Investigation of the Solubility of Elemental Sulfur (S) in Sulfur-Containing Natural Gas with Machine Learning Methods
Abstract
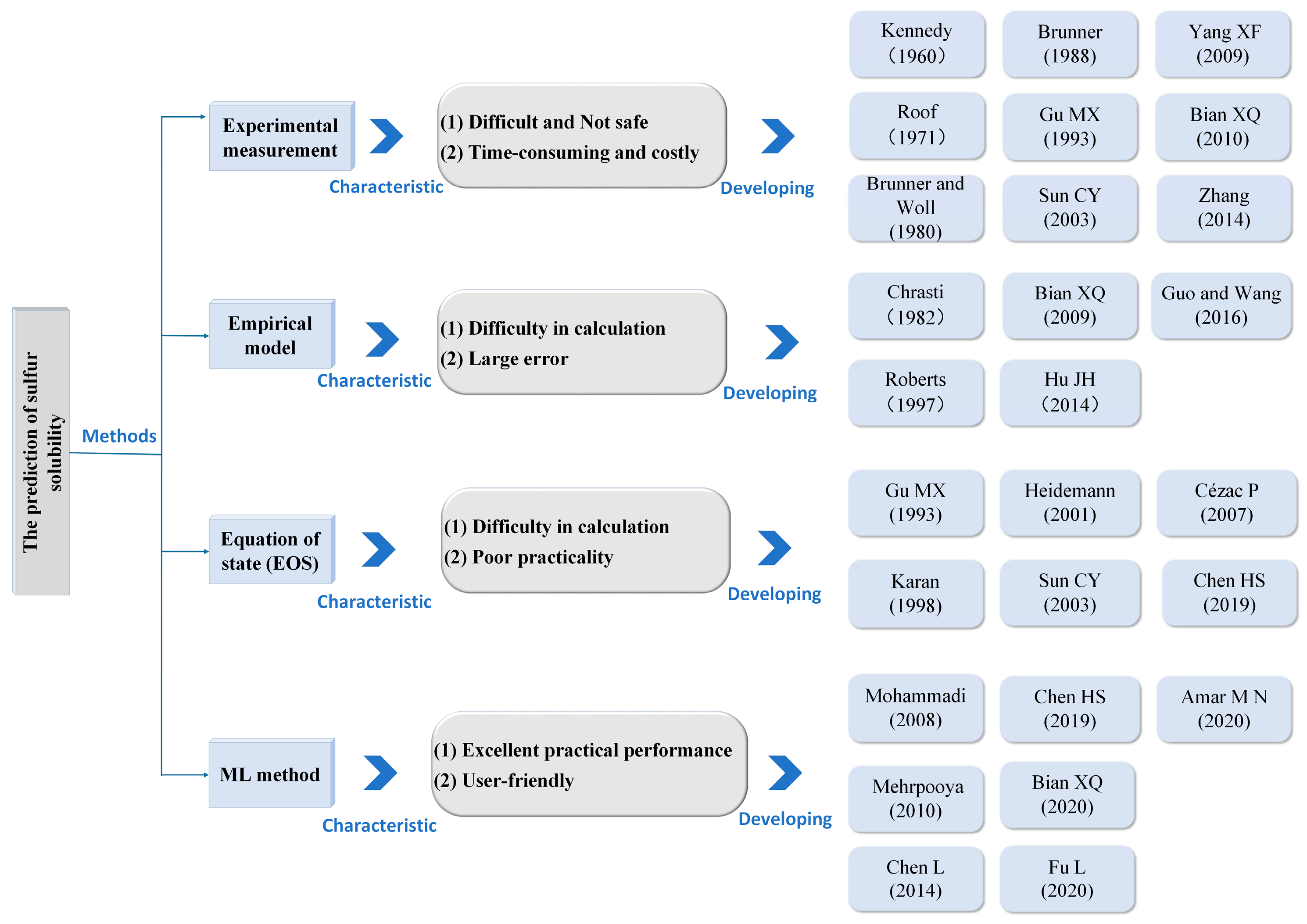
1. Introduction
- (1)
- Researchers appear not to have focused on the impact of hyperparameter optimization in ML models for sulfur solubility prediction on model performance. Moreover, most of the studies typically employ a single algorithm to build ML models. Single algorithms usually have unavoidable drawbacks that may degrade the models’ capabilities.
- (2)
- Despite the limited actual sample of sulfur solubility, researchers have not focused on its limitations in training ML models or the use of WLSSVM and RF for predicting sulfur solubility despite their efficiency and promise.
- (3)
- In previous studies, scholars did not take remedial measures against the black-box characteristics of the ML model. The lack of interpretability of experimental results may limit scholars’ exploration of sulfur solubility variation patterns in practical applications.
- (1)
- For hyperparameter optimization, with the help of c-vOPR and awuST, the new method of a whale optimization–genetic algorithm (WOA-GA) balances accuracy with efficiency, while improving global search capabilities and reducing the risk of slipping into local extremes in the hyperparameter search process.
- (2)
- The WOA-GA-WLSSVM and WOA-GA-RF integrated optimization ML models were created. To train ML models that can accurately predict sulfur solubility, this study uses cnCV as a tool to obtain sufficient information from a limited sample. The performance of the suggested models, as well as their stability and reliability, are analyzed from various angles.
- (3)
- The generic positional oligomer importance matrix (gPOIM) is used to estimate how each variable affects sulfur solubility, from which patterns of variation in sulfur solubility are extracted.
2. Methods
2.1. Optimization Methods
2.1.1. Consensus Nested Cross-Validation (cnCV)
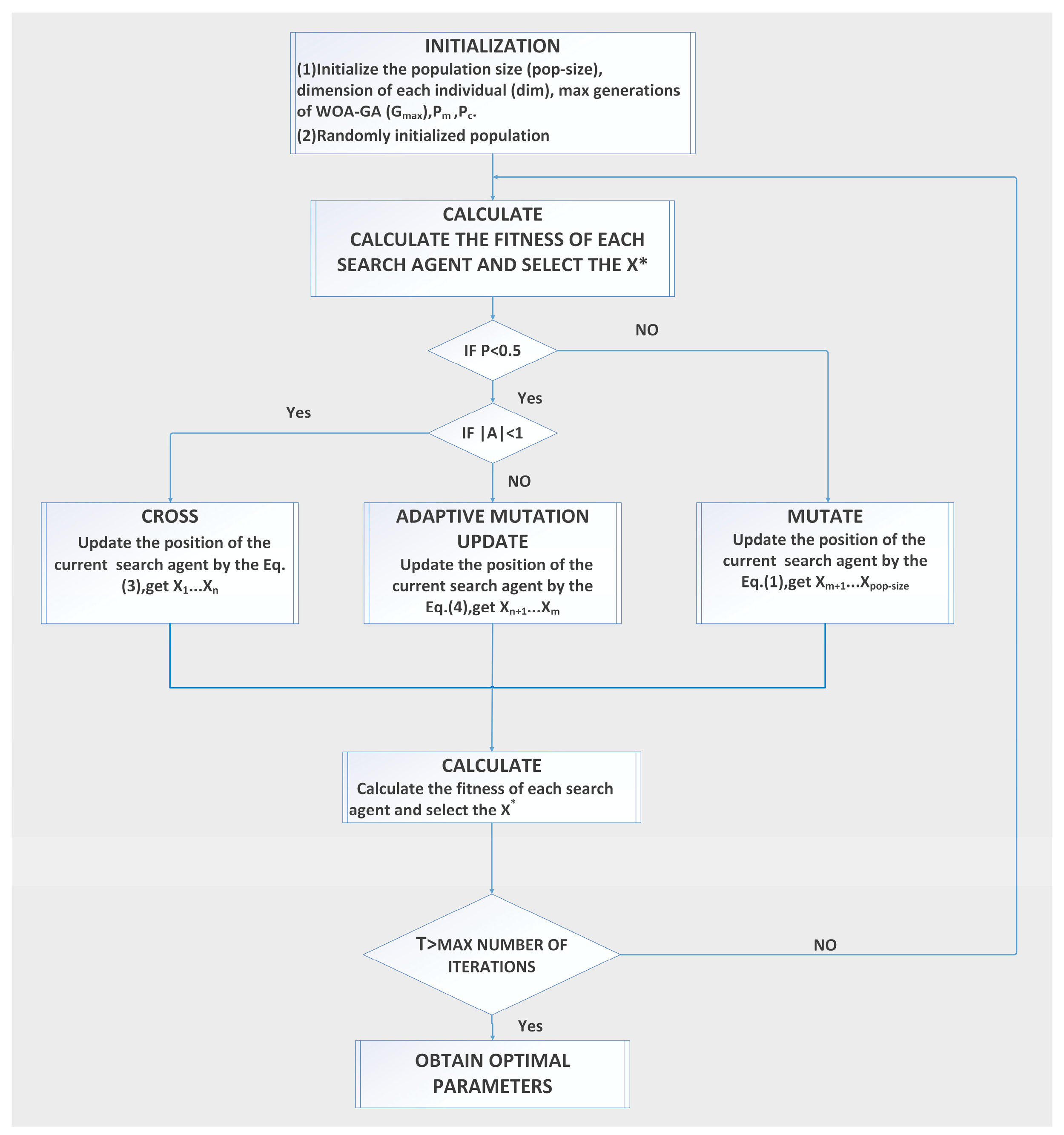
2.1.2. The Hybrid Optimization Algorithm WOA-GA
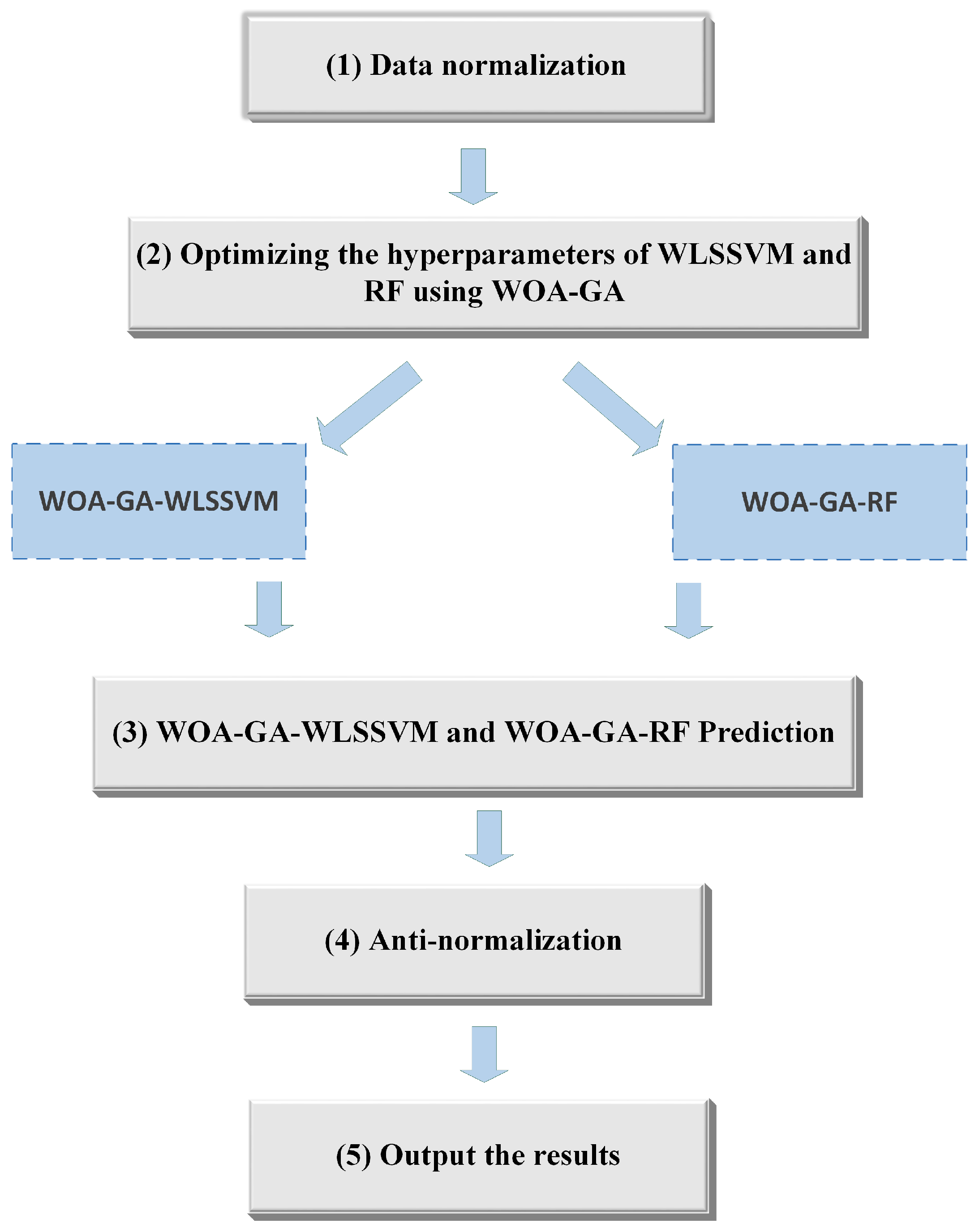
2.2. Modeling of Integrated Optimization
- (1)
- Data preprocessing was performed first. To eliminate the effect of different units and magnitudes of variables on model training, the study normalized all data sets to between −1 and 1.
- (2)
- The optimal hyperparameters of WLSSVM and RF were selected using WOA-GA to build the WOA-GA-WLSSVM and WOA-GA-RF models.
- (3)
- The WOA-GA-WLSSVM and WOA-GA-RF models were trained and tested using cnCV.
- (4)
- The data were anti-normalized.
- (5)
- The final results were output.
2.3. Development of Prediction Models
2.3.1. The Original Data
2.3.2. Model Internal Parameters
3. Results
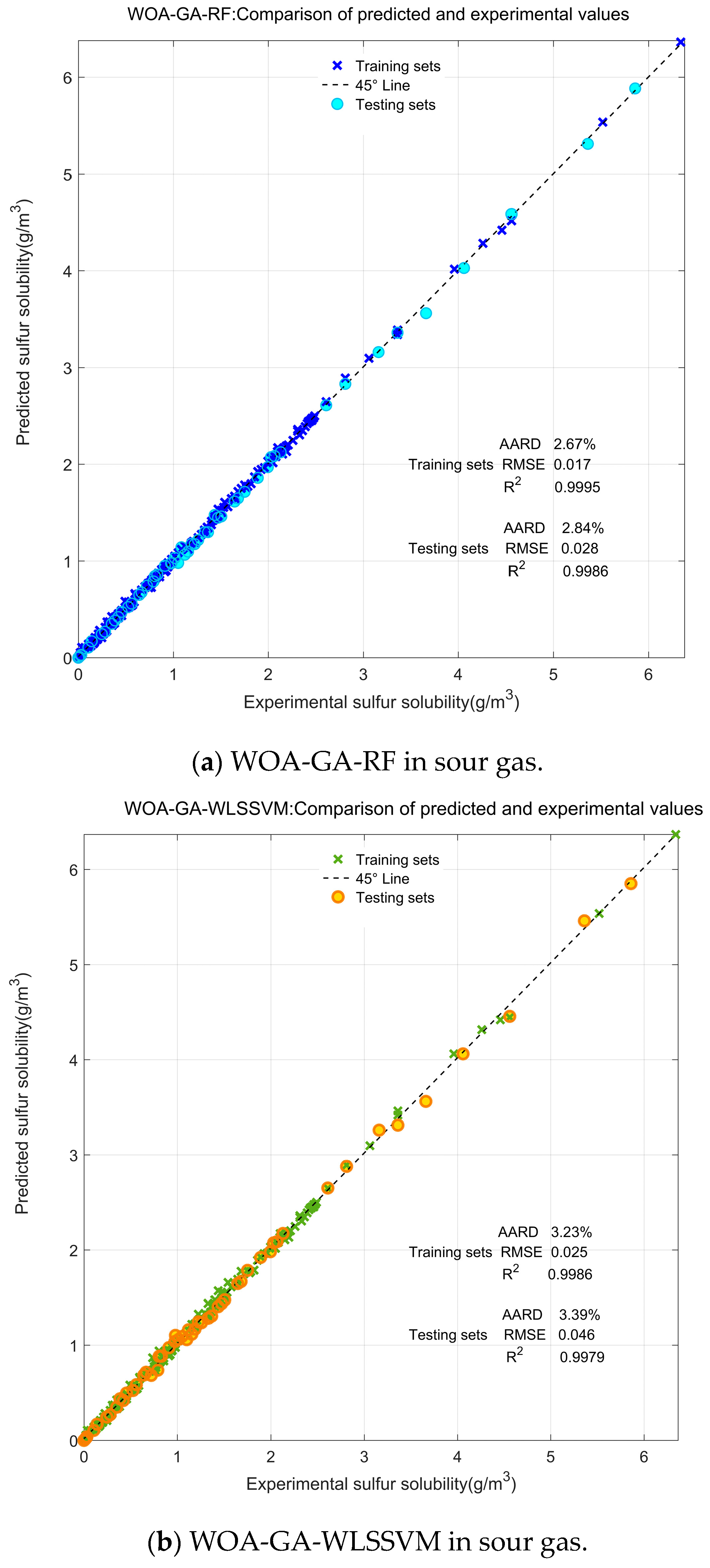
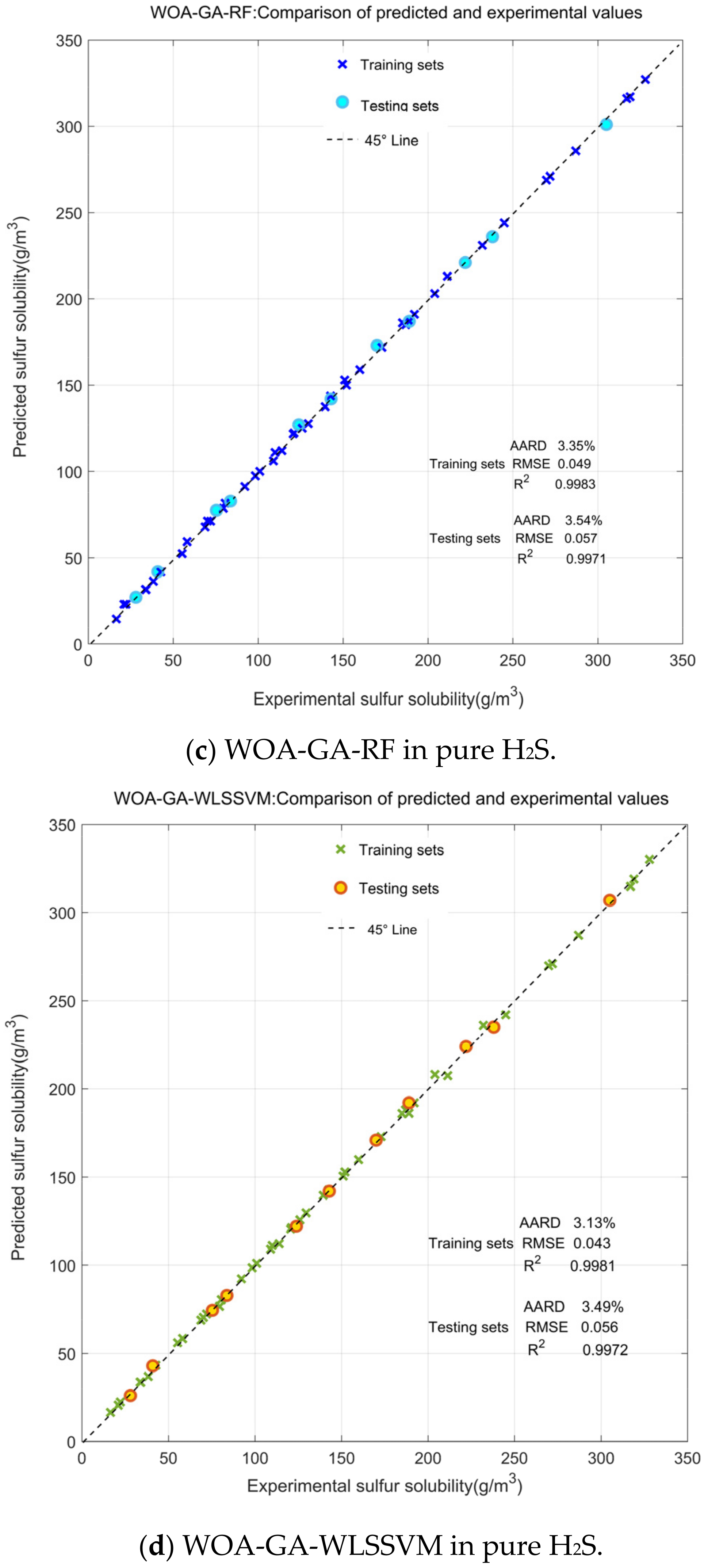
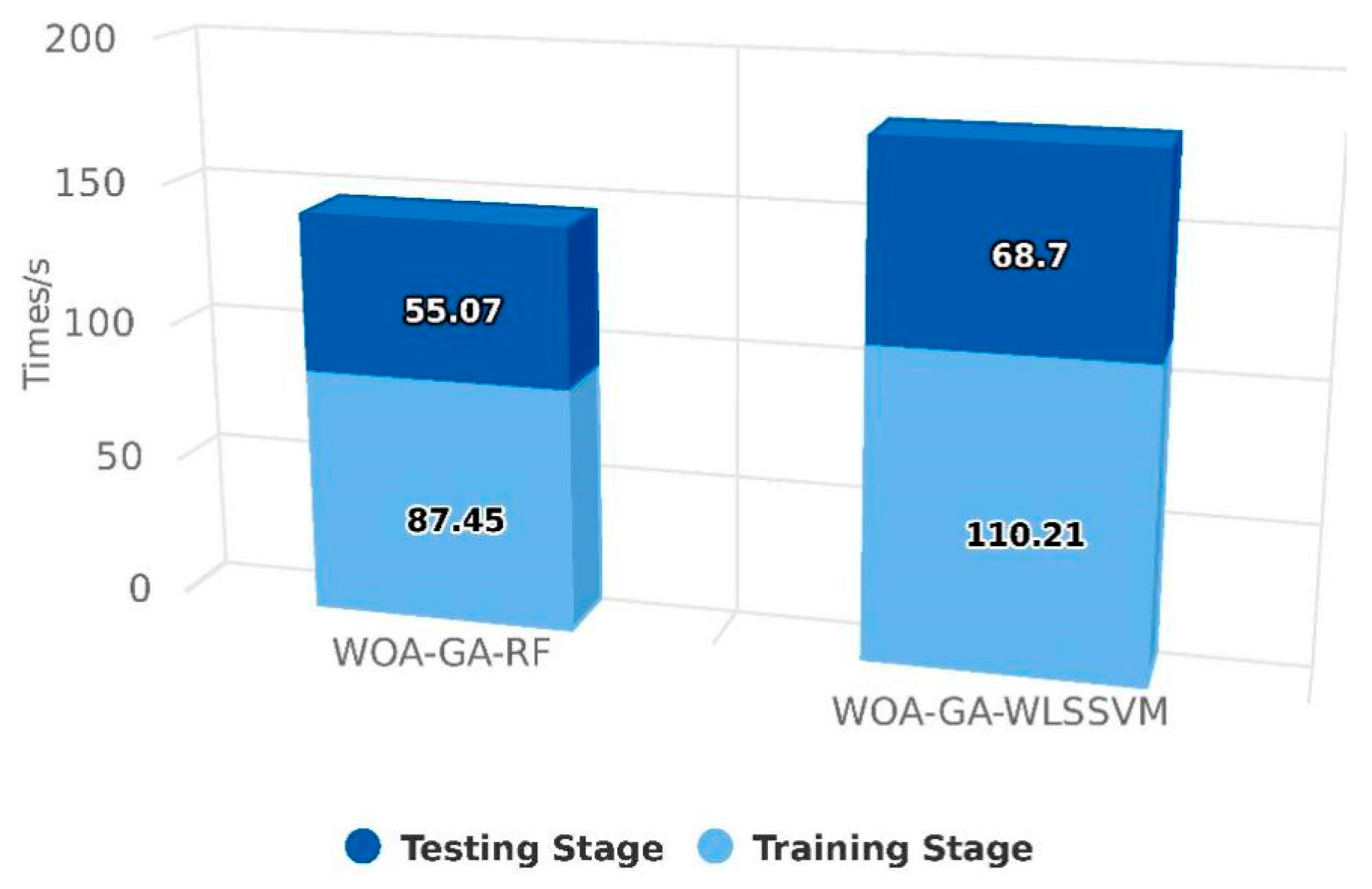
3.1. Comparison and Validation of Models
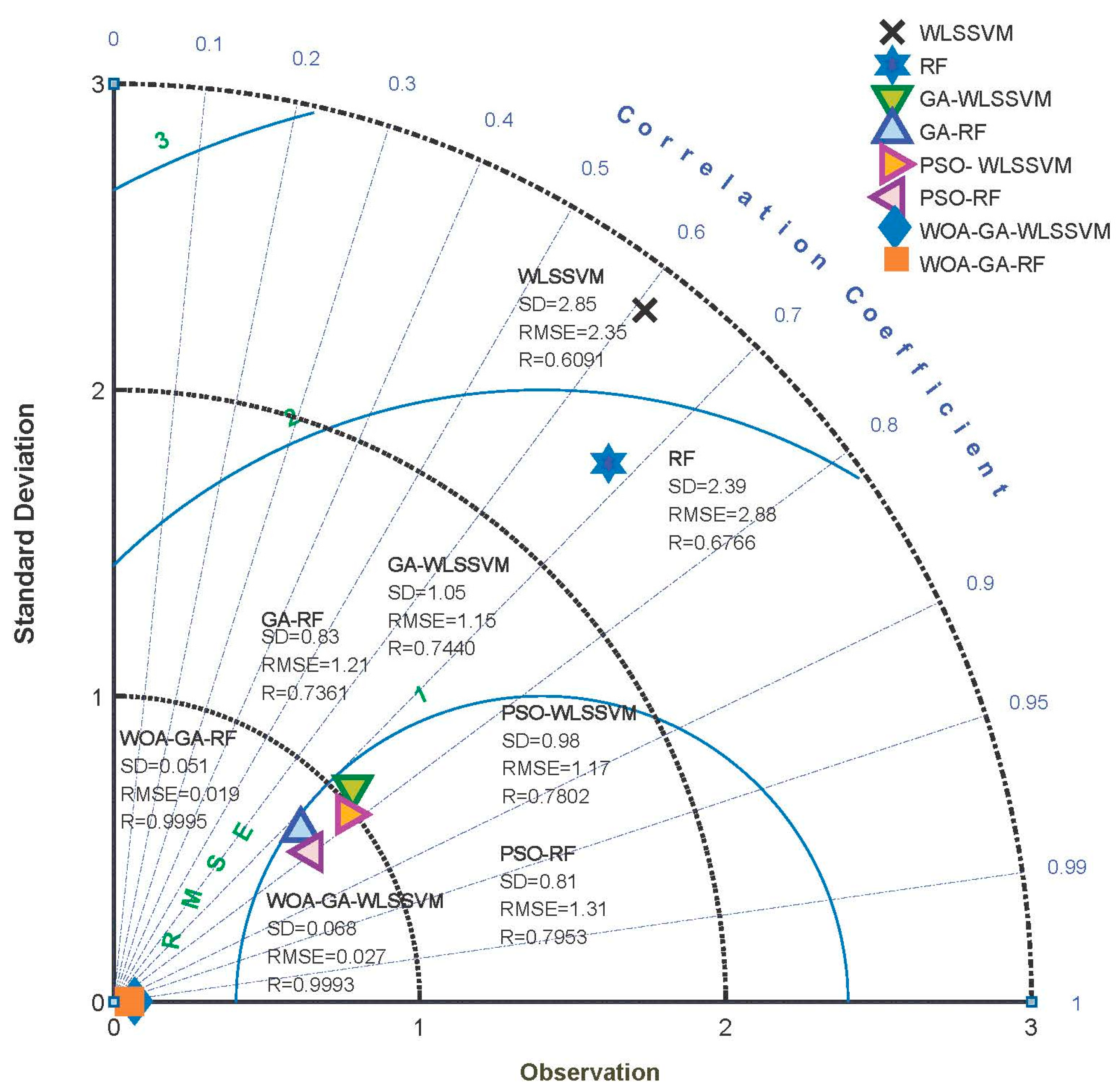
3.2. Stability Analysis
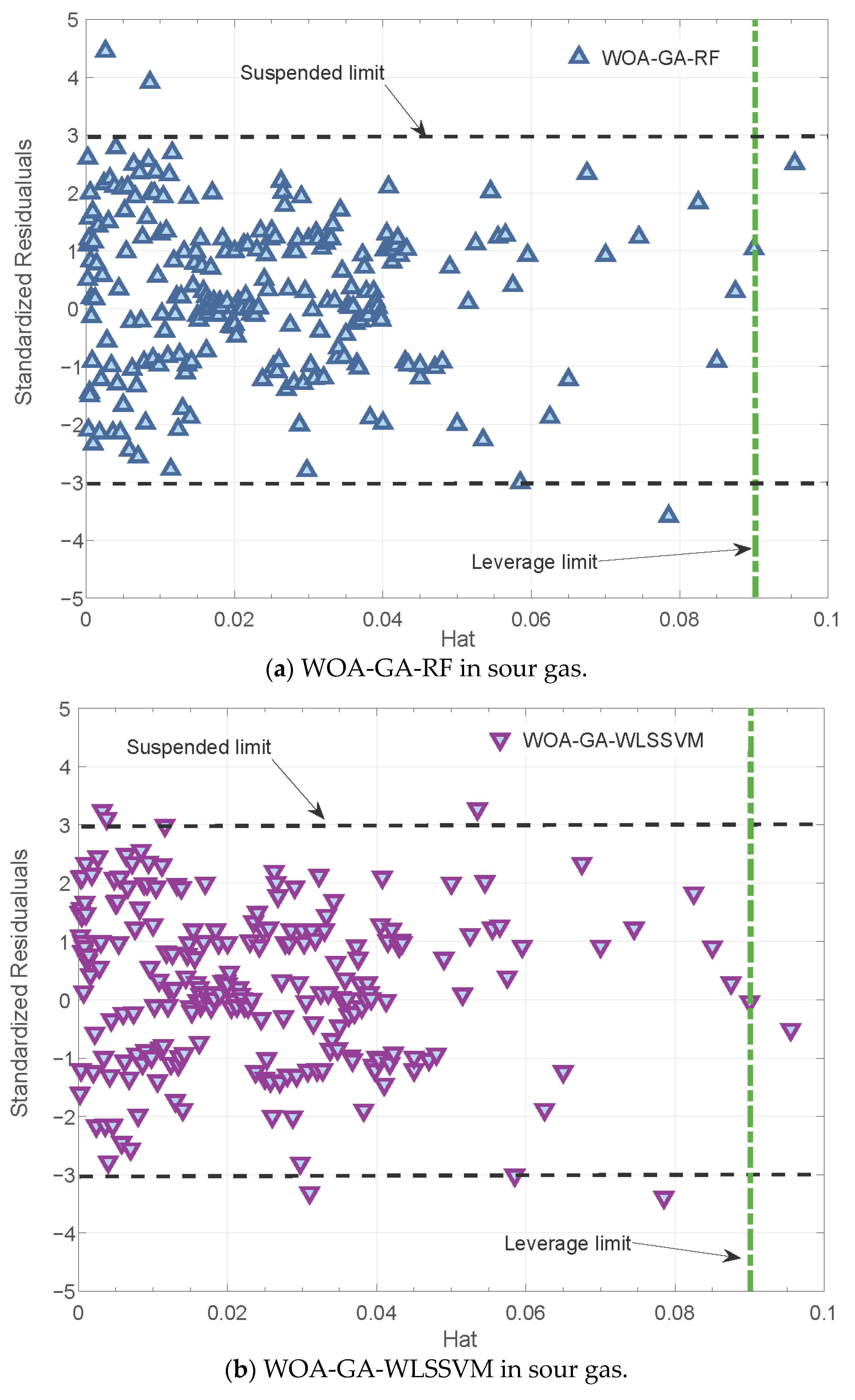
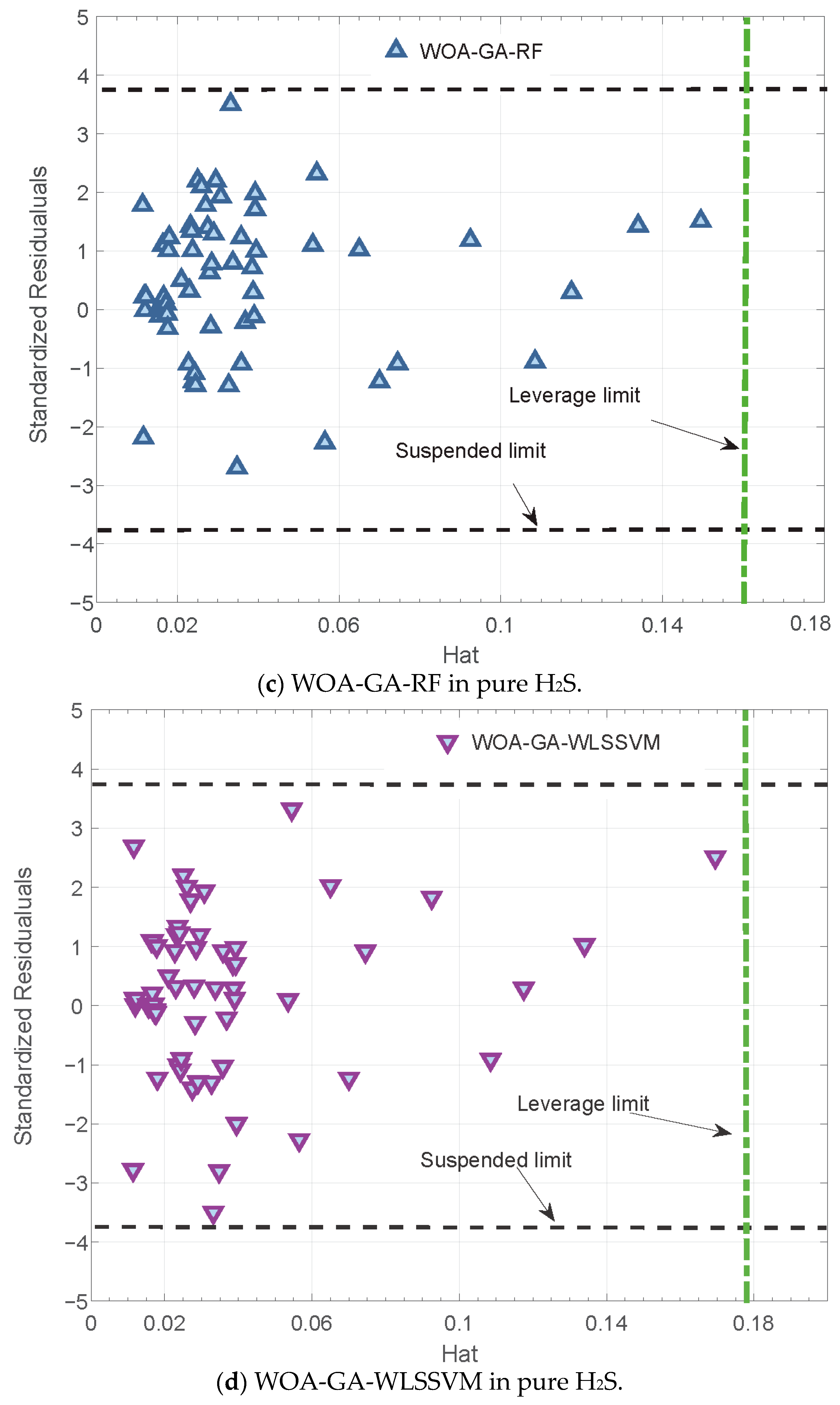
3.3. Reliability Analysis
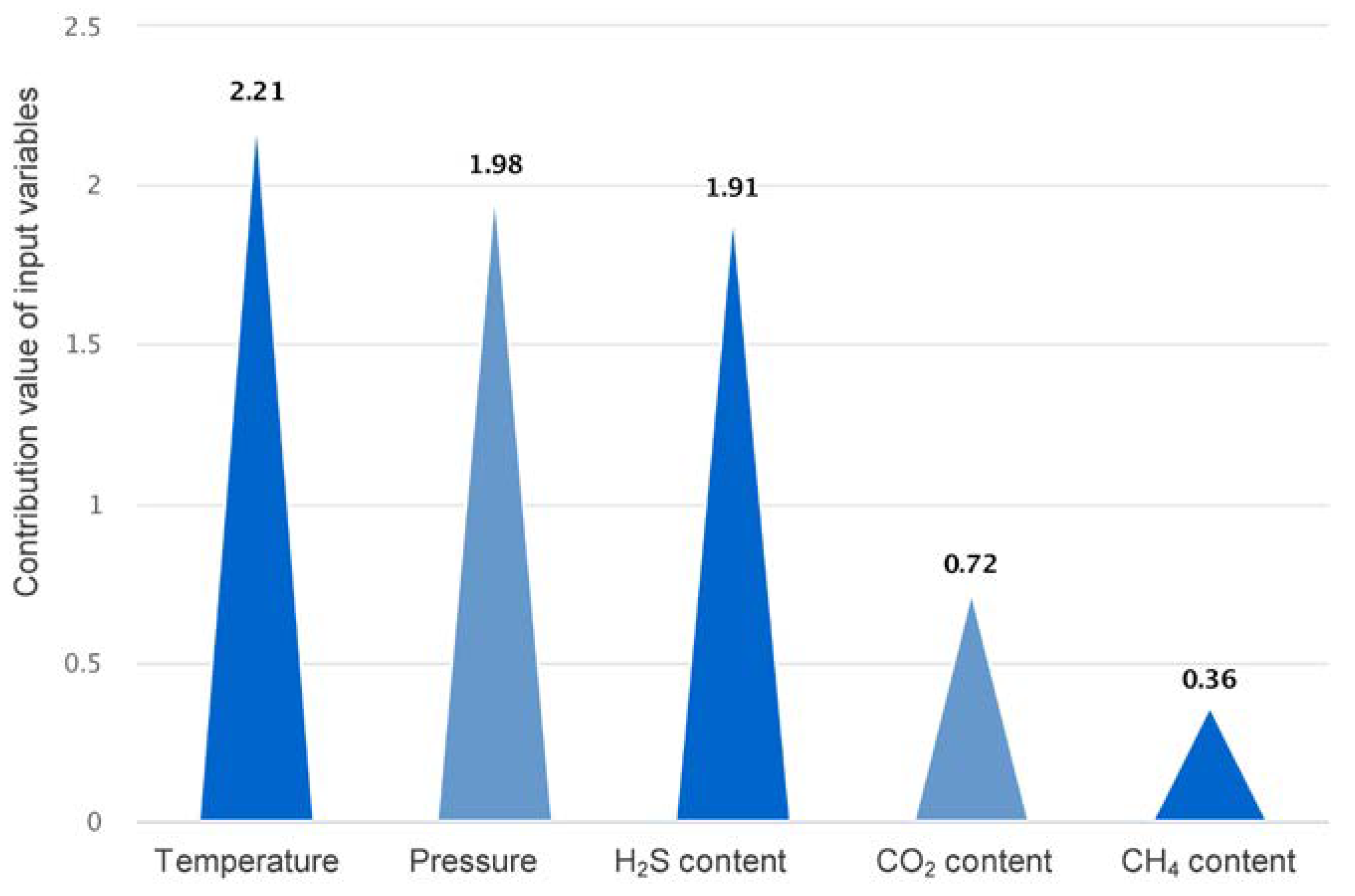
3.4. Analysis of the Contribution of Features
4. Conclusions
- (1)
- In addition to improving the diversity of algorithms, WOA-GA also optimizes the performance of traditional WLSSVM and RF models while avoiding their original drawbacks. By incorporating cnCV in modeling, limited data can provide sufficient information to effectively train the ML model. Researchers should carefully consider the trade-off between computational precision and cost and select ML methods according to the task context, minimizing research costs while ensuring goal completion.
- (2)
- RF was used to predict sulfur solubility for the first time, and its accuracy, stability, and reliability were verified. Compared to the existing ML model, the WOA-GA-RF model has a better comprehensive performance and a greater prediction accuracy in sulfur solubility, with an AARD of 2.69%, SD of 0.051, RMSE of 0.019, and R2 of 0.9991.
- (3)
- Sulfur solubility was found to be more affected by temperature, pressure, and H2S content. Temperature is the most significant element influencing sulfur solubility, followed by pressure. Sulfur solubility significantly increases when the H2S content exceeds 10%, and other conditions remain the same. This pattern can be used to set the relevant parameters in the processing of natural gas containing sulfur.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, S.; Xue, W.; Guo, H.; Li, X.; Zou, G.; Zhao, T.; Dong, H. The Characteristics of Carbon, Nitrogen and Sulfur Transformation During Cattle Manure Composting—Based on Different Aeration Strategies. Int. J. Environ. Res. Public Health 2019, 16, 3930. [Google Scholar] [CrossRef]
- Ding, S.; Chen, Y.; Li, Q.; Li, X.-D. Using Stable Sulfur Isotope to Trace Sulfur Oxidation Pathways during the Winter of 2017–2019 in Tianjin, North China. Int. J. Environ. Res. Public Health 2022, 19, 10966. [Google Scholar] [CrossRef]
- Hongjuan, W.; Lin, Z.; Rong, Z. Progress of Sulfur Deposition Control Measures for High Sulfur-Bearing Gas Reservoirs. Nat. Gas Oil 2012, 30, 5. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Krasavtseva, E.A.; Makarov, D.V. Electrochemistry of Sulfides: Process and Environmental Aspects. Sustainability 2022, 14, 11285. [Google Scholar] [CrossRef]
- Mooyaart, E.A.Q.; Gelderman, E.L.G.; Nijsten, M.W.; de Vos, R.; Hirner, J.M.; de Lange, D.W.; Leuvenink, H.D.G.; van den Bergh, W.M. Outcome after Hydrogen Sulphide Intoxication. Resuscitation 2016, 103, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S. Chemistry of H2S over the Surface of Common Solid Sorbents in Industrial Natural Gas Desulfurization. Catal. Today 2021, 371, 204–220. [Google Scholar] [CrossRef]
- Mansourian, S.H.; Shahhosseini, S.; Maleki, A. Optimization of Oxidative Polymerization-Desulfurization of a Model Fuel Using Polyoxometalate: Effect of Ultrasound Irradiation. J. Ind. Eng. Chem. 2019, 80, 576–589. [Google Scholar] [CrossRef]
- Han, X. Research Progress on Desulfurization Technology of High-Sulfur Natural Gas. Chem. Eng. 2023, 37, 53–57. [Google Scholar] [CrossRef]
- Amar, M.N. Modeling Solubility of Sulfur in Pure Hydrogen Sulfide and Sour Gas Mixtures Using Rigorous Machine Learning Methods. Int. J. Hydrogen Energy 2020, 45, 33274–33287. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Vikrant, K.; Tsang, Y.F.; Singhal, R.K.; Hussain, C.M.; Kim, K.-H. Recent Progress on Solution and Materials Chemistry for the Removal of Hydrogen Sulfide from Various Gas Plants. J. Mol. Liq. 2020, 297, 111886. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Estimating Sulfur Content of Hydrogen Sulfide at Elevated Temperatures and Pressures Using an Artificial Neural Network Algorithm. Ind. Eng. Chem. Res. 2008, 47, 8499–8504. [Google Scholar]
- Brunner, E.; Woll, W. Solubility of Sulfur in Hydrogen Sulfide and Sour Gases. Soc. Pet. Eng. J. 1980, 20, 377–384. [Google Scholar] [CrossRef]
- Bian, X.-Q.; Song, Y.-L.; Mwamukonda, M.K.; Fu, Y. Prediction of the Sulfur Solubility in Pure H2S and Sour Gas by Intelligent Models. J. Mol. Liq. 2020, 299, 112242. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xu, X.; Zhang, L. A New Model for Predicting Sulfur Solubility in Sour Gases Based on Hybrid Intelligent Algorithm. Fuel 2019, 262, 116550. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Leng, M.; Ren, S.; Liu, G.; Ren, Q. Genetic BP Neural Network-Based Prediction of Sulfur Solubility in High Sulfur-Containing Gases. Mod. Chem. 2014, 9, 7. [Google Scholar]
- Fu, L.; Hu, J.; Zhang, Y.; Li, Q. Investigation on Sulfur Solubility in Sour Gas at Elevated Temperatures and Pressures with an Artificial Neural Network Algorithm. Fuel 2020, 262, 116541. [Google Scholar] [CrossRef]
- Hutter, F.; Kotthoff, L.; Vanschoren, J. (Eds.) Automated Machine Learning: Methods, Systems, Challenges; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Jozi, A.; Pinto, T.; Praça, I.; Vale, Z. Decision Support Application for Energy Consumption Forecasting. Appl. Sci. 2019, 9, 699. [Google Scholar] [CrossRef]
- Alshboul, O.; Shehadeh, A.; Mamlook, R.E.A.; Almasabha, G.; Almuflih, A.S.; Alghamdi, S.Y. Prediction Liquidated Damages via Ensemble Machine Learning Model: Towards Sustainable Highway Construction Projects. Sustainability 2022, 14, 9303. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Vafaeinia, M.; Safarzadeh, M.; Ghaemi, A.; Fathalian, F.; Maleki, A. Development of Predictive Models for Activated Carbon Synthesis from Different Biomass for CO2 Adsorption Using Artificial Neural Networks. Ind. Eng. Chem. Res. 2021, 60, 13950–13966. [Google Scholar] [CrossRef]
- DeCastro-García, N.; Muñoz Castañeda, Á.L.; Escudero García, D.; Carriegos, M.V. Effect of the Sampling of a Dataset in the Hyperparameter Optimization Phase over the Efficiency of a Machine Learning Algorithm. Complexity 2019, 2019, e6278908. [Google Scholar] [CrossRef]
- Peng, H.; Zeng, Z.; Deng, C.; Wu, Z. Multi-Strategy Serial Cuckoo Search Algorithm for Global Optimization. Knowl. Based Syst. 2021, 214, 106729. [Google Scholar] [CrossRef]
- Wang, F.; Mao, J.; Liu, Z. Combined Whale Optimization Algorithm and Genetic Algorithm to Optimize GRNN for Predicting Stayed Cable Icing Thickness. J. Civ. Environ. Eng. 2022, 44, 10–19. [Google Scholar] [CrossRef]
- May, S.; Hartmann, S.; Klawonn, F. Combined Pruning for Nested Cross-Validation to Accelerate Automated Hyperparameter Optimization for Embedded Feature Selection in High-Dimensional Data with Very Small Sample Sizes. arXiv 2022, arXiv:2202.00598. [Google Scholar]
- Parvandeh, S.; Yeh, H.-W.; Paulus, M.P.; McKinney, B.A. Consensus Features Nested Cross-Validation. Bioinformatics 2020, 36, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine Learning Algorithm Validation with a Limited Sample Size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Parvandeh, S.; McKinney, B.A. EpistasisRank and EpistasisKatz: Interaction Network Centrality Methods That Integrate Prior Knowledge Networks. Bioinformatics 2019, 35, 2329–2331. [Google Scholar] [CrossRef]
- Elshawi, R.; Maher, M.; Sakr, S. Automated Machine Learning: State-of-The-Art and Open Challenges. arXiv 2019, arXiv:1906.02287. [Google Scholar]
- Yang, L.; Shami, A. On Hyperparameter Optimization of Machine Learning Algorithms: Theory and Practice. Neurocomputing 2020, 415, 295–316. [Google Scholar] [CrossRef]
- Sun, S.; Cao, Z.; Zhu, H.; Zhao, J. A Survey of Optimization Methods From a Machine Learning Perspective. IEEE Trans. Cybern. 2020, 50, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Saihjpal, V.; Singh, N.; Singh, S.B. An Overview of Variants and Advancements of PSO Algorithm. Appl. Sci. 2022, 12, 8392. [Google Scholar] [CrossRef]
- Lin, J.; Zhuang, Y.; Zhao, Y.; Li, H.; He, X.; Lu, S. Measuring the Non-Linear Relationship between Three-Dimensional Built Environment and Urban Vitality Based on a Random Forest Model. Int. J. Environ. Res. Public Health 2023, 20, 734. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, L.; Wang, X.; Zheng, H.; Gu, M.; Li, S.; Fang, X. Risk Assessment of Deep Coal and Gas Outbursts Based on IQPSO-SVM. Int. J. Environ. Res. Public Health 2022, 19, 12869. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.Q.; Zhang, L.; Du, Z.M.; Chen, J.; Zhang, J.Y. Prediction of Sulfur Solubility in Supercritical Sour Gases Using Grey Wolf Optimizer-Based Support Vector Machine. J. Mol. Liq. 2018, 261, S0167732217351760. [Google Scholar] [CrossRef]
- Gu, M.-X.; Li, Q.; Zhou, S.-Y.; Chen, W.-D.; Guo, T.-M. Experimental and Modeling Studies on the Phase Behavior of High H2S-Content Natural Gas Mixturesag]. Fluid Phase Equilibria 1993, 82, 173–182. [Google Scholar] [CrossRef]
- Hu, J.-H.; Zhao, J.-Z.; Wang, L.; Meng, L.-Y.; Li, Y.-M. Prediction Model of Elemental Sulfur Solubility in Sour Gas Mixtures. J. Nat. Gas Sci. Eng. 2014, 18, 31–38. [Google Scholar] [CrossRef]
- Roof, J.G. Solubility of Sulfur in Hydrogen Sulfide and in Carbon Disulfide at Elevated Temperature and Pressure. Soc. Pet. Eng. J. 1971, 11, 272–276. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chen, G.-J. Experimental and Modeling Studies on Sulfur Solubility in Sour Gas. Fluid Phase Equilibria 2003, 214, 187–195. [Google Scholar] [CrossRef]
- Fayed, H.A.; Atiya, A.F. Speed up Grid-Search for Parameter Selection of Support Vector Machines. Appl. Soft Comput. 2019, 80, 202–210. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Q. A New Prediction Model of Elemental Sulfur Solubility in Sour Gas Mixtures. J. Nat. Gas Sci. Eng. 2016, 31, 98–107. [Google Scholar] [CrossRef]
- Roberts, B.E. The Effect of Sulfur Deposition on Gaswell Inflow Performance. SPE Reserv. Eng. 1997, 12, 118–123. [Google Scholar] [CrossRef]
- Eslamimanesh, A.; Gharagheizi, F.; Mohammadi, A.H.; Richon, D. Assessment Test of Sulfur Content of Gases. Fuel Process. Technol. 2013, 110, 133–140. [Google Scholar] [CrossRef]
- El-Melegy, M.T. Model-Wise and Point-Wise Random Sample Consensus for Robust Regression and Outlier Detection. Neural Netw. 2014, 59, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, M.M.-C.; Kloft, M.; Müller, K.-R.; Görnitz, N. ML2Motif—Reliable Extraction of Discriminative Sequence Motifs from Learning Machines. PLoS ONE 2017, 12, e0174392. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Q.; Zhang, X.; Shu, C.; Wang, S.; Wu, K. A Supervised Learning Approach for Accurate Modeling of CO2-Brine Interfacial Tension with Application in Identifying the Optimum Sequestration Depth in Saline Aquifers. Energy Fuels 2020, 34, 7353–7362. [Google Scholar] [CrossRef]
- Amooie, M.A.; Hemmati-Sarapardeh, A.; Karan, K.; Husein, M.M.; Soltanian, M.R.; Dabir, B. Data-Driven Modeling of Interfacial Tension in Impure CO2-Brine Systems with Implications for Geological Carbon Storage. Int. J. Greenh. Gas Control 2019, 90, 102811. [Google Scholar] [CrossRef]



| Authors | Models | Category | Number of Data | Scope of Data | Input Dimension | Results | Possible Disadvantages |
|---|---|---|---|---|---|---|---|
| Chen L (2014) [15] | GA-LM-BP | ANN | 74 | 303.20–363.20 K 11.82–40 Mpa | 5 | AARD = 5.54% | Inefficient and irregular coding of GA leads to inaccurate results |
| Chen HS (2019) [14] | CFA-SVR | SVM | 110 | 316.26–433.15 K 6.89–60 Mpa | 5 | AARD = 4.24% RMSE = 0.0401 | The late convergence speed of CFA is slow and easily falls into the local optimum |
| Bian XQ (2020) [13] | GWO-LSSVM | SVM | 239 | 303.20–433.15 K 10–60 Mpa | 5 | AARD = 3.50% RMSE = 1.0832 | GWO easily falls into the local optimum, and the convergence accuracy is not high |
| Fu L (2020) [16] | T-S FNN | ANN | 167 | 303.15–433.15 K 10–66.52 Mpa | 5 | AARD = 5.35% RMSE = 0.0600 | T S-FNN is slower to learn, prone to local minima, and may not even function properly |
| Amar MN (2020) [9] | CFNN | ANN | 239 | 303.20–433.15 K 7.03–60 Mpa | 5 | RMSE = 0.0488 | The learning speed of CFNN is slow, and the ability to obtain a global optimal solution is weak |
| Disadvantages of k-fold cross-validation (k-fold CV) | Disadvantages of nested cross-validation (nCV) |
| a. Overly optimistic results of the assessment b. Data characteristics cannot be fully learned c. Knowledge leakage | a. Excessive calculation b. Complicates the model c. Selects irrelevant features |
| Symbol | Unit | Min | Max | |
|---|---|---|---|---|
| Temperature | T | K | 303.2 | 433.15 |
| Pressure | P | Mpa | 7 | 66.52 |
| H2S content | XH2S | % | 2.93 | 100 |
| Parameter | Value |
|---|---|
| Input data form | [−1, +1] |
| Input variables | 5 |
| Max iterations | 200 |
| Population | 30 |
| Encoding length | 7 |
| Crossover probability Pc | 0.7 |
| Mutation probability Pm | 0.3 |
| Kernel function | Gaussian radial basis (RBF) |
| Penalty parameter | 2.1089 |
| Kernel function parameter | 12.5165 |
| Models | AARD (%) | SD | RMSE | R2 |
|---|---|---|---|---|
| Roberts model (empirical model) | 65.36 | 0.86 | 0.67 | 0.6792 |
| Guo-Wang model (empirical model) | 12.84 | 0.15 | 0.17 | 0.9833 |
| Hu model (empirical model) | 17.32 | 0.22 | 0.21 | 0.9731 |
| Fu L model (T-S FNN) | 5.35 | 0.08 | 0.06 | 0.9983 |
| Bian XQ model (GWO-LSSVM) | 3.50 | 0.08 | 0.024 | 0.9976 |
| Chen HS model (CFA-SVR) | 4.24 | 0.07 | 0.04 | 0.9978 |
| WOA-GA-WLSSVM | 3.33 | 0.068 | 0.027 | 0.9988 |
| WOA-GA-RF | 2.69 | 0.051 | 0.019 | 0.9991 |
| Number | WOA-GA-WLSSVM | WOA-GA-RF |
|---|---|---|
| 1 | 0.9011 | 0.9302 |
| 2 | 0.9651 | 0.9291 |
| 3 | 0.9016 | 0.9301 |
| 4 | 0.9857 | 0.9681 |
| 5 | 0.9801 | 0.9697 |
| Mean Correctness | 0.9467 | 0.9454 |
| Standard Deviation | 0.0377 | 0.0192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Luo, Z.; Luo, J.; Gao, Y.; Kong, Y.; Wang, Q. Investigation of the Solubility of Elemental Sulfur (S) in Sulfur-Containing Natural Gas with Machine Learning Methods. Int. J. Environ. Res. Public Health 2023, 20, 5059. https://doi.org/10.3390/ijerph20065059
Wang Y, Luo Z, Luo J, Gao Y, Kong Y, Wang Q. Investigation of the Solubility of Elemental Sulfur (S) in Sulfur-Containing Natural Gas with Machine Learning Methods. International Journal of Environmental Research and Public Health. 2023; 20(6):5059. https://doi.org/10.3390/ijerph20065059
Chicago/Turabian StyleWang, Yuchen, Zhengshan Luo, Jihao Luo, Yiqiong Gao, Yulei Kong, and Qingqing Wang. 2023. "Investigation of the Solubility of Elemental Sulfur (S) in Sulfur-Containing Natural Gas with Machine Learning Methods" International Journal of Environmental Research and Public Health 20, no. 6: 5059. https://doi.org/10.3390/ijerph20065059
APA StyleWang, Y., Luo, Z., Luo, J., Gao, Y., Kong, Y., & Wang, Q. (2023). Investigation of the Solubility of Elemental Sulfur (S) in Sulfur-Containing Natural Gas with Machine Learning Methods. International Journal of Environmental Research and Public Health, 20(6), 5059. https://doi.org/10.3390/ijerph20065059








