Association between Metabolic Syndrome Status and Daily Physical Activity Measured by a Wearable Device in Japanese Office Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Wearable Device
2.2.2. Questionnaires
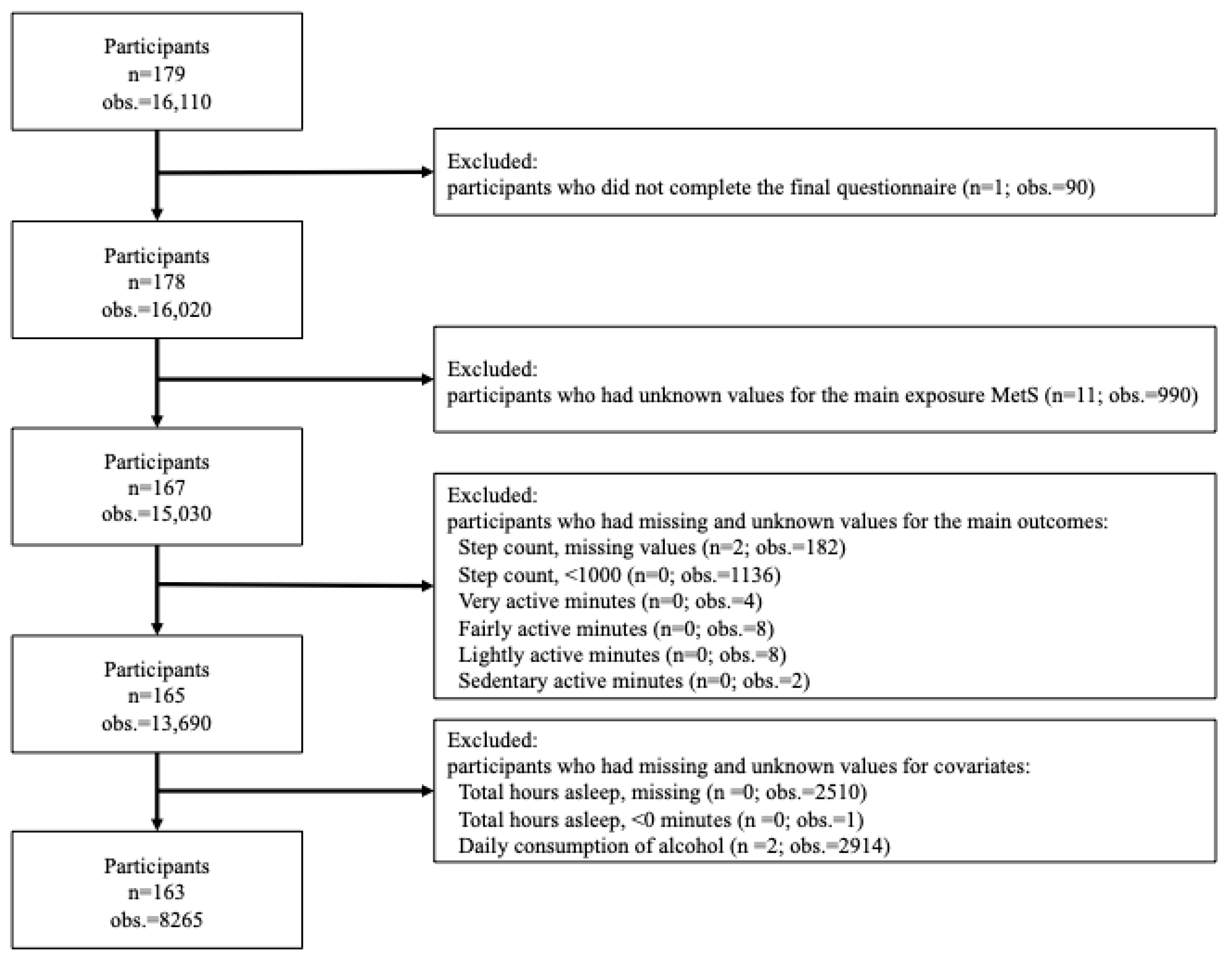
2.3. Study Population
2.4. Metabolic Syndrome Status (Main Exposure)
2.5. Physical Activity (Main Outcome)
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Main Analyses
3.2. Sensitivity Analysis
4. Discussion
4.1. Main Analysis
4.2. Sensitivity Analysis
4.3. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Yu, W.W.; Randhawa, A.K.; Blair, S.N.; Sui, X.; Kuk, J.L. Age- and sex- specific all-cause mortality risk greatest in metabolic syndrome combinations with elevated blood pressure from 7 U.S. cohorts. PLoS ONE 2019, 14, e0218307. [Google Scholar] [CrossRef]
- Oliveira, R.G.; Guedes, D.P. Physical Activity, Sedentary Behavior, Cardiorespiratory Fitness and Metabolic Syndrome in Adolescents: Systematic Review and Meta-Analysis of Observational Evidence. PLoS ONE 2016, 11, e0168503. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef]
- Gaesser, G.A. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr. Diabetes Rep. 2007, 7, 14–19. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. Exercise and Physical Activity Reference for Health Promotion (EPAR) 2013; National Institute of Health and Nutrition, Ed.; Ministry of Health Labour and Welfare: Tokyo, Japan, 2013. [Google Scholar]
- Ministry of Health Labour and Welfare. The National Health and Nutrition Survey (NHNS) Japan, 2019. (In Japanese). Available online: https://www.mhlw.go.jp/content/10900000/000687163.pdf (accessed on 27 February 2022).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart. J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Brickwood, K.J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.A.; Markwald, R.R. Performance of Seven Consumer Sleep-Tracking Devices Compared with Polysomnography. Sleep 2020, 44, zsaa291. [Google Scholar] [CrossRef]
- Nelson, B.W.; Allen, N.B. Accuracy of Consumer Wearable Heart Rate Measurement during an Ecologically Valid 24-Hour Period: Intraindividual Validation Study. JMIR Mhealth Uhealth 2019, 7, e10828. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e18694. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Buman, M.P.; Haskell, W.L.; McConnell, M.V.; Carstensen, L.L. Twenty-four Hours of Sleep, Sedentary Behavior, and Physical Activity with Nine Wearable Devices. Med. Sci. Sports Exerc. 2016, 48, 457–465. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Metabolic syndrome--definition and diagnostic criteria in Japan. J. Atheroscler. Thromb. 2005, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Case, M.A.; Burwick, H.A.; Volpp, K.G.; Patel, M.S. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015, 313, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, Y.; Welk, G.J. Validity of consumer-based physical activity monitors. Med. Sci. Sports Exerc. 2014, 46, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. The National Health and Nutrition Survey. (In Japanese). Available online: https://www.mhlw.go.jp/content/000681200.pdf (accessed on 12 March 2022).
- Carpenter, C.; Yang, C.H.; West, D. A Comparison of Sedentary Behavior as Measured by the Fitbit and ActivPAL in College Students. Int. J. Environ. Res. Public Health 2021, 18, 3914. [Google Scholar] [CrossRef] [PubMed]
- Semanik, P.; Lee, J.; Pellegrini, C.A.; Song, J.; Dunlop, D.D.; Chang, R.W. Comparison of Physical Activity Measures Derived From the Fitbit Flex and the ActiGraph GT3X+ in an Employee Population with Chronic Knee Symptoms. ACR Open Rheumatol. 2020, 2, 48–52. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare. Health Japan 21 (Kenko Nippon 21) Alcohol Section. (In Japanese). Available online: https://www.mhlw.go.jp/www1/topics/kenko21_11/pdf/b5.pdf (accessed on 19 April 2022).
- Noh, J.W.; Chang, Y.; Park, M.; Kwon, Y.D.; Ryu, S. Self-rated health and the risk of incident type 2 diabetes mellitus: A cohort study. Sci. Rep. 2019, 9, 3697. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare. The Standard of National Health Screening and Intervention Program. (In Japanese). Available online: https://www.mhlw.go.jp/content/10900000/000496784.pdf (accessed on 8 May 2022).
- Owei, I.; Umekwe, N.; Ceesay, F.; Dagogo-Jack, S. Awareness of Prediabetes Status and Subsequent Health Behavior, Body Weight, and Blood Glucose Levels. J. Am. Board Fam. Med. 2019, 32, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Garriga, A.; Sempere-Rubio, N.; Molina-Prados, M.J.; Faubel, R. Impact of Seasonality on Physical Activity: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 19, 2. [Google Scholar] [CrossRef]
- Kim, J.; Tanabe, K.; Yokoyama, N.; Zempo, H.; Kuno, S. Association between physical activity and metabolic syndrome in middle-aged Japanese: A cross-sectional study. BMC Public Health 2011, 11, 624. [Google Scholar] [CrossRef]
- Ko, K.J.; Kim, E.H.; Baek, U.H.; Gang, Z.; Kang, S.J. The relationship between physical activity levels and metabolic syndrome in male white-collar workers. J. Phys. Ther. Sci. 2016, 28, 3041–3046. [Google Scholar] [CrossRef]
- Xu, F.; Cohen, S.A.; Lofgren, I.E.; Greene, G.W.; Delmonico, M.J.; Greaney, M.L. The Association between Physical Activity and Metabolic Syndrome in Older Adults with Obesity. J. Frailty Aging 2019, 8, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, N.; Rockette-Wagner, B.; Azuma, K.; Ueshima, H.; Hisamatsu, T.; Takamiya, T.; El-Saed, A.; Miura, K.; Kriska, A.; Sekikawa, A. Physical activity levels in American and Japanese men from the ERA-JUMP Study and associations with metabolic syndrome. J. Sport Health Sci. 2020, 9, 170–178. [Google Scholar] [CrossRef]
- Redenius, N.; Kim, Y.; Byun, W. Concurrent validity of the Fitbit for assessing sedentary behavior and moderate-to-vigorous physical activity. BMC Med. Res. Methodol. 2019, 19, 29. [Google Scholar] [CrossRef]
- Degroote, L.; De Bourdeaudhuij, I.; Verloigne, M.; Poppe, L.; Crombez, G. The Accuracy of Smart Devices for Measuring Physical Activity in Daily Life: Validation Study. JMIR Mhealth Uhealth 2018, 6, e10972. [Google Scholar] [CrossRef]
- So, R.; Matsuo, T. The Effect of Domain-Specific Sitting Time and Exercise Habits on Metabolic Syndrome in Japanese Workers: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 3883. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Alfaro, L.; Bibiloni, M.D.M.; Mateos, D.; Ugarriza, L.; Tur, J.A. Leisure-Time Physical Activity and Metabolic Syndrome in Older Adults. Int. J. Environ. Res. Public Health 2019, 16, 3358. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Li, R.H.; Huang, S.L.; Sia, H.K.; Lee, S.S.; Wang, W.H.; Tang, F.C. Relationships between different types of physical activity and metabolic syndrome among Taiwanese workers. Sci. Rep. 2017, 7, 13735. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | no-MetS n = 52 | pre-MetS n = 60 | MetS n = 51 | p Value a |
|---|---|---|---|---|
| Men, n (%) | 43 (82.7) | 60 (100.0) | 48 (94.1) | 0.002 |
| Age at screening [years (median ± iqr)] | 43.0 ± 10.0 | 43.5 ± 13.5 | 47.0 ±10.0 | 0.012 |
| Weekday obs (%) | 1670 (69.9) | 2205 (68.8) | 1825 (68.4) | 0.52 |
| Lifestyle factors | ||||
| Smoking status, n (%) | 0.47 | |||
| Non-smoker | 28 (53.9) | 24 (40.0) | 22 (43.1) | |
| Past smoker | 11 (21.2) | 21 (35.0) | 17 (33.3) | |
| Current smoker (<20 cigarettes/day) | 5 (9.6) | 10 (16.7) | 6 (11.8) | |
| Current smoker (≥20 cigarettes/day) | 8 (15.4) | 5 (8.3) | 6 (11.8) | |
| Living arrangement, n (%) Living with someone | 47 (90.4) | 54 (90.0) | 46 (90.2) | 1.00 |
| Total hours of overtime/month [hours (median ± iqr)] | 30 ± 27.5 | 42.5 ± 30.0 | 40 ± 32.0 | 0.24 |
| Income, n (%) | 0.42 | |||
| <10 million JPY | 37 (71.2) | 39 (65.0) | 30 (58.8) | |
| ≥10 million JPY | 15 (28.9) | 21 (35.0) | 21 (41.2) | |
| Diet (eating habit), n (%) Balanced food intake | 36 (69.2) | 40 (66.7) | 36 (70.6) | 0.90 |
| Health awareness | ||||
| Health awareness score (7-point scale) [score (median ± iqr)] | 2.5 ± 3.0 | 3.0 ± 2.0 | 3.0 ± 3.0 | 0.65 |
| Health condition (5-point scale) [score (median ± iqr)] | 3.0 ± 1.0 | 2.0 ± 1.0 | 2.0 ± 2.0 | 0.34 |
| Daily components | ||||
| Number of steps/day [median ± iqr] | 11,023 ± 5058 | 10,276 ± 5024 | 11,195 ± 5262 | <0.001 |
| Total active minutes/day [median ± iqr] | 54 ± 53.6 | 48 ± 50.4 | 56 ± 60.2 | <0.001 |
| Total sleep time, hours/day [median ± iqr] | 5.7 ± 1.4 | 5.6 ± 1.3 | 5.5 ± 1.3 | 0.001 |
| Alcohol consumption, obs (%) | <0.001 | |||
| <20 ethanol g/day | 1323 (55.3) | 1934 (60.3) | 1640 (61.5) | |
| ≥20 ethanol g/day | 1068 (44.7) | 1272 (39.7) | 1028 (38.5) |
| Step Count | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|
| >9000/day in men and >8500/day in women | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| no-MetS | Reference | Reference | Reference | |
| pre-MetS | 0.63 (0.38, 1.04) | 0.60 (0.36, 1.01) | 0.60 * (0.36, 0.99) | |
| MetS | 0.81 (0.47, 1.37) | 0.86 (0.50, 1.49) | 0.81 (0.47, 1.40) | |
| Active minutes | Model 1 a | Model 2 b | Model 3 c | |
| >3 METs h/day | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| no-MetS | Reference | Reference | Reference | |
| pre-MetS | 0.63 (0.39, 1.01) | 0.61 * (0.39, 0.97) | 0.62 * (0.40, 0.96) | |
| MetS | 0.94 (0.57, 1.54) | 1.02 (0.63, 1.64) | 0.94 (0.59, 1.49) |
| Step Count | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|
| >9000/day in men and >8500/day in women | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Weekday | no-MetS | Reference | Reference | Reference |
| pre-MetS | 0.61 (0.35, 1.06) | 0.57 * (0.33, 0.96) | 0.57 * (0.34, 0.95) | |
| MetS | 1.06 (0.59, 1.90) | 1.09 (0.62, 1.92) | 1.03 (0.59, 1.79) | |
| weekend/holiday | no-MetS | 0.32 ** (0.26, 0.40) | 0.35 ** (0.27, 0.44) | 0.35 ** (0.27, 0.44) |
| pre-MetS | 0.22 ** (0.12, 0.39) | 0.23 ** (0.13, 0.39) | 0.23 ** (0.13, 0.38) | |
| MetS | 0.18 ** (0.10, 0.33) | 0.22 ** (0.12, 0.39) | 0.21 ** (0.12, 0.36) | |
| Active minutes | Model 1 a | Model 2 b | Model 3 c | |
| >3 METs h/day | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| weekday | no-MetS | Reference | Reference | Reference |
| weekend/holiday | pre-MetS | 0.62 (0.38, 1.00) | 0.60 * (0.38, 0.96) | 0.61 * (0.39, 0.95) |
| MetS | 1.07 (0.64, 1.77) | 1.19 (0.73, 1.94) | 1.09 (0.68, 1.75) | |
| no-MetS | 1.03 (0.85, 1.26) | 1.12 (0.90, 1.38) | 1.12 (0.91, 1.38) | |
| pre-MetS | 0.66 (0.40, 1.09) | 0.70 (0.43, 1.12) | 0.71 (0.45, 1.12) | |
| MetS | 0.72 (0.43, 1.21) | 0.84 (0.51, 1.38) | 0.76 (0.47, 1.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaga, Y.; Svensson, T.; Chung, U.-i.; Svensson, A.K. Association between Metabolic Syndrome Status and Daily Physical Activity Measured by a Wearable Device in Japanese Office Workers. Int. J. Environ. Res. Public Health 2023, 20, 4315. https://doi.org/10.3390/ijerph20054315
Yamaga Y, Svensson T, Chung U-i, Svensson AK. Association between Metabolic Syndrome Status and Daily Physical Activity Measured by a Wearable Device in Japanese Office Workers. International Journal of Environmental Research and Public Health. 2023; 20(5):4315. https://doi.org/10.3390/ijerph20054315
Chicago/Turabian StyleYamaga, Yukako, Thomas Svensson, Ung-il Chung, and Akiko Kishi Svensson. 2023. "Association between Metabolic Syndrome Status and Daily Physical Activity Measured by a Wearable Device in Japanese Office Workers" International Journal of Environmental Research and Public Health 20, no. 5: 4315. https://doi.org/10.3390/ijerph20054315
APA StyleYamaga, Y., Svensson, T., Chung, U.-i., & Svensson, A. K. (2023). Association between Metabolic Syndrome Status and Daily Physical Activity Measured by a Wearable Device in Japanese Office Workers. International Journal of Environmental Research and Public Health, 20(5), 4315. https://doi.org/10.3390/ijerph20054315







