Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use
Abstract
1. Introduction
2. Study Area
3. Materials and Methods
4. Results
5. Discussion
5.1. Geochemical Properties of Sediments—Comparison with Literature Data
5.2. Interpretation of Geochemical Indicators
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rzętała, M.A. Assessment of toxic metal contamination of bottom sediments in water bodies in urban areas. Soil Sediment Contam. Int. J. 2015, 24, 49–63. [Google Scholar] [CrossRef]
- Moynier, F.; Vance, D.; Fujii, T.; Savage, P. The Isotope Geochemistry of Zinc and Copper. Rev. Mineral. Geochem. 2017, 82, 543–600. [Google Scholar] [CrossRef]
- Clark, G.; Jacks, D. Coal and the industrial revolution, 1700–1869. Eur. Rev. Econ. Hist. 2007, 11, 39–72. [Google Scholar] [CrossRef]
- Solarski, M.; Machowski, R.; Rzetala, M.; Rzetala, M.A. Hypsometric changes in urban areas resulting from multiple years of mining activity. Sci. Rep. 2022, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Manna, K. Sources and toxicological effects of lead on human health. Indian J. Med. Spec. 2019, 10, 66–71. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; M.M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Dokmeci, A.H.; Ongen, A.; Dagdeviren, S. Environmental toxicity of cadmium and health effect. J. Environ. Prot. Ecol. 2009, 10, 84–93. [Google Scholar]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Machowski, R.; Rzetala, M.A.; Rzetala, M.; Solarski, M. Anthropogenic enrichment of the chemical composition of bottom sediments of water bodies in the neighborhood of a non-ferrous metal smelter (Silesian Upland, Southern Poland). Sci. Rep. 2019, 9, 14445. [Google Scholar] [CrossRef] [PubMed]
- Sojka, M.; Jaskuła, J.; Siepak, M. Heavy Metals in Bottom Sediments of Reservoirs in the Lowland Area of Western Poland: Concentrations, Distribution, Sources and Ecological Risk. Water 2019, 11, 56. [Google Scholar] [CrossRef]
- Dwucet, K.; Rzetala, M.; Snieszko, Z. Regeneration and adaptation of strongly anthropogenically altered areas for recreation and tourism purposes–case study of the Silesian Upland. In Proceedings of the 3rd International Multidisciplinary Scientific Conferences on Social Sciences & Arts SGEM 2016, Economics & Tourism, Sofia, Bulgaria, 24–31 August 2016; pp. 551–558. [Google Scholar]
- Rzetala, M. The new evaluation proposal of tourist-recreational attractiveness of water reservoirs. In Proceedings of the 3rd International Multidisciplinary Scientific Conferences on Social Sciences & Arts SGEM 2016, Sofia, Bulgaria, 24–31 August 2016; pp. 773–780. [Google Scholar]
- Kuś, S.; Sierka, E.; Jelonek, I.; Jelonek, Z. Synthetic Analysis of Thematic Studies towards Determining the Recreational Potential of Anthropogenic Reservoirs. Environ. Ecol. Res. 2022, 10, 355–369. [Google Scholar] [CrossRef]
- Dorevitch, S.; Pratap, P.; Wroblewski, M.; Hryhorczuk, D.O.; Li, H.; Liu, L.C.; Scheff, P.A. Health Risks of Limited-Contact Water Recreation. Environ. Health Perspect. 2012, 120, 192–197. [Google Scholar] [CrossRef]
- De Florio-Barker, S.; Wade, T.J.; Turyk, M.; Dorevitch, S. Water recreation and illness severity. J. Water Health 2016, 14, 713–726. [Google Scholar] [CrossRef]
- Moksness, E.; Giosaeter, J.; Lagaillarde, G.; Mikkelsen, E.; Olsen, E.M.; Sandersen, H.T.; Volstad, J.H. Effects of Fishing Tourism in a Coastal Municipality: A Case Study from Risor, Norway. Ecol. Soc. 2011, 16, 11. [Google Scholar] [CrossRef]
- Gonzales, R.C.L.; Antelo, M.D.P. Fishing Tourism as an Opportunity for Sustainable Rural Development-The Case of Galicia, Spain. Land 2020, 9, 437. [Google Scholar] [CrossRef]
- Hall, C.M. Tourism and fishing. Scand. J. Hosp. Tour. 2021, 21, 361–373. [Google Scholar] [CrossRef]
- Tsafoutis, D.; Metaxas, T. Fishing Tourism in Greece: Defining Possibilities and Prospects. Sustainability 2021, 13, 13847. [Google Scholar] [CrossRef]
- Crase, L.; Gillespie, R. The impact of water quality and water level on the recreation values of Lake Hume. Australas. J. Environ. Manag. 2008, 15, 21–29. [Google Scholar] [CrossRef]
- Vesterinen, J.; Pouta, E.; Huhtala, A.; Neuvonen, M. Impacts of changes in water quality on recreation behavior and benefits in Finland. J. Environ. Manag. 2010, 91, 984–994. [Google Scholar] [CrossRef]
- Lopes, F.W.A.; Davies-Colley, R.J.; Von Sperling, E.; Magalhaes, A.P. A water quality index for recreation in Brazilian freshwaters. J. Water Health 2016, 14, 243–254. [Google Scholar] [CrossRef]
- Tandyrak, R.; Parszuto, K.; Grochowska, J. Water Quality of Lake Elk as a Factor Connected with Tourism, Leisure And Recreation on an Urban Area. Quaest. Geogr. 2016, 35, 51–59. [Google Scholar] [CrossRef]
- Lankia, T.; Neuvonen, M.; Pouta, E. Effects of water quality changes on the recreation benefits of swimming in Finland: Combined travel cost and contingent behavior model. Water Resour. Econ. 2019, 25, 2–12. [Google Scholar] [CrossRef]
- Lopes, F.A.; Davies-Colley, R.; Piazi, J.; Silveira, J.S.; Leite, A.C.; Lopes, N.I.A. Challenges for contact recreation in a tropical urban lake: Assessment by a water quality index. Environ. Dev. Sustain. 2020, 22, 5409–5423. [Google Scholar] [CrossRef]
- Hashim, M.; Michael, J.; Nayan, N.; Mahat, H.; Saleh, Y.; See, K.L.; Said, Z.M. Lake water quality and its suitability for water-based recreation activities in Tasik Embayu, Tanjong Malim, Perak. Geogr.-Malays. J. Soc. Space 2022, 18, 59–70. [Google Scholar] [CrossRef]
- Icemer, G.T.; Okudan, E.S.; Goktug, T.H. Assessment of Environmental Impacts of Recreational Yacht / Boat Activities On Marine Water Quality And Marine Vegetation Variation In Phaselis Bay In Turkey. Fresenius Environ. Bull. 2022, 31, 6793–6799. [Google Scholar]
- Riungu, G.K.; Hallo, J.C.; Backman, K.F.; Brownlee, M.; Beeco, J.A.; Larson, L.R. Water-based recreation management: A normative approach to reviewing boating threFlds. Lake Reserv. Manag. 2020, 36, 139–154. [Google Scholar] [CrossRef]
- Jankowski, A.T.; Molenda, T.; Rzetala, M.A.; Rzetala, M. Heavy metals in bottom deposits of artificial water reservoirs of the Silesian Upland an as indicator of human impact into the environment. Limnol. Rev. 2002, 2, 171–180. [Google Scholar]
- Kostecki, M.; Suschka, J. The Successful Results of Plawniowice Reservoir (Upper Silesia Region–South of Poland) Restoration By Hypoliminetic Withdrawal. Arch. Environ. Prot. 2013, 39, 17–25. [Google Scholar] [CrossRef]
- Rzetala, M.; Jaguś, A. New lake district in Europe: Origin and hydrochemical characteristics. Water Environ. J. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- ActLabs. Available online: http://www.actlabs.com (accessed on 4 October 2018).
- Müller, G. Schwermetalle in den sedimenten des Rheins—Veränderungen seit 1971. Umsch. Wiss. Tech. 1979, 79, 778–783. [Google Scholar]
- Förstner, U.; Müller, G. Concentrations of Heavy Metals and Polycyclic Aromatic Hydrocarbons in River Sediments: Geochemical Background, Man’s Influence and Environmental Impact. GeoJournal 1981, 5, 417–432. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Rzetala, M.A. Basic components and trace elements in sediments in the inactive channel of the Dunajec river (Pieniny Mts.) and their geo-ecological significance. Carpathian J. Earth Environ. Sci. 2015, 10, 85–94. [Google Scholar]
- Rzetala, M.A. Cadmium contamination of sediments in the water reservoirs in Silesian Upland (southern Poland). J. Soils Sediments 2016, 16, 2458–2470. [Google Scholar] [CrossRef]
- Li, Y.H.; Schoonmaker, J.E. Chemical composition and mineralogy of marine sediments. In Sediments, Diagenesis, and Sedimentary Rocks 7; Mackenzie, F.T., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–35. [Google Scholar]
- Lis, J.; Pasieczna, A. Geochemical Atlas of Upper Silesia, 1:200000; Polish Geological Institute: Warsaw, Poland, 1995. [Google Scholar]
- Lis, J.; Pasieczna, A. Geochemical Atlas of Poland, 1:2500000; Polish Geological Institute: Warsaw, Poland, 1995. [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundances of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Rzetala, M.; Jaguś, A.; Rzetała, M.A.; Rahmonov, O.; Rahmonov, G.; Khak, V. Variations in the Chemical Composition of Bottom Deposits in Anthropogenic Lakes. Pol. J. Environ. Stud. 2013, 22, 1799–1805. [Google Scholar]
- Sojka, M.; Ptak, M.; Jaskuła, J.; Krasniqi, V. Ecological and Health Risk Assessments of Heavy Metals Contained in Sediments of Polish Dam Reservoirs. Int. J. Environ. Res. Public Health 2023, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-K.; Kang, M.-J.; Yu, S.; Ko, K.-S.; Ha, K.; Shin, S.-C.; Park, J.H. Enrichment and geochemical mobility of heavy metals in bottom sediment of the Hoedong reservoir, Korea and their source apportionment. Chemosphere 2017, 184, 74–85. [Google Scholar] [CrossRef]
- Chrastný, V.; Komárek, M.; Tlustoš, P.; Švehla, J. Effects of Flooding on Lead and Cadmium Speciation in Sediments from a Drinking Water Reservoir. Environ. Monit. Assess. 2006, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Şener, Ş.; Davraz, A.; Karagüzel, R. Assessment of trace metal contents in water and bottom sediments from Eğirdir Lake, Turkey. Environ. Earth Sci. 2014, 71, 2807–2819. [Google Scholar] [CrossRef]
- Linnik, P.M.; Zubenko, I.B. Role of bottom sediments in the secondary pollution of aquatic environments by heavy-metal compounds. Lakes Reserv. Res. Manag. 2000, 5, 11–21. [Google Scholar] [CrossRef]
- Dampilova, B.V.; Khazheeva, Z.I.; Plyusnin, A.M. Heavy Metal Species in the Bottom Sediments of the Aquatic System of Lake Gusinoe (Buryatia). Geochem. Int. 2022, 60, 279–285. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, J.J.; Chen, X.; Liu, J.T.; Sun, J.C. Heavy Metal Ecological Risk in Bottom Sludges of Yangzong Lake, China. Asian J. Chem. 2014, 26, 3325–3330. [Google Scholar] [CrossRef]
- Saleh, A.H.; Gad, M.; Khalifa, M.M.; Elsayed, S.; Moghanm, F.S.; Ghoneim, A.M.; Danish, S.; Datta, R.; Moustapha, M.E.; Abou El-Safa, M.M. Environmental Pollution Indices and Multivariate Modeling Approaches for Assessing the Potentially Harmful Elements in Bottom Sediments of Qaroun Lake, Egypt. J. Mar. Sci. Eng. 2021, 9, 1443. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Yu, Q.; Wei, F.; Zhang, Q. Ecological assessment of heavy metals in sediments from Jianhu Lake in Yunnan Province, China. Pol. J. Environ. Stud. 2020, 29, 4139–4150. [Google Scholar] [CrossRef]
- Ismukhanova, L.; Choduraev, T.; Opp, C.; Madibekov, A. Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan. Appl. Sci. 2022, 12, 11474. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, Z.; Wei, M.; Xiaona, H. Characterization of heavy metals in water and sediments in Taihu Lake, China. Environ. Monit. Assess. 2012, 184, 4367–4382. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Bui, T.; Kessler, M.; Weber, C.J.; Beier, T.; Mildenberger, A.; Traub, M.; Opp, C. Catchment Soil Properties Affect Metal(loid) Enrichment in Reservoir Sediments of German Low Mountain Regions. Appl. Sci. 2022, 12, 2277. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Leermakers, M.; Osán, J.; Török, S.; Baeyens, W. Heavy metals in Lake Balaton: Water column, suspended matter, sediment and biota. Sci. Total Environ. 2005, 340, 213–230. [Google Scholar] [CrossRef]
- Grinham, A.; O’Sullivan, C.; Dunbabin, M.; Sturm, K.; Gale, D.; Clarke, W.; Albert, S. Drivers of Anaerobic Methanogenesis in Sub-Tropical Reservoir Sediments. Front. Environ. Sci. 2022, 10, 852344. [Google Scholar] [CrossRef]
- Reis, A.R.; Roboredo, M.; Pinto, J.P.R.M.; Vieira, B.; Varandas, S.G.P.; Fernandes, L.F.S.; Pacheco, F.A.L. Distribution and Potential Availability of As, Metals and P in Sediments from a Riverine Reservoir in a Rural Mountainous Catchment (NE Portugal). Int. J. Environ. Res. Public Health 2021, 18, 5616. [Google Scholar] [CrossRef] [PubMed]
- Mrozińska, N.; Bąkowska, M. Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast. Int. J. Environ. Res. Public Health 2020, 17, 6848. [Google Scholar] [CrossRef]
- Malsiu, A.; Shehu, I.; Stafilov, T.; Faiku, F. Assessment of Heavy Metal Concentrations with Fractionation Method in Sediments and Waters of the Badovci Lake (Kosovo). J. Environ. Public Health 2020, 2020, 3098594. [Google Scholar] [CrossRef]
- Šestinova, O.; Findoráková, L.; Hančuľák, J.; Šestinova, L. Study of metal mobility and phytotoxicity in bottom sediments that have been influenced by former mining activities in Eastern Slovakia. Environ. Earth Sci. 2015, 74, 6017–6025. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Santos, J.A.; Lopes, D.V. Fate of copper in intensive shrimp farms: Bioaccumulation and deposition in pond sediments. Braz. J. Biol. 2009, 69, 851–858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szarek-Gwiazda, E.; Mazurkiewicz-Boroń, G. Deposition of Copper in the Eutrophic, Submontane Dobczyce Dam Reservoir (Southern Poland)–Role of Speciation. Water Air Soil Pollut. 2002, 140, 203–218. [Google Scholar] [CrossRef]
- Mustafa, M.; Maulana, A.; Irfan, U.R.; Tonggiroh, A. Determination of heavy metal elements concentration in soils and tailing sediments from lateritic nickel post-mining areas in Motui District, Southeast Sulawesi. J. Degrad. Min. Lands Manag. 2022, 9, 3273–3279. [Google Scholar] [CrossRef]
- Javed, T.; Ahmad, N.; Mashiatullah, A.; Khan, K. Chronological record, source identification and ecotoxicological impact assessment of heavy metals in sediments of Kallar Kahar Lake, Salt Range-Punjab, Pakistan. Environ. Earth Sci. 2021, 80, 546. [Google Scholar] [CrossRef]
- de Andrade, L.C.; Coelho, F.F.; Hassan, S.M.; Morris, L.A.; de Oliveira Camargo, F.A. Sediment pollution in an urban water supply lake in southern Brazil. Environ. Monit. Assess. 2019, 191, 12. [Google Scholar] [CrossRef]
- Lima, G.F.C.; Bento, C.C.; Horn, A.H.; Marques, E.D.; Filho, H.B. Geochemical signature and environmental background of bottom sediments in a tropical aquatic system: The Três Marias Reservoir, Brazil. Environ. Monit. Assess. 2021, 193, 85. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, C.; Feng, H.; Hong, Z.; Zhu, Q.; Kolenčík, M.; Chang, X. Assessment of metal mobility in sediment, commercial fish accumulation and impact on human health risk in a large shallow plateau lake in southwest of China. Ecotoxicol. Environ. Saf. 2020, 194, 110346. [Google Scholar] [CrossRef]
- Dvořák, T.; Száková, J.; Vondráčková, S.; Košnář, Z.; Holečková, Z.; Najmanová, J.; Tlustoš, P. Content of Inorganic and Organic Pollutants and Their Mobility in Bottom Sediment from the Orlík Water Reservoir (Vltava River, Czech Republic). Soil Sediment Contam. Int. J. 2017, 26, 584–604. [Google Scholar] [CrossRef]
- El-Radaideh, N.; Al-Taani, A.A.; Al Khateeb, W.M. Characteristics and quality of reservoir sediments, Mujib Dam, Central Jordan, as a case study. Environ. Monit. Assess. 2017, 189, 143. [Google Scholar] [CrossRef]
- Chatterjee, S.; Datta, S.; Das, T.K.; Veer, V.; Mishra, D.; Chakraborty, A.; Chattopadhyay, B.; Datta, S.; Mukhopadhyay, S.K.; Gupta, D.K. Metal accumulation and metallothionein induction in Oreochromis niloticus grown in wastewater fed fishponds. Ecol. Eng. 2016, 90, 405–416. [Google Scholar] [CrossRef]
- Linge, K.L.; Oldham, C.E. Arsenic Remobilization in a Shallow Lake: The Role of Sediment Resuspension. J. Environ. Qual. 2002, 31, 822–828. [Google Scholar] [CrossRef]
- Szara, M.; Baran, A.; Klimkowicz-Pawlas, A.; Tarnawski, M. Ecotoxicological characteristics and ecological risk assessment of trace elements in the bottom sediments of the Rożnów reservoir (Poland). Ecotoxicology 2020, 29, 45–57. [Google Scholar] [CrossRef]
- Shadrin, N.; Mirzoeva, N.; Kravchenko, N.; Miroshnichenko, O.; Tereshchenko, N.; Anufriieva, E. Trace Elements in the Bottom Sediments of the Crimean Saline Lakes. Is It Possible to Explain Their Concentration Variability? Water 2020, 12, 2364. [Google Scholar] [CrossRef]
- Bertolino, S.R.A.; Zimmermann, U.; Sattler, F.J. Mineralogy and geochemistry of bottom sediments from water reservoirs in the vicinity of Córdoba, Argentina: Environmental and health constraints. Appl. Clay Sci. 2007, 36, 206–220. [Google Scholar] [CrossRef]
- Alemayehu, D.; McAlister, J.; Fox, W. Sediment core sampling and analysis of Kaw Lake. Am. J. Environ. Sci. 2014, 10, 458–468. [Google Scholar] [CrossRef]
- Jaguś, A.; Khak, V.; Rzętała, M.A.; Rzętała, M. Trace elements in the bottom sediments of the Irkutsk Reservoir. Ecol. Chem. Eng. A 2012, 19, 939–950. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Yang, Z.; Wu, P.; Zhang, K.; Chen, S. Study on antimony and arsenic cycling, transformation and contrasting mobility in river-type reservoir. Appl. Geochem. 2022, 136, 105132. [Google Scholar] [CrossRef]
- Michalski, R.; Jabłońska-Czapla, M.; Szopa, S.; Łyko, A.; Grygoyć, K. Variability in different antimony, arsenic and chromium species in waters and bottom sediments of three water reservoirs in Upper Silesia (Poland): A comparative study. Int. J. Environ. Anal. Chem. 2016, 96, 682–693. [Google Scholar] [CrossRef]
- Tzoraki, O.; Dörflinger, G.; Demetriou, C. Nutrient and heavy metal storage and mobility within sediments in Kouris Reservoir, Cyprus. Lakes Reserv. Res. Manag. 2017, 22, 74–84. [Google Scholar] [CrossRef]
- Filippelli, G.M.; Morrison, D.; Cicchella, D. Urban Geochemistry and Human Health. Elements 2012, 8, 439–444. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Yang, Z.; Mao, C.; Ji, J. Characteristics of lead geochemistry and the mobility of Pb isotopes in the system of pedogenic rock–pedosphere–irrigated riverwater–cereal–atmosphere from the Yangtze River delta region, China. Chemosphere 2013, 93, 1927–1935. [Google Scholar] [CrossRef]
- Ciszewski, D. Flood-related changes of heavy metal concentrations in the Biała Przemsza River bottom sediments (SW Poland). Pol. Geol. Rev. 1999, 47, 993–998. (In Polish) [Google Scholar]
- Ciszewski, D. Heavy metals in vertical profiles of the middle Odra River overbank sediments: Evidence for pollution changes. Water Air Soil Pollut. 2003, 143, 81–98. [Google Scholar] [CrossRef]
- Niemitz, J.; Haynes, C.; Lasher, G. Legacy sediments and historic land use: Chemostratigraphic evidence for excess nutrient and heavy metal sources and remobilization. Geology 2012, 41, 47–50. [Google Scholar] [CrossRef]
- Hutton, M. Sources of cadmium in the environment. Ecotoxicol. Environ. Saf. 1983, 7, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Karikari, A.Y.; Asmah, R.; Anku, W.W.; Amisah, S.; Agbo, N.W.; Telfer, T.C.; Ross, L.G. Heavy metal concentrations and sediment quality of a cage farm on Lake Volta, Ghana. Aquac. Res. 2020, 51, 2041–2051. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel impact on human health: An intrinsic disorder perspective. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warszawa, Poland, 1993; pp. 1–364. [Google Scholar]
- Stern, B.R.; Solioz, M.; Krewski, D.; Aggett, P.; Aw, T.-C.; Baker, S.; Crump, K.; Dourson, M.; Haber, L.; Hertzberg, R.; et al. Copper and Human Health: Biochemistry, Genetics, and Strategies for Modeling Dose-response Relationships. J. Toxicol. Environ. Health 2007, 10, 157–222. [Google Scholar] [CrossRef]
- Schulz, K.J.; DeYoung, J.H., Jr.; Seal, R.R., II; Bradley, D.C. Chapter F: Cobalt. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply: U.S. Geological Survey Professional Paper 1802; U.S. Geological Survey: Reston, VA, USA, 2017; pp. F1–F40. [Google Scholar] [CrossRef]
- Yamada, K. Cobalt: Its Role in Health and Disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Morales-Simfors, N.; Bundschuh, J.; Herath, I.; Inguaggiato, C.; Caselli, A.T.; Tapia, J.; Choquehuayta, F.E.A.; Armienta, M.A.; Ormachea, M.; Joseph, E.; et al. Arsenic in Latin America: A critical overview on the geochemistry of arsenic originating from geothermal features and volcanic emissions for solving its environmental consequences. Sci. Total Environ. 2020, 716, 135564. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, B.; Wang, B.; Li, S.; Wu, D.; Hu, J. Arsenic concentrations in Chinese coals. Sci. Total Environ. 2006, 357, 96–102. [Google Scholar] [CrossRef]
- O’Day, P. Chemistry and Mineralogy of Arsenic. Elements 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Singh, R.P.; Singh, N.; Yunus, M. Arsenic hazards in coal fly ash and its fate in Indian scenario. Resour. Conserv. Recycl. 2011, 55, 819–835. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Vaughan, D.J. Arsenic. Elements 2006, 2, 71–75. [Google Scholar] [CrossRef]
- Hopenhayn, C. Arsenic in drinking water: Impact on human health. Elements 2006, 2, 103–107. [Google Scholar] [CrossRef]
- Rebelo, F.M.; Caldas, E.D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Thornton, I.; Farago, M. The Geochemistry of Arsenic. In Arsenic; Springer: Dordrecht, The Netherlands, 1997; pp. 1–16. Available online: https://link.springer.com/content/pdf/10.1007/978-94-011-5864-0_1.pdf (accessed on 28 December 2022).
- Aziz, H.A.; Ghazali, M.F.; Hung, Y.-T.; Wang, L.K. Toxicity, Source, and Control of Barium in the Environment. In Advanced Industrial and Hazardous Wastes Management; CRC Press: Boca Raton, FL, USA, 2017; pp. 463–482. [Google Scholar]
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Antimony Toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Harden, F.A.; Toms, L.-M.L.; Norman, R.E. Health consequences of exposure to brominated flame retardants: A systematic review. Chemosphere 2014, 106, 1–19. [Google Scholar] [CrossRef]
- Scott, V.; Juran, L.; Ling, E.J.; Benham, B.; Spiller, A. Assessing Strontium and Vulnerability to Strontium in Private Drinking Water Systems in Virginia. Water 2020, 12, 1053. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Wang, L.; Wang, W.; Xu, J. Concentrations and potential health risks of strontium in drinking water from Xi’an, Northwest China. Ecotoxicol. Environ. Saf. 2018, 164, 181–188. [Google Scholar] [CrossRef]
- Komarnisky, L.A.; Christopherson, R.J.; Basu, T.K. Sulfur: Its clinical and toxicologic aspects. Nutrition 2003, 19, 54–61. [Google Scholar] [CrossRef]
- Kostecki, M.; Domurad, A.; Kowalski, E.; Kozłowski, J. Acidification of water in the Nakło-Chechło Reservoir (commune Świerklaniec): An attempt of causes explanation. Arch. Environm. Prot. 1999, 25, 65–80. [Google Scholar]
- Kostecki, M. Heavy metals in flesh and liver of some fish species in Dzierżno Duże dam-reservoir (Upper Silesia). Arch. Environ. Prot. 2000, 26, 109–125. Available online: https://journals.pan.pl/Content/124317/PDF/11_AE_VOL_26_4_2000_Kostecki_Zawartosc.pdf (accessed on 13 February 2023).
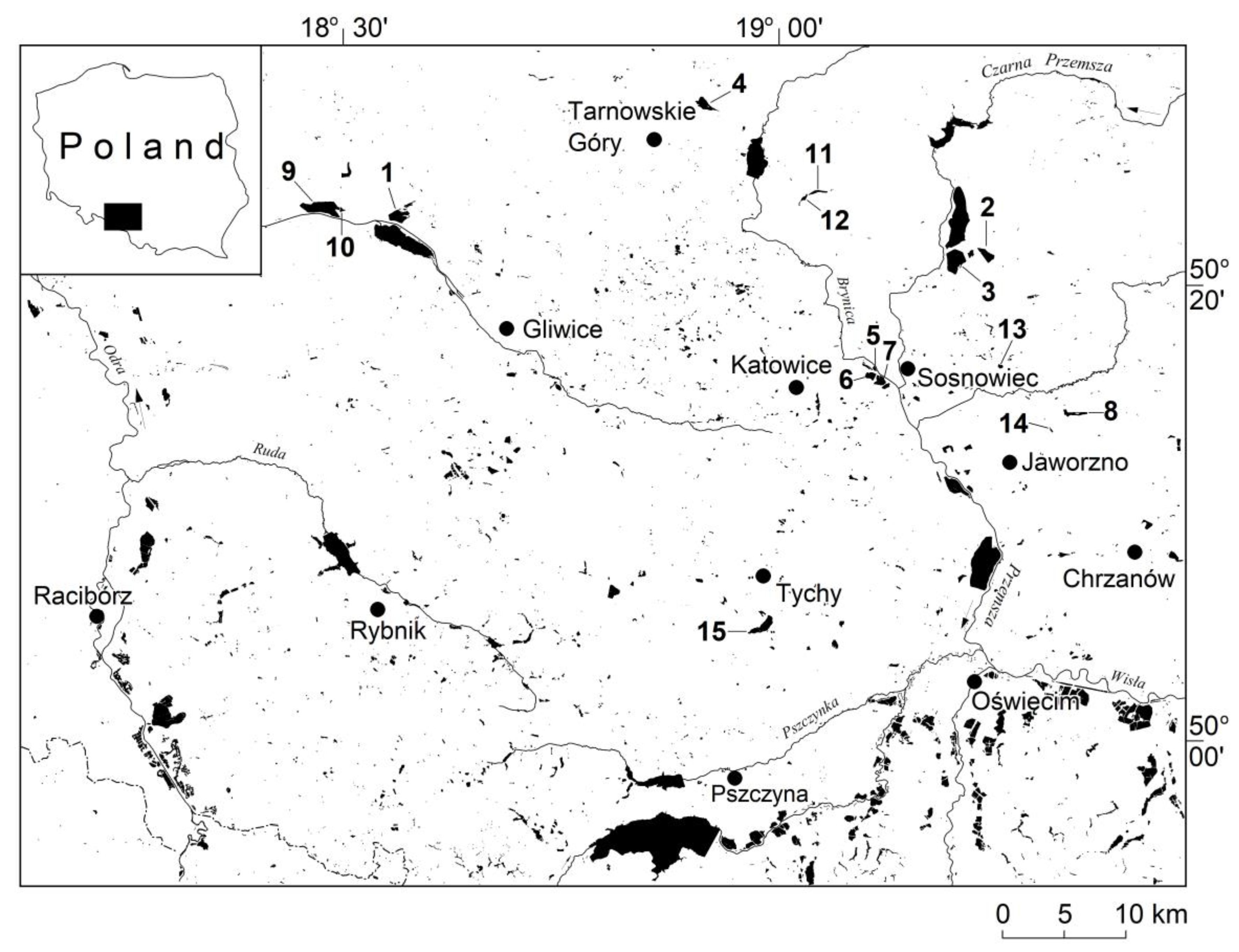
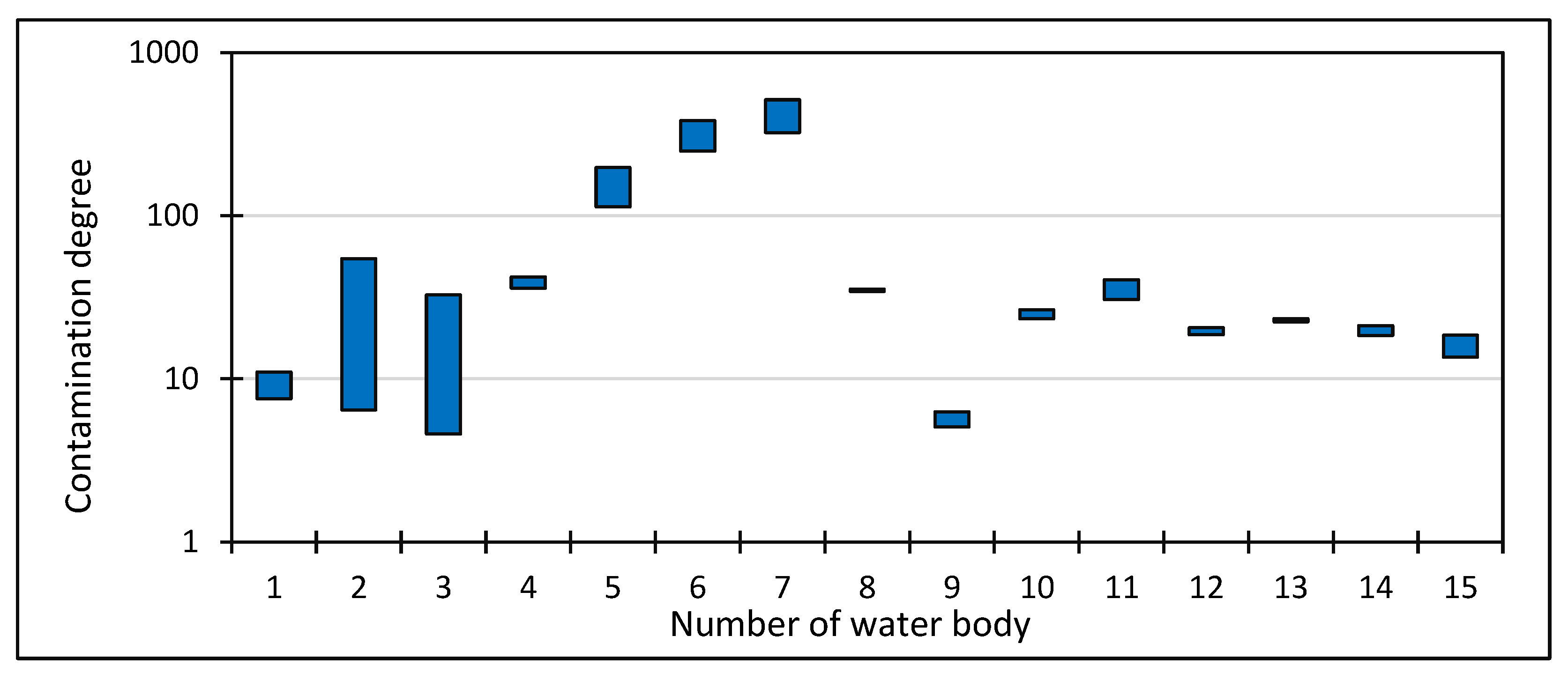
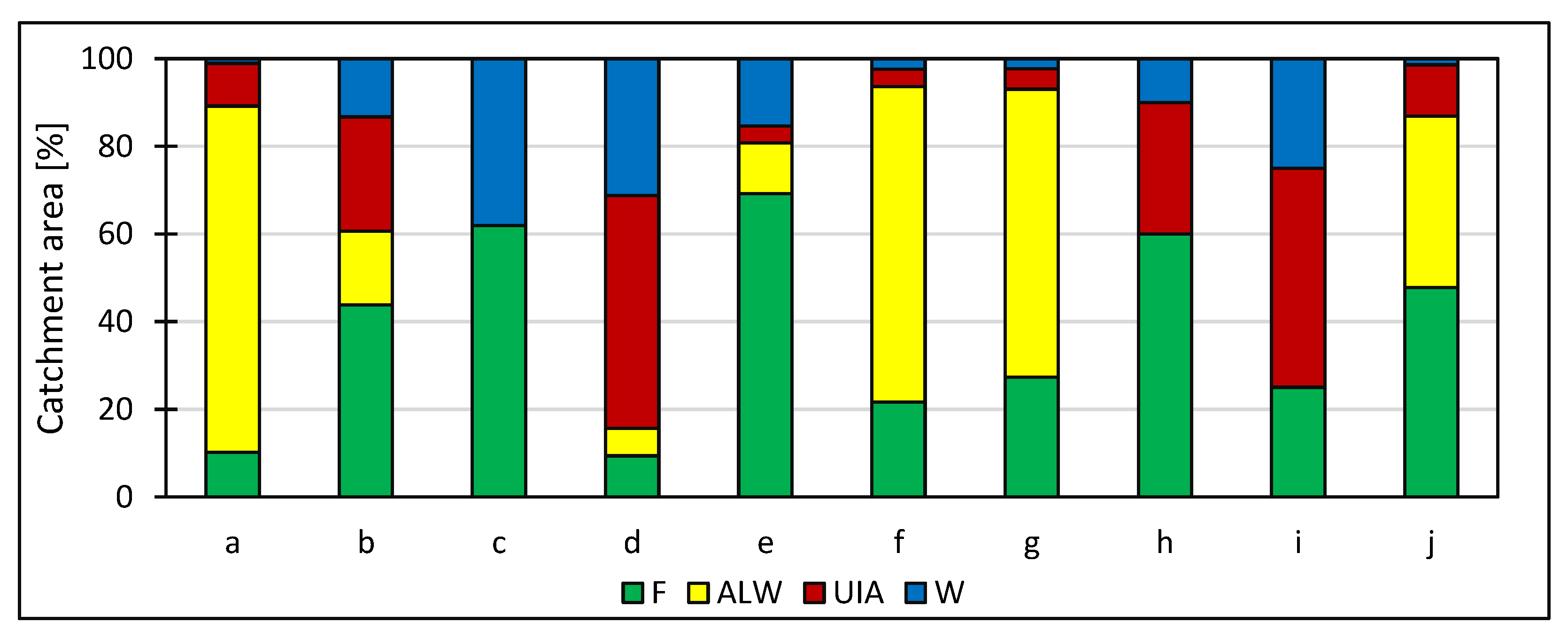
| Water Body Name | Geographical Coordinates | TC | MA | EC | WR | NO3− | PO43− | Recreational Functions of the Water Body | |
|---|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | [dam3] | [ha] | [μS/cm] | [pH] | [mg/dm3] | |||
| Dzierżno Małe | 50°23′16.30″ N | 18°33′51.10″ E | 12,600 | 160.0 | 679.0 | 7.5–8.7 | 37.1 | 0.09 | S, C, F, W, M, B, O |
| Pogoria I | 50°21′27.00″ N | 19°14′15.00″ E | 3600 | 75.0 | 736.0 | 7.8–8.5 | 36.6 | 0.04 | S, C, F, M, B, N, O |
| Pogoria III | 50°21′13.11″ N | 19°12′05.00″ E | 12,000 | 208.0 | 483.0 | 7.4–8.2 | 38.3 | 0.04 | S, C, F, D, M, B, O |
| Chechło | 50°28′04.00″ N | 18°54′49.10″ E | 1300 | 90.0 | 183.0 | 6.9–7.6 | 8.4 | 0.02 | S, C, F, M, B, O |
| Stawiki | 50°16′25.56″ N | 19°06′35.59″ E | 131 | 7.6 | 784.5 | 7.9–8.3 | 22.0 | 0.11 | C, F, W, M, B, N, O |
| Morawa | 50°16′24.56″ N | 19°07′19.57″ E | 693 | 34.7 | 380.0 | 8.0–8.6 | 35.5 | 2.82 | S, C, F, M, B, N, O |
| Gliniak | 50°15′53.55″ N | 19°07′00.54″ E | 824 | 38.7 | 512.1 | 8.0–8.4 | 6.3 | 0.04 | S, C, F, W, M, B, N, O |
| Sosina | 50°14′27.00″ N | 19°19′50.05″ E | 1000 | 50.0 | 547.4 | 8.0–8.6 | 31.3 | 1.32 | S, C, F, W, M, B, O |
| Pławniowice | 50°23′29.23″ N | 18°28′08.00″ E | 29,100 | 240.0 | 617.0 | 7.8–9.1 | 9.8 | 0.08 | S, C, F, W, M, B, O |
| Mały Zalew | 50°23′20.45″ N | 18°29′55.77″ E | 143 | 6.5 | 488.0 | 7.6–8.2 | 18.9 | 3.43 | C, F, W, M, B |
| Rogoźnik II | 50°24′13.40″ N | 19°02′40.03″ E | 340 | 25.0 | 651.0 | 8.0–8.2 | 24.3 | 0.09 | F, M, B, O |
| Rogoźnik I | 50°23′54.59″ N | 19°01′43.58″ E | 360 | 12.1 | 644.0 | 7.9–8.5 | 14.0 | 0.11 | S, C, F, M, B, O |
| Balaton | 50°16′31.21″ N | 19°15′11.16″ E | 71 | 9.0 | 535.5 | 7.9–8.2 | 25.1 | 0.11 | C, F, M, B, O |
| Koparki | 50°13′42.52″ N | 19°18′40.77″ E | 440 | 4.0 | 707.0 | 8.1–8.7 | 4.5 | 0.00 | D, B, O |
| Paprocany | 50°05′05.59″ N | 18°59′02.22″ E | 1600 | 110.0 | 315.0 | 7.3–8.4 | 14.2 | 0.14 | S, C, F, W, M, B, N, O |
| Parameter | Cu | Pb | Zn | Ni | Cd | Co | Cr | Ba | Sr | As | Sb | Br | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/kg] | [%] | ||||||||||||
| Minimum | 11.0 | 30.0 | 142.0 | 10.0 | 0.7 | 3.0 | 22.0 | 261.0 | 63.0 | 8.0 | 0.9 | 1.0 | 0.001 |
| 25% quartile | 19.5 | 56.5 | 302.3 | 19.5 | 2.1 | 10.5 | 59.8 | 407.5 | 112.5 | 13.0 | 1.4 | 4.8 | 0.038 |
| Median | 25.0 | 145.0 | 940.0 | 34.0 | 11.9 | 17.0 | 101.5 | 459.0 | 149.0 | 20.0 | 1.9 | 10.0 | 0.692 |
| 75% quartile | 55.5 | 425.5 | 1390.0 | 54.0 | 17.6 | 25.5 | 122.0 | 654.5 | 236.5 | 41.0 | 5.5 | 20.0 | 1.670 |
| Maximum | 298.0 | 3020.0 | 35,300.0 | 115.0 | 286.0 | 40.0 | 203.0 | 19,300.0 | 510.0 | 178.0 | 52.5 | 31.0 | 4.590 |
| Arithmetic mean | 56.4 | 481.8 | 3204.7 | 39.2 | 27.7 | 19.0 | 94.4 | 1795.2 | 191.3 | 36.5 | 6.7 | 12.4 | 1.043 |
| Standard deviation | 71.4 | 813.9 | 7316.7 | 23.0 | 54.6 | 10.2 | 42.8 | 4824.8 | 116.2 | 39.0 | 11.2 | 9.2 | 1.134 |
| No. of Water Bodies (see Figure 1) | Parameter | Cu | Pb | Zn | Ni | Cd | Co | Cr | Ba | Sr | As | Sb | Br | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/kg] | [%] | |||||||||||||
| 1 | min | 19 | 56 | 288 | 19 | 2.1 | 10 | 45 | 386 | 203 | 12 | 1.5 | 13.0 | 0.001 |
| max | 28 | 88 | 480 | 32 | 3.3 | 17 | 86 | 483 | 434 | 13 | 2.0 | 27.0 | 1.170 | |
| 2 | min | 26 | 46 | 232 | 44 | 1.2 | 21 | 122 | 563 | 89 | 9 | 1.2 | 1.0 | 0.001 |
| max | 67 | 429 | 2338 | 54 | 29.0 | 23 | 150 | 750 | 114 | 30 | 5.6 | 10.0 | 0.001 | |
| 3 | min | 12 | 35 | 142 | 19 | 0.7 | 11 | 92 | 388 | 100 | 8 | 0.9 | 1.0 | 0.001 |
| max | 58 | 467 | 1220 | 61 | 13.1 | 26 | 118 | 668 | 127 | 36 | 7.7 | 28.0 | 0.347 | |
| 4 | min | 63 | 478 | 1360 | 40 | 16.0 | 14 | 78 | 19,100 | 476 | 39 | 5.4 | 11.0 | 0.500 |
| max | 79 | 510 | 1480 | 56 | 20.0 | 34 | 105 | 19,300 | 510 | 44 | 8.8 | 14.0 | 0.790 | |
| 5 | min | 123 | 1070 | 5940 | 47 | 56.9 | 33 | 120 | 459 | 152 | 45 | 19.6 | 10.0 | 2.040 |
| max | 156 | 2250 | 9210 | 56 | 99.9 | 35 | 203 | 480 | 235 | 92 | 37.8 | 24.0 | 3.330 | |
| 6 | min | 204 | 2580 | 13,800 | 96 | 119.0 | 34 | 153 | 741 | 151 | 105 | 31.6 | 7.0 | 2.750 |
| max | 298 | 3020 | 35,300 | 115 | 119.0 | 40 | 182 | 817 | 209 | 135 | 52.5 | 10.0 | 4.590 | |
| 7 | min | 250 | 2560 | 16,300 | 67 | 172.0 | 23 | 130 | 524 | 155 | 152 | 20.4 | 10.0 | 3.240 |
| max | 270 | 2680 | 28,900 | 73 | 286.0 | 38 | 140 | 659 | 165 | 178 | 28.3 | 17.0 | 3.510 | |
| 8 | min | 47 | 265 | 1630 | 62 | 17.5 | 28 | 109 | 394 | 194 | 20 | 3.3 | 6.0 | 1.690 |
| max | 48 | 293 | 1690 | 67 | 17.6 | 33 | 113 | 409 | 206 | 23 | 3.5 | 6.0 | 1.890 | |
| 9 | min | 18 | 30 | 165 | 18 | 1.8 | 8 | 36 | 347 | 227 | 14 | 1.4 | 18.0 | 1.320 |
| max | 21 | 36 | 199 | 18 | 1.9 | 10 | 62 | 385 | 286 | 20 | 1.4 | 30.0 | 2.100 | |
| 10 | min | 25 | 51 | 1025 | 36 | 13.0 | 18 | 57 | 430 | 131 | 32 | 1.6 | 23.0 | 0.610 |
| max | 35 | 63 | 1035 | 38 | 15.7 | 20 | 65 | 474 | 149 | 32 | 2.1 | 31.0 | 0.880 | |
| 11 | min | 20 | 408 | 762 | 20 | 16.9 | 10 | 67 | 619 | 119 | 30 | 2.6 | 3.4 | 0.900 |
| max | 24 | 422 | 838 | 22 | 25.7 | 14 | 77 | 678 | 132 | 34 | 2.6 | 4.6 | 1.000 | |
| 12 | min | 11 | 225 | 418 | 10 | 11.4 | 5 | 50 | 411 | 140 | 11 | 1.4 | 5.0 | 0.500 |
| max | 17 | 237 | 450 | 14 | 12.3 | 7 | 60 | 421 | 142 | 17 | 1.4 | 5.0 | 0.660 | |
| 13 | min | 22 | 134 | 1001 | 30 | 11.9 | 15 | 122 | 409 | 83 | 13 | 1.6 | 3.0 | 0.692 |
| max | 22 | 145 | 1033 | 34 | 12.5 | 15 | 127 | 449 | 96 | 14 | 1.7 | 3.0 | 0.730 | |
| 14 | min | 16 | 162 | 1090 | 12 | 5.5 | 3 | 22 | 261 | 63 | 50 | 1.4 | 9.0 | 0.074 |
| max | 19 | 166 | 1130 | 13 | 6.9 | 3 | 23 | 262 | 65 | 63 | 1.5 | 13.0 | 0.080 | |
| 15 | min | 18 | 49 | 693 | 34 | 5.4 | 25 | 122 | 502 | 102 | 23 | 1.8 | 4.0 | 0.640 |
| max | 35 | 96 | 799 | 51 | 6.4 | 40 | 157 | 694 | 136 | 48 | 3.1 | 6.0 | 1.990 | |
| No. of Water Bodies (see Figure 1) | Parameter | Cu | Pb | Zn | Ni | Cd | Co | Cr | Ba | Sr | As | Sb | Br | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Igeo | ||||||||||||||
| 1 | min | −1.62 | 1.13 | 1.52 | −2.12 | 3.81 | −1.35 | −1.20 | −1.15 | −1.37 | 2.32 | 2.32 | 2.05 | −6.31 |
| max | −1.06 | 1.79 | 2.26 | −1.37 | 4.46 | −0.58 | −0.27 | −0.82 | −0.27 | 2.44 | 2.74 | 3.10 | 3.88 | |
| 2 | min | −1.17 | 0.85 | 1.21 | −0.91 | 3.00 | −0.28 | 0.24 | −0.60 | −2.56 | 1.91 | 2.00 | −1.66 | −6.31 |
| max | 0.20 | 4.07 | 4.54 | −0.61 | 7.59 | −0.15 | 0.54 | −0.19 | −2.20 | 3.64 | 4.22 | 1.67 | −6.31 | |
| 3 | min | −2.29 | 0.46 | 0.50 | −2.12 | 2.22 | −1.21 | −0.17 | −1.14 | −2.39 | 1.74 | 1.58 | −1.66 | −6.31 |
| max | −0.01 | 4.19 | 3.60 | −0.44 | 6.45 | 0.03 | 0.19 | −0.36 | −2.05 | 3.91 | 4.68 | 3.15 | 2.13 | |
| 4 | min | 0.11 | 4.23 | 3.76 | −1.04 | 6.74 | −0.87 | −0.41 | 4.48 | −0.14 | 4.02 | 4.17 | 1.80 | 2.65 |
| max | 0.43 | 4.32 | 3.88 | −0.56 | 7.06 | 0.42 | 0.02 | 4.50 | −0.04 | 4.20 | 4.87 | 2.15 | 3.31 | |
| 5 | min | 1.07 | 5.39 | 5.89 | −0.81 | 8.57 | 0.37 | 0.21 | −0.90 | −1.79 | 4.23 | 6.03 | 1.67 | 4.68 |
| max | 1.42 | 6.46 | 6.52 | −0.56 | 9.38 | 0.46 | 0.97 | −0.83 | −1.16 | 5.26 | 6.98 | 2.93 | 5.39 | |
| 6 | min | 1.80 | 6.66 | 7.10 | 0.22 | 9.63 | 0.42 | 0.56 | −0.21 | −1.80 | 5.45 | 6.72 | 1.15 | 5.11 |
| max | 2.35 | 6.89 | 8.46 | 0.48 | 9.63 | 0.65 | 0.81 | −0.07 | −1.33 | 5.81 | 7.45 | 1.67 | 5.85 | |
| 7 | min | 2.10 | 6.65 | 7.34 | −0.30 | 10.16 | −0.15 | 0.33 | −0.71 | −1.76 | 5.98 | 6.09 | 1.67 | 5.35 |
| max | 2.21 | 6.72 | 8.17 | −0.18 | 10.90 | 0.58 | 0.44 | −0.38 | −1.67 | 6.21 | 6.56 | 2.43 | 5.46 | |
| 8 | min | −0.32 | 3.38 | 4.02 | −0.41 | 6.87 | 0.13 | 0.07 | −1.12 | −1.44 | 3.06 | 3.46 | 0.93 | 4.41 |
| max | −0.29 | 3.52 | 4.07 | −0.30 | 6.87 | 0.37 | 0.13 | −1.06 | −1.35 | 3.26 | 3.54 | 0.93 | 4.57 | |
| 9 | min | −1.70 | 0.23 | 0.72 | −2.20 | 3.58 | −1.67 | −1.52 | −1.30 | −1.21 | 2.54 | 2.22 | 2.51 | 4.05 |
| max | −1.48 | 0.48 | 0.99 | −2.20 | 3.66 | −1.35 | −0.74 | −1.15 | −0.88 | 3.06 | 2.22 | 3.25 | 4.72 | |
| 10 | min | −1.23 | 1.00 | 3.35 | −1.20 | 6.44 | −0.50 | −0.86 | −0.99 | −2.00 | 3.74 | 2.42 | 2.87 | 2.94 |
| max | −0.74 | 1.30 | 3.36 | −1.12 | 6.71 | −0.35 | −0.67 | −0.85 | −1.82 | 3.74 | 2.81 | 3.30 | 3.47 | |
| 11 | min | −1.55 | 4.00 | 2.92 | −2.04 | 6.82 | −1.35 | −0.63 | −0.47 | −2.14 | 3.64 | 3.12 | 0.11 | 3.50 |
| max | −1.29 | 4.05 | 3.06 | −1.91 | 7.42 | −0.87 | −0.43 | −0.33 | −1.99 | 3.82 | 3.12 | 0.55 | 3.65 | |
| 12 | min | −2.41 | 3.14 | 2.06 | −3.04 | 6.25 | −2.35 | −1.05 | −1.06 | −1.91 | 2.20 | 2.22 | 0.67 | 2.65 |
| max | −1.78 | 3.22 | 2.16 | −2.56 | 6.36 | −1.87 | −0.79 | −1.02 | −1.89 | 2.82 | 2.22 | 0.67 | 3.05 | |
| 13 | min | −1.41 | 2.39 | 3.32 | −1.46 | 6.31 | −0.77 | 0.24 | −1.06 | −2.66 | 2.44 | 2.42 | −0.07 | 3.12 |
| max | −1.41 | 2.51 | 3.36 | −1.28 | 6.38 | −0.77 | 0.30 | −0.93 | −2.45 | 2.54 | 2.50 | −0.07 | 3.20 | |
| 14 | min | −1.87 | 2.67 | 3.44 | −2.78 | 5.20 | −3.09 | −2.23 | −1.71 | −3.06 | 4.38 | 2.22 | 1.51 | −0.10 |
| max | −1.62 | 2.70 | 3.49 | −2.67 | 5.52 | −3.09 | −2.17 | −1.71 | −3.01 | 4.71 | 2.32 | 2.05 | 0.01 | |
| 15 | min | −1.70 | 0.94 | 2.79 | −1.28 | 5.17 | −0.03 | 0.24 | −0.77 | −2.36 | 3.26 | 2.58 | 0.34 | 3.01 |
| max | −0.74 | 1.91 | 2.99 | −0.69 | 5.42 | 0.65 | 0.60 | −0.30 | −1.95 | 4.32 | 3.37 | 0.93 | 4.65 | |
| Explanations: | ||||||||||||||
| practically uncontaminated (class 0: Igeo ≤ 0.0) | ||||||||||||||
| uncontaminated to moderately contaminated (class I: 0.0 < Igeo ≤ 1.0) | ||||||||||||||
| moderately contaminated (class II: 1.0 < Igeo ≤ 2.0) | ||||||||||||||
| moderately to heavily contaminated (class III: 2.0 < Igeo ≤ 3.0) | ||||||||||||||
| heavily contaminated (class IV: 3.0 < Igeo ≤ 4.0) | ||||||||||||||
| heavily to extremely contaminated (class V: 4.0 < Igeo ≤ 5.0) | ||||||||||||||
| extremely contaminated (class VI: Igeo > 5.0) | ||||||||||||||
| No. of Water Bodies (see Figure 1) | Parameter | Cu | Pb | Zn | Ni | Cd | Co | Cr | Ba | Sr | As | Sb | Br | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRE | ||||||||||||||
| 1 | min | 1.3 | 0.9 | 1.1 | 1.7 | 0.8 | 2.5 | 5.0 | 3.9 | 8.5 | 2.0 | (–) | (–) | 0.0 |
| max | 1.9 | 1.5 | 1.9 | 2.9 | 1.3 | 4.3 | 9.6 | 4.9 | 18.1 | 2.2 | (–) | (–) | 22.5 | |
| 2 | min | 1.7 | 0.8 | 0.9 | 4.0 | 0.5 | 5.3 | 13.6 | 5.7 | 3.7 | 1.5 | (–) | (–) | 0.0 |
| max | 4.5 | 7.3 | 9.0 | 4.9 | 11.6 | 5.8 | 16.7 | 7.7 | 4.8 | 5.0 | (–) | (–) | 0.0 | |
| 3 | min | 0.8 | 0.6 | 0.5 | 1.7 | 0.3 | 2.8 | 10.2 | 4.0 | 4.2 | 1.3 | (–) | (–) | 0.0 |
| max | 3.9 | 7.9 | 4.7 | 5.5 | 5.2 | 6.5 | 13.1 | 6.8 | 5.3 | 6.0 | (–) | (–) | 6.7 | |
| 4 | min | 4.2 | 8.1 | 5.3 | 3.6 | 6.4 | 3.5 | 8.7 | 194.9 | 19.8 | 6.5 | (–) | (–) | 9.6 |
| max | 5.3 | 8.6 | 5.7 | 5.1 | 8.0 | 8.5 | 11.7 | 196.9 | 21.3 | 7.3 | (–) | (–) | 15.2 | |
| 5 | min | 8.2 | 18.1 | 22.9 | 4.3 | 22.8 | 8.3 | 13.3 | 4.7 | 6.3 | 7.5 | (–) | (–) | 39.2 |
| max | 10.4 | 38.1 | 35.6 | 5.1 | 40.0 | 8.8 | 22.6 | 4.9 | 9.8 | 15.3 | (–) | (–) | 64.0 | |
| 6 | min | 13.6 | 43.7 | 53.3 | 8.7 | 47.6 | 8.5 | 17.0 | 7.6 | 6.3 | 17.5 | (–) | (–) | 52.9 |
| max | 19.9 | 51.2 | 136.3 | 10.5 | 47.6 | 10.0 | 20.2 | 8.3 | 8.7 | 22.5 | (–) | (–) | 88.3 | |
| 7 | min | 16.7 | 43.4 | 62.9 | 6.1 | 68.8 | 5.8 | 14.4 | 5.3 | 6.5 | 25.3 | (–) | (–) | 62.3 |
| max | 18.0 | 45.4 | 111.6 | 6.6 | 114.4 | 9.5 | 15.6 | 6.7 | 6.9 | 29.7 | (–) | (–) | 67.5 | |
| 8 | min | 3.1 | 4.5 | 6.3 | 5.6 | 7.0 | 7.0 | 12.1 | 4.0 | 8.1 | 3.3 | (–) | (–) | 32.5 |
| max | 3.2 | 5.0 | 6.5 | 6.1 | 7.0 | 8.3 | 12.6 | 4.2 | 8.6 | 3.8 | (–) | (–) | 36.3 | |
| 9 | min | 1.2 | 0.5 | 0.6 | 1.6 | 0.7 | 2.0 | 4.0 | 3.5 | 9.5 | 2.3 | (–) | (–) | 25.4 |
| max | 1.4 | 0.6 | 0.8 | 1.6 | 0.8 | 2.5 | 6.9 | 3.9 | 11.9 | 3.3 | (–) | (–) | 40.4 | |
| 10 | min | 1.7 | 0.9 | 4.0 | 3.3 | 5.2 | 4.5 | 6.3 | 4.4 | 5.5 | 5.3 | (–) | (–) | 11.7 |
| max | 2.3 | 1.1 | 4.0 | 3.5 | 6.3 | 5.0 | 7.2 | 4.8 | 6.2 | 5.3 | (–) | (–) | 16.9 | |
| 11 | min | 1.3 | 6.9 | 2.9 | 1.8 | 6.8 | 2.5 | 7.4 | 6.3 | 5.0 | 5.0 | (–) | (–) | 17.3 |
| max | 1.6 | 7.2 | 3.2 | 2.0 | 10.3 | 3.5 | 8.6 | 6.9 | 5.5 | 5.7 | (–) | (–) | 19.2 | |
| 12 | min | 0.7 | 3.8 | 1.6 | 0.9 | 4.6 | 1.3 | 5.6 | 4.2 | 5.8 | 1.8 | (–) | (–) | 9.6 |
| max | 1.1 | 4.0 | 1.7 | 1.3 | 4.9 | 1.8 | 6.7 | 4.3 | 5.9 | 2.8 | (–) | (–) | 12.7 | |
| 13 | min | 1.5 | 2.3 | 3.9 | 2.7 | 4.8 | 3.8 | 13.6 | 4.2 | 3.5 | 2.2 | (–) | (–) | 13.3 |
| max | 1.5 | 2.5 | 4.0 | 3.1 | 5.0 | 3.8 | 14.1 | 4.6 | 4.0 | 2.3 | (–) | (–) | 14.0 | |
| 14 | min | 1.1 | 2.7 | 4.2 | 1.1 | 2.2 | 0.8 | 2.4 | 2.7 | 2.6 | 8.3 | (–) | (–) | 1.4 |
| max | 1.3 | 2.8 | 4.4 | 1.2 | 2.8 | 0.8 | 2.6 | 2.7 | 2.7 | 10.5 | (–) | (–) | 1.5 | |
| 15 | min | 1.2 | 0.8 | 2.7 | 3.1 | 2.2 | 6.3 | 13.6 | 5.1 | 4.3 | 3.8 | (–) | (–) | 12.3 |
| max | 2.3 | 1.6 | 3.1 | 4.6 | 2.6 | 10.0 | 17.4 | 7.1 | 5.7 | 8.0 | (–) | (–) | 38.3 | |
| Explanations: (–)—lack of data. | ||||||||||||||
| 0.0 < IRE ≤ 1.0 | ||||||||||||||
| 1.0 < IRE ≤ 5.0 | ||||||||||||||
| 5.0 < IRE ≤ 10.0 | ||||||||||||||
| 10.0 < IRE ≤ 100.0 | ||||||||||||||
| IRE > 100.0 | ||||||||||||||
| Item | Water Bodies Used for Recreational Purposes on the Silesian Upland | Water Bodies Used for Recreational Purposes Worldwide and the Concentration of Metals, Non-Metals and Metalloids |
|---|---|---|
| Pb | 30.0–3020.0 mg/kg | Hoedong Reservoir (South Korea)—53.6–69.2 mg/kg [49]; Římov Reservoir (Czech Republic)—up to 42.0 mg/kg [50]; Lake Eğirdir (Turkey)—0.8–22.1 mg/kg [51]; the water bodies forming the Dnieper reservoir cascade (Ukraine)—from a minimum of 17.2 mg/kg (Kremenchug Reservoir) to a maximum of 63.3 mg/kg (Kakhovka Reservoir) [52]. |
| Zn | 142.0–35,300.0 mg/kg | Lake Gusinoe (Russia)—74.2–598.0 mg/kg [53]; Lake Yangzong (China)—149.2 mg/kg (average) [54]; Lake Qaroun (Egypt)—0.01–92.6 mg/kg [55]. |
| Cd | 0.7–286.0 mg/kg | Lake Jianhu (China)—0.29–0.42 mg/kg [56]; Kapshagay Reservoir (Kazakhstan)—0.46 mg/kg [57]; Hoedong Reservoir (South Korea)—1.4–1.8 mg/kg [48]; Lake Taihu (China)—0.23–3.07 mg/kg [58]; several reservoirs in Germany—4.03 mg/kg (average) [59]. |
| Ni | 10.0–115.0 mg/kg | Lake Balaton (Hungary)—4.4–5.5 mg/kg [60]; Wivenhoe Reservoir (Australia)—23.5–26.5 mg/kg; Little Nerang Reservoir (Australia)—18.5–19.0 mg/kg [61]; Terragido Reservoir (Portugal)—18.0–80.0 mg/kg [62]; Lake Łebsko (Poland)—13.7–184.4 mg/kg [63]; Badovci Lake (Kosovo)—139.0–666.0 mg/kg [64]. |
| Cu | 11.0–298.0 mg/kg | Ružín Reservoir (Slovakia)—196.0–310.7 mg/kg [65]; Kapshagay Reservoir (Kazakhstan)—0.12–0.38 mg/kg [57]; the ponds used for intensive shrimp farming (Brazil)—10.0–20.0 mg/kg [66]; Dobczyce Reservoir (Poland)—5.5–45.4 mg/kg [67]. |
| Co | 3.0–40.0 mg/kg | Tailings ponds (Indonesia)—255.0 mg/kg [68]; Kallar Kahar Lake (Pakistan)—4.03–11.34 mg/kg [69]; Wivenhoe Reservoir (Australia)—20.7–21.8 mg/kg; Little Nerang (Australia)—16.6–19.1 mg/kg [61]; Guaíba Lake (Brasil)—6.4–97.6 mg/kg [70]. |
| Cr | 22.0–203.0 mg/kg | Três Marias Reservoir (Brasil)—2.0–150.6 mg/kg [71]; Dianchi Lake (China)—68.6–95.3 mg/kg [72]; Orlík Reservoir (Czech Republic)—72.4–123 mg/kg (108 mg/kg—average) [73]; Mujib Reservoir (Jordan)—79.8–136.8 mg/kg (114.2 mg/kg—average) [74]; ponds in Kolkata (India)—up to 882.2 mg/kg [75]. |
| As | 8.0–178.0 mg/kg | Yangebup Lake (Australia)—21.8 mg/kg (average) [76]; Badovci Lake (Kosovo)—10.0–29.9 mg/kg (24.2 mg/kg—average) [64]; Rożnów Lake (Poland)—5.2 mg/kg (average) [77]; 15 lakes located on the Crimean Peninsula—from 3.05 mg/kg (Dzharylgach Lake) to 20.41 mg/kg (Adjigol Lake) [78]. |
| Ba | 263.0–19,300.0 mg/kg | Los Molinos and San Roque reservoirs (Argentina)—383–400 mg/kg [79]; Kaw Reservoir (USA)—280–420 mg/kg [80]; Irkutsk Reservoir (Russia)—582–633 mg/kg [81]; Guaíba Lake (Brasil)—139–1448 mg/kg [70]. |
| Sb | 0.9–52.5 mg/kg | Lengshuigou Reservoir (China)—258.8–466.6 mg/kg [82]; Goczałkowice Reservoir (Poland)—80.0–120.0 mg/kg [83]. |
| Br | 1.0–31.0 mg/kg | lack of data |
| Sr | 63.0–510.0 mg/kg | Los Molinos and San Roque reservoirs (Argentina)—94.0–99.0 mg/kg [79]; Irkutsk Reservoir (Russia)—186.0–274.0 mg/kg [81]; Kouris Reservoir (Cyprus)—706.0 mg/kg (average) [84]. |
| S | 0.001–4.590% | lack of data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzetala, M.A.; Machowski, R.; Solarski, M.; Bakota, D.; Płomiński, A.; Rzetala, M. Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use. Int. J. Environ. Res. Public Health 2023, 20, 4334. https://doi.org/10.3390/ijerph20054334
Rzetala MA, Machowski R, Solarski M, Bakota D, Płomiński A, Rzetala M. Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use. International Journal of Environmental Research and Public Health. 2023; 20(5):4334. https://doi.org/10.3390/ijerph20054334
Chicago/Turabian StyleRzetala, Martyna A., Robert Machowski, Maksymilian Solarski, Daniel Bakota, Arkadiusz Płomiński, and Mariusz Rzetala. 2023. "Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use" International Journal of Environmental Research and Public Health 20, no. 5: 4334. https://doi.org/10.3390/ijerph20054334
APA StyleRzetala, M. A., Machowski, R., Solarski, M., Bakota, D., Płomiński, A., & Rzetala, M. (2023). Toxic Metals, Non-Metals and Metalloids in Bottom Sediments as a Geoecological Indicator of a Water Body’s Suitability for Recreational Use. International Journal of Environmental Research and Public Health, 20(5), 4334. https://doi.org/10.3390/ijerph20054334









