRef]


| Experimental Preparation | Blank Control Experiment | Formal Experiment | Disinfection Sterilization | |
|---|---|---|---|---|
| Temperature (C) Mean ± SD | 26.1 ± 0.27 | 26.1 ± 0.27 | 26.1 ± 0.27 | 26.1 ± 0.27 |
| Relative humidity (%) Mean ± SD | 50.3 ± 0.013 | 50.3 ± 0.013 | 50.4 ± 0.013 | 50.5 ± 0.013 |
| Airspeed (m/s) Mean ± SD | 0.10 ± 0.135 | 0.03 ± 0.002 | 0.03 ± 0.002 | 0.10 ± 0.135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lv, J.; Zhang, Z.; Ma, J.; Song, Y.; Wu, M.; Cao, G.; Guo, J. Three Experimental Common High-Risk Procedures: Emission Characteristics Identification and Source Intensity Estimation in Biosafety Laboratory. Int. J. Environ. Res. Public Health 2023, 20, 4479. https://doi.org/10.3390/ijerph20054479
Liu Z, Lv J, Zhang Z, Ma J, Song Y, Wu M, Cao G, Guo J. Three Experimental Common High-Risk Procedures: Emission Characteristics Identification and Source Intensity Estimation in Biosafety Laboratory. International Journal of Environmental Research and Public Health. 2023; 20(5):4479. https://doi.org/10.3390/ijerph20054479
Chicago/Turabian StyleLiu, Zhijian, Jiabin Lv, Zheng Zhang, Juntao Ma, Yangfan Song, Minnan Wu, Guoqing Cao, and Jiacheng Guo. 2023. "Three Experimental Common High-Risk Procedures: Emission Characteristics Identification and Source Intensity Estimation in Biosafety Laboratory" International Journal of Environmental Research and Public Health 20, no. 5: 4479. https://doi.org/10.3390/ijerph20054479
APA StyleLiu, Z., Lv, J., Zhang, Z., Ma, J., Song, Y., Wu, M., Cao, G., & Guo, J. (2023). Three Experimental Common High-Risk Procedures: Emission Characteristics Identification and Source Intensity Estimation in Biosafety Laboratory. International Journal of Environmental Research and Public Health, 20(5), 4479. https://doi.org/10.3390/ijerph20054479










