Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach
Abstract
1. Introduction
1.1. Substance-Use Disorders
1.2. The Endocannabinoid System
2. Materials and Methods
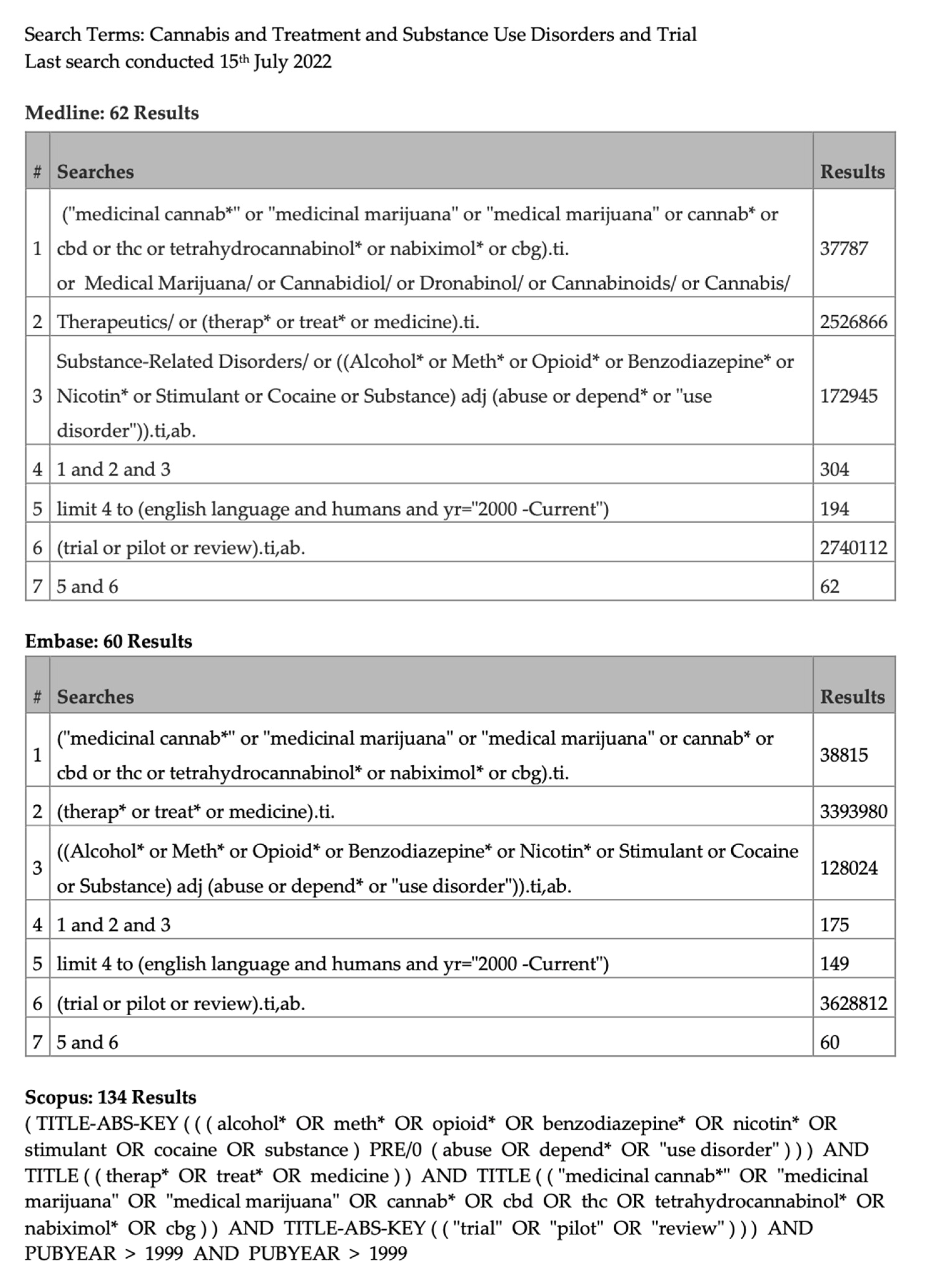
2.1. Search Strategy
2.2. Study Inclusion
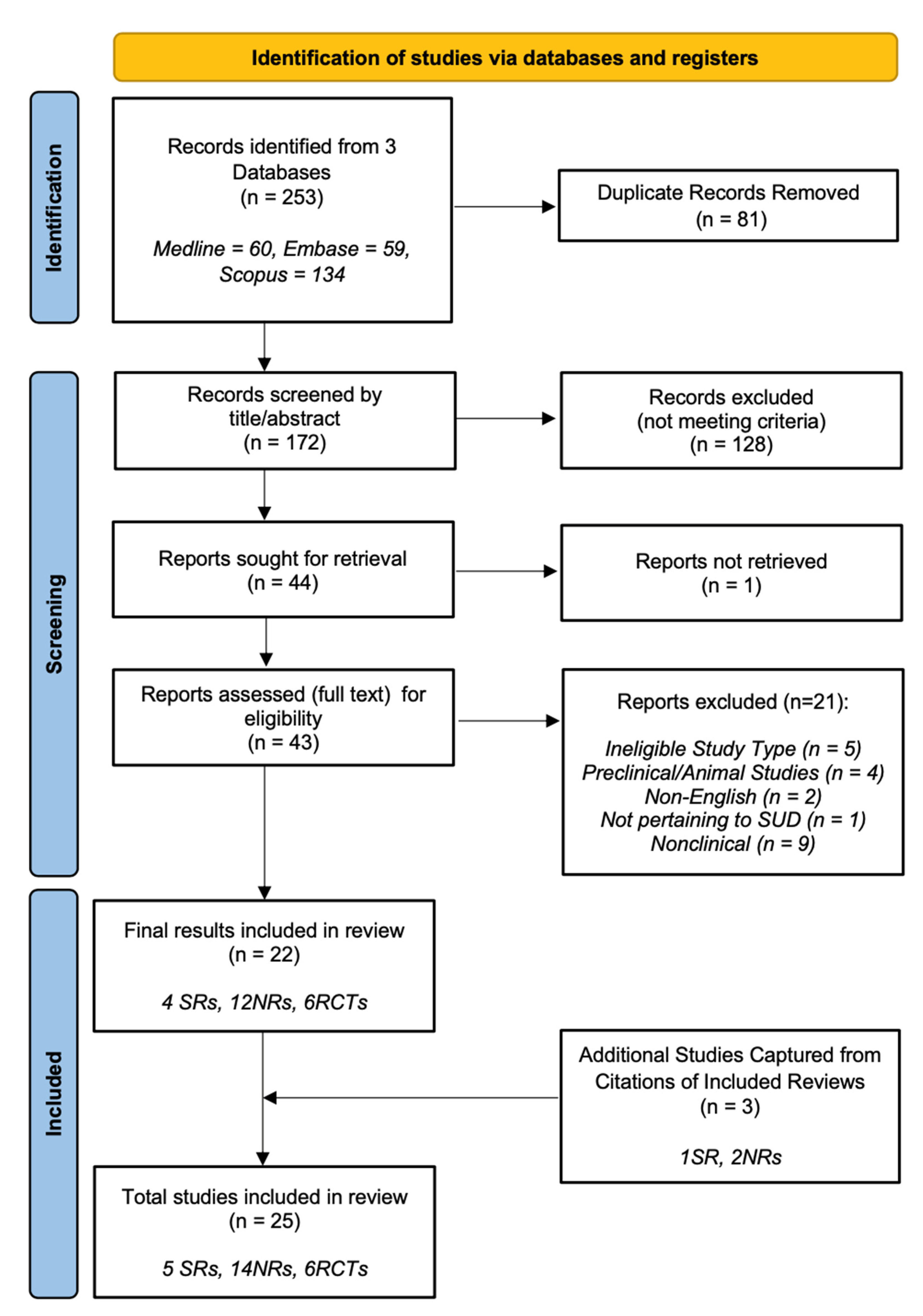
3. Results
3.1. Participants
3.2. Interventions
3.3. Cannabis-Use Disorder
3.3.1. Tetrahydrocannabinol (THC)
3.3.2. Dronabinol
3.3.3. Cannabidiol
3.3.4. Nabiximols
3.3.5. Fatty-Acid Amide Hydrolase Inhibitor—PF-04457845
3.4. Opioid-Use Disorder
3.4.1. Dronabinol
3.4.2. Cannabidiol (CBD)
3.5. Cocaine-Use Disorder
Cannabidiol
3.6. Nicotine-Use Disorder
3.6.1. Rimonabant
3.6.2. Taranabant
3.6.3. Surinabant
3.6.4. Cannabidiol
3.7. Alcohol-Use Disorder
4. Discussion
4.1. Cannabis-Use Disorder
4.1.1. THC and Dronabinol
4.1.2. Cannabidiol (CBD)
4.1.3. Nabiximols
4.1.4. Fatty-Acid Amide Hydrolase Inhibitor—PF-04457845
4.2. Opioid-Use Disorder
4.2.1. Dronabinol
4.2.2. Cannabidiol
4.3. Cocaine-Use Disorder
Cannabidiol
4.4. Nicotine-Use Disorder
4.4.1. Rimonabant
4.4.2. Taranabant
4.4.3. Surinabant
4.4.4. Cannabidiol
4.5. Alcohol-Use Disorder
Rimonabant
4.6. Limitations
4.7. Future Research Directions
4.8. Implications for Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| NO. | Author | Reason for exclusion |
|---|---|---|
| 1 | Bhardwaj et. al., 2018 [84] | Ineligible study type—study protocol. |
| 2 | Calpe-López et. al., 2019 [31] | Nonclinical—pharmacological review of mechanisms. |
| 3 | Calpe-López et. al., 2019 [31] | Duplicate. |
| 4 | Cohen et. al., 2020 [85] | Not pertaining to SUD treatment, only PTSD. |
| 5 | De Ternay et. al., 2019 [86] | Animal studies—no human trials. |
| 6 | George 2007 [87] | Ineligible study type—book chapter. |
| 7 | Janero 2012 [88] | Ineligible study type—short survey. |
| 8 | Janero & Makriyannis, 2007 [89] | Nonclinical—pharmacological review of mechanisms. |
| 9 | Khurana et. al., 2017 [90] | Nonclinical—pharmacological review of mechanisms. |
| 10 | Lake et. al., 2021 [91] | Ineligible study type—cohort study. |
| 11 | Lee et. al., 2017 [39] | Nonclinical—pharmacological review of mechanisms. |
| 12 | Luján & Valverde, 2020 [92] | Nonclinical—pharmacological review of mechanisms. |
| 13 | Mackie 2006 [93] | Nonclinical—pharmacological review of mechanisms. |
| 14 | Onaivi 2009 [94] | Nonclinical—pharmacological review of mechanisms. |
| 15 | Pietrzak et. al., 2011 [95] | Non-English (Polish). |
| 16 | Preedy 2017 [96] | Ineligible study type—book chapter. |
| 17 | Rodrigues et. al., 2020 [97] | Animal studies—no human trials. |
| 18 | Sholler et. al., 2020 [41] | Nonclinical—pharmacological review of mechanisms. |
| 19 | Śmiarowska et. al., 2022 [98] | Nonclinical—pharmacological review of mechanisms. |
| 20 | Weidenauer et. al., 2021 [99] | Non-English (German). |
| 21 | Yang et. al., 2012 [100] | Animal studies—no human trials. |
References
- Australian Bureau of Statistics. National Study of Mental Health and Wellbeing, 2020-21. Available online: https://www.abs.gov.au/statistics/health/mental-health/national-study-mental-health-and-wellbeing/latest-release (accessed on 21 September 2022).
- Kalin, N.H. Substance Use Disorders and Addiction: Mechanisms, Trends, and Treatment Implications. Am. J. Psychiatry 2020, 177, 1015–1018. [Google Scholar] [CrossRef]
- Commission on Narcotic Drugs: 65th Session. Comorbidities in Drug Use Disorders: No Wrong Door; United Nations Office on Drugs and Crime: Vienna, Austria, 2022. [Google Scholar]
- Australian Institute of Health and Welfare. Mental Health: Prevalence and Impact. 2022. Available online: https://www.aihw.gov.au/reports/mental-health-services/mental-health (accessed on 25 September 2022).
- National Institute on Drug Abuse. Effective Treatments for Opioid Addiction. 2022. Available online: https://nida.nih.gov/publications/effective-treatments-opioid-addiction (accessed on 16 September 2022).
- National Institute on Drug Abuse. Tobacco Addiction. 2022. Available online: https://nida.nih.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapies/tobacco-addiction (accessed on 16 September 2022).
- National Institute on Drug Abuse. Alcohol Addiction. 2022. Available online: https://nida.nih.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapies/alcohol (accessed on 20 September 2022).
- Hodgkin, D.; Connery, H.S. Effectiveness and Availability of Treatment for Substance Use Disorders. Oxf. Res. Encycl. Econ. Financ. 2018. [Google Scholar] [CrossRef]
- Sloan, M.E.; Gowin, J.L.; Ramchandani, V.A.; Hurd, Y.L.; Le Foll, B. The Endocannabinoid System as a Target for Addiction Treatment: Trials and Tribulations. Neuropharmacology 2017, 124, 73–83. [Google Scholar] [CrossRef]
- Sviženska, I.; Dubový, P.; Sulcova, A. Cannabinoid Receptors 1 and 2 (CB1 and CB2), Their Distribution, Ligands and Functional Involvement in Nervous System Structures—A Short Review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol Is an Allosteric Modulator at Mu- and Delta-Opioid Receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Karschner, E.L.; Darwin, W.D.; Goodwin, R.S.; Wright, S.; Huestis, M.A. Plasma Cannabinoid Pharmacokinetics Following Controlled Oral Δ9-Tetrahydrocannabinol and Oromucosal Cannabis Extract Administration. Clin. Chem. 2011, 57, 66–75. [Google Scholar] [CrossRef]
- Schlicker, E.; Kathmann, M. Modulation of Transmitter Release via Presynaptic Cannabinoid Receptors. Trends Pharmacol. Sci. 2001, 22, 565–572. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M.; Telang, F. Dopamine in Drug Abuse and Addiction. Arch. Neurol. 2007, 64, 1575–1579. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The Prisma Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Hallinan, C.M.; Habibabadi, S.K.; Conway, M.; Bonomo, Y.A. Social Media Discourse and Internet Search Queries on Cannabis as a Medicine: A Systematic Scoping Review. PLoS ONE 2023, 18, e0269143. [Google Scholar] [CrossRef]
- Pawliuk, C.; Chau, B.; Rassekh, S.R.; McKellar, T.; Siden, H. Efficacy and Safety of Paediatric Medicinal Cannabis Use: A Scoping Review. Paediatr. Child Health 2020, 26, 228–233. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Wohlin, C.; Kalinowski, M.; Felizardo, K.R.; Mendes, E. Successful Combination of Database Search and Snowballing for Identification of Primary Studies in Systematic Literature Studies. Inf. Softw. Technol. 2022, 147, 106908. [Google Scholar] [CrossRef]
- Batalla, A.; Janssen, H.; Gangadin, S.S.; Bossong, M.G. The Potential of Cannabidiol as a Treatment for Psychosis and Addiction: Who Benefits Most? A Systematic Review. J. Clin. Med. 2019, 8, 1058. [Google Scholar] [CrossRef]
- Hoch, E.; Niemann, D.; von Keller, R.; Schneider, M.; Friemel, C.M.; Preuss, U.W.; Hasan, A.; Pogarell, O. Correction to: How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 995. [Google Scholar] [CrossRef]
- Paulus, V.; Billieux, J.; Benyamina, A.; Karila, L. Cannabidiol in the context of substance use disorder treatment: A systematic review. Addict. Behav. 2022, 132, 107360. [Google Scholar] [CrossRef]
- Pavel, A.; Paun, R.; Valentin, M.P. 316 The use of cannabidiol in treating psychiatric disorder: A systematic review. Eur. Neuropsychopharmacol. 2021, 44, S50. [Google Scholar] [CrossRef]
- Prud’Homme, M.; Cata, R.; Jutras-Aswad, D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Subst. Abus. Res. Treat. 2015, 9, 33–38. [Google Scholar] [CrossRef]
- Babalonis, S.; Walsh, S.L. Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence. Eur. Neuropsychopharmacol. 2020, 36, 206–216. [Google Scholar] [CrossRef]
- Beardsley, P.M.; Thomas, B.F.; McMahon, L.R. Cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Int. Rev. Psychiatry 2009, 21, 134–142. [Google Scholar] [CrossRef]
- Calpe-López, C.; García-Pardo, M.P.; Aguilar, M.A. Cannabidiol Treatment Might Promote Resilience to Cocaine and Methamphetamine Use Disorders: A Review of Possible Mechanisms. Molecules 2019, 24, 2583. [Google Scholar] [CrossRef]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The Endocannabinoid System and Cannabidiol’s Promise for the Treatment of Substance Use Disorder. Front. Psychiatry 2019, 10, 63. [Google Scholar] [CrossRef]
- Femenía, T.; Portillo, P.; Pérez-Ortiz, J.M.; Aracil-Fernández, A.; Rubio, G.; Manzanares, J. Opioid and Cannabinoid Systems as Therapeutic Targets for the Treatment of Alcohol Dependence: From Animal Models to Clinical Practice. Open Neuropsychopharmacol. J. 2009, 2, 53–63. [Google Scholar] [CrossRef]
- Fischer, B.; Kuganesan, S.; Gallassi, A.; Malcher-Lopes, R.; Brink, W.V.D.; Wood, E. Addressing the stimulant treatment gap: A call to investigate the therapeutic benefits potential of cannabinoids for crack-cocaine use. Int. J. Drug Policy 2015, 26, 1177–1182. [Google Scholar] [CrossRef]
- Le Foll, B.; Forget, B.; Aubin, H.-J.; Goldberg, S.R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: Iinsights from pre-clinical and clinical studies. Addict. Biol. 2008, 13, 239–252. [Google Scholar] [CrossRef]
- Galaj, E.; Xi, Z.-X. Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs 2019, 33, 1001–1030. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Smaga, I.; Filip, M.; Bujalska-Zadrozny, M. Cannabinoid Ligands and Alcohol Addiction: A Promising Therapeutic Tool or a Humbug? Neurotox. Res. 2015, 29, 173–196. [Google Scholar] [CrossRef]
- Kolongowski, B.; Tjiattas-Saleski, L. Cannabidiol: Background and Literature Review of Potential Treatments. Osteopat. Fam. Physician 2021, 16–23. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Bertoglio, L.J.; Guimarães, F.S.; Stevenson, C.W. Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Role of Cannabidiol in the Therapeutic Intervention for Substance Use Disorders. Front. Pharmacol. 2021, 12, 626010. [Google Scholar] [CrossRef]
- Sholler, D.J.; Huestis, M.A.; Amendolara, B.; Vandrey, R.; Cooper, Z.D. Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids. Pharmacol. Biochem. Behav. 2020, 199, 173059. [Google Scholar] [CrossRef]
- Lintzeris, N.; Bhardwaj, A.; Mills, L.; Dunlop, A.; Copeland, J.; Mcgregor, I.; Bruno, R.; Gugusheff, J.; Phung, N.; Montebello, M.; et al. Nabiximols for the Treatment of Cannabis Dependence. JAMA Intern. Med. 2019, 179, 1242–1253. [Google Scholar] [CrossRef]
- Lintzeris, N.; Mills, L.; Dunlop, A.; Copeland, J.; Mcgregor, I.; Bruno, R.; Kirby, A.; Montebello, M.; Hall, M.; Jefferies, M.; et al. Cannabis Use in Patients 3 Months after Ceasing Nabiximols for the Treatment of Cannabis Dependence: Results from a Placebo-Controlled Randomised Trial. Drug Alcohol Depend. 2020, 215, 108220. [Google Scholar] [CrossRef]
- Bisaga, A.; Sullivan, M.A.; Glass, A.; Mishlen, K.; Pavlicova, M.; Haney, M.; Raby, W.N.; Levin, F.R.; Carpenter, K.M.; Mariani, J.J.; et al. The Effects of Dronabinol during Detoxification and the Initiation of Treatment with Extended Release Naltrexone. Drug Alcohol Depend. 2015, 154, 38–45. [Google Scholar] [CrossRef]
- Soyka, M.; Koller, G.; Schmidt, P.; Lesch, O.-M.; Leweke, M.; Fehr, C.; Gann, H.; Mann, K.F. Cannabinoid Receptor 1 Blocker Rimonabant (SR 141716) for Treatment of Alcohol Dependence. J. Clin. Psychopharmacol. 2008, 28, 317–324. [Google Scholar] [CrossRef]
- de Meneses-Gaya, C.; Crippa, J.A.; Hallak, J.E.; Miguel, A.Q.; Laranjeira, R.; Bressan, R.A.; Zuardi, A.W.; Lacerda, A.L. Cannabidiol for the Treatment of Crack-Cocaine Craving: An Exploratory Double-Blind Study. Braz. J. Psychiatry 2021, 43, 467–476. [Google Scholar] [CrossRef]
- Mongeau-Pérusse, V.; Brissette, S.; Bruneau, J.; Conrod, P.; Dubreucq, S.; Gazil, G.; Stip, E.; Jutras-Aswad, D. Cannabidiol as a Treatment for Craving and Relapse in Individuals with Cocaine Use Disorder: A Randomized Placebo-Controlled Trial. Addiction 2021, 116, 2431–2442. [Google Scholar] [CrossRef]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an Agonist Replacement Therapy During Cannabis Withdrawal. JAMA Psychiatry 2014, 71, 281–291. [Google Scholar] [CrossRef]
- Trigo, J.M.; Lagzdins, D.; Rehm, J.; Selby, P.; Gamaleddin, I.; Fischer, B.; Barnes, A.J.; Huestis, M.A.; Le Foll, B. Effects of Fixed or Self-Titrated Dosages of Sativex on Cannabis Withdrawal and Cravings. Drug Alcohol Depend. 2016, 161, 298–306. [Google Scholar] [CrossRef]
- Trigo, J.M.; Soliman, A.; Quilty, L.C.; Fischer, B.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; George, T.P.; Streiner, D.L.; et al. Nabiximols Combined with Motivational Enhancement/Cognitive Behavioral Therapy for the Treatment of Cannabis Dependence: A Pilot Randomized Clinical Trial. PLoS ONE 2018, 13, e0190768. [Google Scholar] [CrossRef]
- Haney, M.; Hart, C.L.; Vosburg, S.K.; Nasser, J.; Bennett, A.; Zubaran, C.; Foltin, R.W. Marijuana Withdrawal in Humans: Effects of Oral THC or Divalproex. Neuropsychopharmacology 2003, 29, 158–170. [Google Scholar] [CrossRef]
- Budney, A.J.; Vandrey, R.G.; Hughes, J.R.; Moore, B.A.; Bahrenburg, B. Oral Delta-9-Tetrahydrocannabinol Suppresses Cannabis Withdrawal Symptoms. Drug Alcohol Depend. 2007, 86, 22–29. [Google Scholar] [CrossRef]
- Haney, M.; Hart, C.L.; Vosburg, S.K.; Comer, S.D.; Reed, S.C.; Foltin, R.W. Effects of THC and Lofexidine in a Human Laboratory Model of Marijuana Withdrawal and Relapse. Psychopharmacology 2007, 197, 157–168. [Google Scholar] [CrossRef]
- Levin, F.R.; Mariani, J.J.; Brooks, D.J.; Pavlicova, M.; Cheng, W.; Nunes, E. Dronabinol for the Treatment of Cannabis Dependence: A Randomized, Double-Blind, Placebo-Controlled Trial. Drug Alcohol Depend. 2011, 116, 142–150. [Google Scholar] [CrossRef]
- Vandrey, R.; Stitzer, M.L.; Mintzer, M.Z.; Huestis, M.A.; Murray, J.A.; Lee, D. The Dose Effects of Short-Term Dronabinol (Oral THC) Maintenance in Daily Cannabis Users. Drug Alcohol Depend. 2013, 128, 64–70. [Google Scholar] [CrossRef]
- Levin, F.R.; Mariani, J.J.; Pavlicova, M.; Brooks, D.; Glass, A.; Mahony, A.; Nunes, E.V.; Bisaga, A.; Dakwar, E.; Carpenter, K.M.; et al. Dronabinol and Lofexidine for Cannabis Use Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Drug Alcohol Depend. 2016, 159, 53–60. [Google Scholar] [CrossRef]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D.; et al. Cannabidiol for the Treatment of Cannabis Use Disorder: A Phase 2a, Double-Blind, Placebo-Controlled, Randomised, Adaptive Bayesian Trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Cortes-Briones, J.; Creatura, G.; Bluez, G.; Thurnauer, H.; Deaso, E.; Bielen, K.; Surti, T.; Radhakrishnan, R.; Gupta, A.; et al. Efficacy and Safety of a Fatty Acid Amide Hydrolase Inhibitor (PF-04457845) in the Treatment of Cannabis Withdrawal and Dependence in Men: A Double-Blind, Placebo-Controlled, Parallel Group, Phase 2a Single-Site Randomised Controlled Trial. Lancet Psychiatry 2019, 6, 35–45. [Google Scholar] [CrossRef]
- Jicha, C.J.; Lofwall, M.R.; Nuzzo, P.A.; Babalonis, S.; Elayi, S.C.; Walsh, S.L. Safety of Oral Dronabinol during Opioid Withdrawal in Humans. Drug Alcohol Depend. 2015, 157, 179–183. [Google Scholar] [CrossRef]
- Lofwall, M.R.; Babalonis, S.; Nuzzo, P.A.; Elayi, S.C.; Walsh, S.L. Opioid Withdrawal Suppression Efficacy of Oral Dronabinol in Opioid Dependent Humans. Drug Alcohol Depend. 2016, 164, 143–150. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef]
- George, D.T.; Herion, D.W.; Jones, C.L.; Phillips, M.J.; Hersh, J.; Hill, D.; Heilig, M.; Ramchandani, V.A.; Geyer, C.; Spero, D.E.; et al. Rimonabant (SR141716) Has No Effect on Alcohol Self-Administration or Endocrine Measures in Nontreatment-Seeking Heavy Alcohol Drinkers. Psychopharmacology 2009, 208, 37–44. [Google Scholar] [CrossRef]
- Rigotti, N.A.; Gonzales, D.; Dale, L.C.; Lawrence, D.; Chang, Y. A Randomized Controlled Trial of Adding the Nicotine Patch to Rimonabant for Smoking Cessation: Efficacy, Safety and Weight Gain. Addiction 2009, 104, 266–276. [Google Scholar] [CrossRef]
- Cahill, K.; Ussher, M.H. Cannabinoid Type 1 Receptor Antagonists for Smoking Cessation. Cochrane Database Syst. Rev. 2011, 2011, CD005353. [Google Scholar] [CrossRef]
- Robinson, J.D.; Cinciripini, P.M.; Karam-Hage, M.; Aubin, H.-J.; Dale, L.C.; Niaura, R.; Anthenelli, R.M. Pooled Analysis of Three Randomized, Double-Blind, Placebo Controlled Trials with Rimonabant for Smoking Cessation. Addict. Biol. 2017, 23, 291–303. [Google Scholar] [CrossRef]
- Tonstad, S.; Aubin, H.-J. Efficacy of a Dose Range of Surinabant, a Cannabinoid Receptor Blocker, for Smoking Cessation: A Randomized Controlled Clinical Trial. J. Psychopharmacol. 2012, 26, 1003–1009. [Google Scholar] [CrossRef]
- Morrison, M.F.; Ceesay, P.; Gantz, I.; Kaufman, K.D.; Lines, C.R. Randomized, controlled, double-blind trial of taranabant for smoking cessation. Psychopharmacology 2010, 209, 245–253. [Google Scholar] [CrossRef]
- Morgan, C.J.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol Reduces Cigarette Consumption in Tobacco Smokers: Preliminary Findings. Addict. Behav. 2013, 38, 2433–2436. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Crudgington, H.; Davies, A.C.; Stroud, J.B.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. The Effects of Cannabidiol on Impulsivity and Memory during Abstinence in Cigarette Dependent Smokers. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Stroud, J.B.; Crudgington, H.; Davies, A.C.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. Cannabidiol Reverses Attentional Bias to Cigarette Cues in a Human Experimental Model of Tobacco Withdrawal. Addiction 2018, 113, 1696–1705. [Google Scholar] [CrossRef]
- Chiang, K.P. Reduced Cellular Expression and Activity of the P129T Mutant of Human Fatty Acid Amide Hydrolase: Evidence for a Link between Defects in the Endocannabinoid System and Problem Drug Use. Hum. Mol. Genet. 2004, 13, 2113–2119. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Carlson, B.L.A.; Ramesh, D.; Abdullah, R.A.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Inhibitors of Endocannabinoid-Metabolizing Enzymes Reduce Precipitated Withdrawal Responses in THC-Dependent Mice. AAPS J. 2009, 11, 342–352. [Google Scholar] [CrossRef]
- Ren, Y.; Whittard, J.; Higuera-Matas, A.; Morris, C.V.; Hurd, Y.L. Cannabidiol, a Nonpsychotropic Component of Cannabis, Inhibits Cue-Induced Heroin Seeking and Normalizes Discrete Mesolimbic Neuronal Disturbances. J. Neurosci. 2009, 29, 14764–14769. [Google Scholar] [CrossRef]
- Peña, I.D.; Gevorkiana, R.; Shi, W.-X. Psychostimulants Affect Dopamine Transmission through Both Dopamine Transporter-Dependent and Independent Mechanisms. Eur. J. Pharmacol. 2015, 764, 562–570. [Google Scholar] [CrossRef]
- Conrad, K.; Ford, K.; Marinelli, M.; Wolf, M. Dopamine Receptor Expression and Distribution Dynamically Change in the Rat Nucleus Accumbens after Withdrawal from Cocaine Self-Administration. Neuroscience 2010, 169, 182–194. [Google Scholar] [CrossRef]
- Kramar, C.P.; I Chefer, V.; A Wise, R.; Medina, J.H.; Barbano, M.F. Dopamine in the Dorsal Hippocampus Impairs the Late Consolidation of Cocaine-Associated Memory. Neuropsychopharmacology 2014, 39, 1645–1653. [Google Scholar] [CrossRef]
- Gessa, G.; Melis, M.; Muntoni, A.; Diana, M. Cannabinoids Activate Mesolimbic Dopamine Neurons by an Action on Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 1998, 341, 39–44. [Google Scholar] [CrossRef]
- Fanarioti, E.; Mavrikaki, M.; Panagis, G.; Mitsacos, A.; Nomikos, G.G.; Giompres, P. Behavioral and Neurochemical Changes in Mesostriatal Dopaminergic Regions of the Rat after Chronic Administration of the Cannabinoid Receptor Agonist WIN55,212-2. Int. J. Neuropsychopharmacol. 2015, 18, pyu097. [Google Scholar] [CrossRef]
- Jones, D. End of the Line for Cannabinoid Receptor 1 as an Anti-Obesity Target? Nat. Rev. Drug Discov. 2008, 7, 961–962. [Google Scholar] [CrossRef]
- Gardner, E. Endocannabinoid Signaling System and Brain Reward: Emphasis on Dopamine. Pharmacol. Biochem. Behav. 2005, 81, 263–284. [Google Scholar] [CrossRef]
- Lallemand, F.; De Witte, P. Ethanol Induces Higher BEC in CB1 Cannabinoid Receptor Knockout Mice While Decreasing Ethanol Preference. Alcohol Alcohol. 2004, 40, 54–62. [Google Scholar] [CrossRef]
- Economidou, D.; Mattioli, L.; Cifani, C.; Perfumi, M.; Massi, M.; Cuomo, V.; Trabace, L.; Ciccocioppo, R. Effect of the Cannabinoid CB1 Receptor Antagonist SR-141716A on Ethanol Self-Administration and Ethanol-Seeking Behaviour in Rats. Psychopharmacology 2005, 183, 394–403. [Google Scholar] [CrossRef]
- Hill, K.P.; Gold, M.S.; Nemeroff, C.B.; McDonald, W.; Grzenda, A.; Widge, A.S.; Rodriguez, C.; Kraguljac, N.V.; Krystal, J.H.; Carpenter, L.L. Risks and Benefits of Cannabis and Cannabinoids in Psychiatry. Am. J. Psychiatry 2022, 179, 98–109. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Agonist Replacement for Cannabis Dependence (ARCD) study group; Allsop, D.J.; Copeland, J.; McGregor, I.S.; Dunlop, A.; Shanahan, M.; Bruno, R.; Phung, N.; Montebello, M.; et al. Randomised Controlled Trial (RCT) of Cannabinoid Replacement Therapy (Nabiximols) for the Management of Treatment-Resistant Cannabis Dependent Patients: A Study Protocol. BMC Psychiatry 2018, 18, 140. [Google Scholar] [CrossRef]
- Cohen, J.; Wei, Z.; Phang, J.; LaPrairie, R.B.; Zhang, Y. Cannabinoids as an Emerging Therapy for Posttraumatic Stress Disorder and Substance Use Disorders. J. Clin. Neurophysiol. 2020, 37, 28–34. [Google Scholar] [CrossRef]
- De Ternay, J.; Naassila, M.; Nourredine, M.; Louvet, A.; Bailly, F.; Sescousse, G.; Maurage, P.; Cottencin, O.; Carrieri, P.; Rolland, B. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 2019, 10, 627. [Google Scholar] [CrossRef]
- George, T.P. Medication Treatments for Nicotine Dependence; CRC/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Janero, D.R. Cannabinoid-1 Receptor (CB1R) Blockers as Medicines: Beyond Obesity and Cardiometabolic Disorders to Substance Abuse/Drug Addiction with CB1R Neutral Antagonists. Expert Opin. Emerg. Drugs 2012, 17, 17–29. [Google Scholar] [CrossRef]
- Janero, D.R.; Makriyannis, A. Targeted Modulators of the Endogenous Cannabinoid System: Future Medications to Treat Addiction Disorders and Obesity. Curr. Psychiatry Rep. 2007, 9, 365–373. [Google Scholar] [CrossRef]
- Khurana, L.; Mackie, K.; Piomelli, D.; Kendall, D.A. Modulation of CB1 Cannabinoid Receptor by Allosteric Ligands: Pharmacology and Therapeutic Opportunities. Neuropharmacology 2017, 124, 3–12. [Google Scholar] [CrossRef]
- Lake, S.; Kerr, T.; Buxton, J.; Walsh, Z.; Cooper, Z.D.; Socías, M.E.; Fairbairn, N.; Hayashi, K.; Milloy, M.-J. The Cannabis-Dependent Relationship Between Methadone Treatment Dose and Illicit Opioid Use in a Community-Based Cohort of People Who Use Drugs. Cannabis Cannabinoid Res. 2021, 8, 155–165. [Google Scholar] [CrossRef]
- Luján, M.; Valverde, O. The Pro-Neurogenic Effects of Cannabidiol and Its Potential Therapeutic Implications in Psychiatric Disorders. Front. Behav. Neurosci. 2020, 14, 109. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid Receptors as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef]
- Onaivi, E.S. Cannabinoid Receptors in Brain. Pharmacogenetics, Neuropharmacology, Neurotoxicology, and Potential Therapeutic Applications. New Concepts Psychostimulant Induc. Neurotox. 2009, 88, 335–369. [Google Scholar] [CrossRef]
- Pietrzak, B.; Dunaj, A.; Piątkowska, K. The Role of the Cannabinoid System in the Pathogenesis and Treatment of Alcohol Dependence. Postępy Hig. I Med. Doświadczalnej 2011, 65, 606–615. [Google Scholar] [CrossRef]
- Preedy, V. Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Rodrigues, L.A.; Caroba, M.E.S.; Taba, F.K.; Filev, R.; Gallassi, A.D. Evaluation of the Potential Use of Cannabidiol in the Treatment of Cocaine Use Disorder: A Systematic Review. Pharmacol. Biochem. Behav. 2020, 196, 172982. [Google Scholar] [CrossRef]
- Śmiarowska, M.; Białecka, M.; Machoy-Mokrzyńska, A. Cannabis and Cannabinoids: Pharmacology and Therapeutic Potential. Neurol. I Neurochir. Pol. 2022, 56, 4–13. [Google Scholar] [CrossRef]
- Weidenauer, A.; Sauerzopf, U.; Praschak-Rieder, N.; Keimpema, E.; Kasper, S.; Willeit, M. Cannabidiol in for the Treatment of Psychiatric Disorders: A New Hope or Smoke on the Water? J. Fur Neurol. Neurochir. Und Psychiatr. 2021, 22, 136–140. [Google Scholar]
- Yang, P.; Wang, L.; Xie, X.-Q. Latest Advances in Novel Cannabinoid CB2 Ligands for Drug Abuse and Their Therapeutic Potential. Future Med. Chem. 2012, 4, 187–204. [Google Scholar] [CrossRef]
| Search Parameter | Inclusion Criterion |
|---|---|
| Participants | Studies were included if their participants met the criteria for a substance-use disorder (DSM-5), abuse, or dependence (DSM-IV). |
| Interventions | Experimental condition: any formulation with primary mechanism of action upon the endocannabinoid system (receptors, ligands, or enzymes) with the intention to treat a substance-use disorder, abuse, or dependence. |
| Control | Placebo or any other intervention differing from the experimental condition. |
| Outcome | The primary outcome was the effectiveness of the intervention for the treatment of any substance-use disorder. |
| Study design | The studies considered for inclusion in this review were systematic reviews (SRs), narrative reviews (NRs), and randomised-control trials (RCTs) |
| Primary Papers N = 29 | Systematic Scoping Review Search Results N = 25 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systematic Reviews n = 5 | Narrative Reviews n = 14 | Randomised Controlled Trials n = 6 | |||||||||||||||||||||||
| Batalla et. al. 2019, [24] | Hoch et. al., 2019 [25] | Paulus et. al., 2022 [26] | Pavel et. al., 2021 [27] | Prud’hommeet. al., 2015 [28] | Babalonis & Walsh 2020 [29] | Beardsley et. al., 2009 [30] | Calpe-López et al., 2019 [31] | Chye et. al., 2019 [32] | Femenia et. al., 2009 [33] | Fischer et. al., 2015 [34] | Foll et. al., 2008 [35] | Galaj & Xi 2019 [36] | Kleczko-ska et. al., 2015 [37] | Kolongo-wski et. al., 2021 [38] | Lee et. al., 2017 [39] | Navarrete et. al., 2021 [40] | Sholler et. al., 2020 [41] | Sloan et. al., 2017 [9] | Lintzeris et. al., 2019 [42] | Lintzeris et. al., 2020 [43] | Bisaga et. al., 2015 [44] | Soyka et. al., 2008 [45] | Meneses-Gaya et. al., 2021 [46] | Mongeau-Pérusse et. al., 2021 [47] | |
| Allsop et. al. 2014 [48] | |||||||||||||||||||||||||
| Trigo et. al., 2016 [49] | |||||||||||||||||||||||||
| Trigo et. al., 2018 [50] | |||||||||||||||||||||||||
| Lintzeris et. al., 2019 [42] | |||||||||||||||||||||||||
| Lintzeris et. al., 2020 [43] | |||||||||||||||||||||||||
| Haney et. al., 2003 [51] | |||||||||||||||||||||||||
| Budney et. al., 2007 [52] | |||||||||||||||||||||||||
| Haney et. al., 2007 [53] | |||||||||||||||||||||||||
| Levin et. al., 2011 [54] | |||||||||||||||||||||||||
| Vandrey et. al., 2013 [55] | |||||||||||||||||||||||||
| Levin et. al., 2016 [56] | |||||||||||||||||||||||||
| Freeman et. al., 2020 [57] | |||||||||||||||||||||||||
| D’Souza et. al., 2019 [58] | |||||||||||||||||||||||||
| Bisaga et. al., 2015 [44] | |||||||||||||||||||||||||
| Jicha et. al., 2015 [59] | |||||||||||||||||||||||||
| Lofwall et. al., 2016 [60] | |||||||||||||||||||||||||
| Hurd et. al., 2019 [61] | |||||||||||||||||||||||||
| Soyka et. al., 2008 [45] | |||||||||||||||||||||||||
| George et. al., 2009 [62] | |||||||||||||||||||||||||
| Meneses-Gaya et. al., 2021 [46] | |||||||||||||||||||||||||
| Mongeau-Pérusse et. al., 2021 [47] | |||||||||||||||||||||||||
| Rigotti et. al., 2009 [63] | |||||||||||||||||||||||||
| Cahill & Ussher 2011 [64] | |||||||||||||||||||||||||
| Robinson et. al., 2017 [65] | |||||||||||||||||||||||||
| Tonstad & Aubin 2012 [66] | |||||||||||||||||||||||||
| Morrison et. al., 2010 [67] | |||||||||||||||||||||||||
| Morgan et. al., 2013 [68] | |||||||||||||||||||||||||
| Hindocha et. al., 2018a [69] | |||||||||||||||||||||||||
| Hindocha et. al., 2018b [70] | |||||||||||||||||||||||||
| Study | Type | N | Population | Intervention | Adjunct Intervention | Duration | Follow Up | Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Nabiximols | ||||||||||
| Allsop et. al., 2014 [48] | Randomised Control Trial (RCT) | 51 | Cannabis Dependence (DSM-IV), Treatment Seeking | Nabixi-mols, oro-mucosal spray, 86.4 mg tetrahydrocannabinol (THC):80 mg can-nabidiol (CBD) (max dai-ly dose) | CBT Workbook, Standard Detoxification Care | 6 days | 28 days | Placebo | Primary: Withdrawal Severity (CWS) Secondary: Cannabis Use (28 day follow-up) Treatment Retention | ↓ Withdrawal Severity =Cannabis Use ↑ Treatment Retention |
| Trigo et. al., 2016 [49] | RCT | 9 | Cannabis Dependence (DSM-IV), Non-Treatment Seeking | Nabiximols, Oromucosal Spray, 100 mg CBD:108 mg THC (max daily dose) | n/a | 8 weeks | n.a. | Placebo | Primary: Withdrawal Severity (CWS) Secondary: Craving (MCQ) | ↓ Withdrawal Severity (dose-dependent) =Craving (MCQ) |
| Trigo et. al., 2018 [50] | RCT | 40 | Cannabis Dependence (DSM-IV), Treatment Seeking | Nabiximols Oromucusal Spray, 113.4 mg THC:105 mg CBD (max daily dose) | MET CBT | 12 weeks | n.a. | Placebo | Primary Tolerability Abstinence (EOT) Secondary Cannabis Use (days/week) Withdrawal Severity (CWS) Craving (MCQ) | =Tolerability =Abstinence ↓ Cannabis Use = Withdrawal Severity ↓ Craving |
| Lintzeris et. al., 2019 [42] | RCT | 128 | Cannabis Dependence (DSM-IV), Treatment Seeking | Nabiximols, Oromucosal Spray, 80 mg CBD:86.4 mg THC (maximum daily doses), | CBT, Case Management | 12 weeks | n.a. | Placebo | Primary: Cannabis Use (days/trial) Secondary: Craving (MCQ) Withdrawal Severity (CWS) | ↓ Cannabis Use = Craving = Withdrawal Severity |
| Lintzeris et. al., 2020 [43] | RCT | 128 | Cannabis Dependence (DSM-IV), Treatment Seeking | Nabiximols, Oromucosal Spray, 80 mg CBD:86.4 mg THC (maximum daily doses), | CBT, Case Management | 12 weeks | 24weeks | Placebo | Primary: Cannabis Use Secondary: Abstinence (previous 28 days) | ↓ Cannabis Use ↑ Abstinence |
| Tetrahydrocannabinol (THC) | ||||||||||
| Haney et. al., 2003 [51] | Placebo Controlled, Within Subject Study | 7 | Cannabis Users, Non-Treatment Seeking | THC, Oral Capsules, 5 × 10 mg | n/a | 6 days | n.a. | Placebo | Primary: Withdrawal Severity (Marijuana Withdrawal Checklist) Secondary: Craving | ↓ Withdrawal Severity ↓ Craving |
| Budney et. al., 2007 [52] | Placebo Controlled, Within Subject Study | 8 | Cannabis Dependence (DSM-IV), Non-Treatment Seeking | THC, Oral Capsules, 30 mg vs. 90 mg | n/a | 5 days | n.a. | Placebo | Primary: Withdrawal Severity (Marijuana Withdrawal Checklist) Secondary: Craving (Marijuana Craving Questionnaire) | ↓ Withdrawal Severity (dose-dependent) ↓ Craving |
| Haney et. al., 2007 [53] | Placebo Controlled, Within Subject Study | 8 | Cannabis Users, Non-Treatment Seeking | THC 3x20 mg vs Lofexidine 2.4 mg vs THC + Lofexidine | n/a | 7 days | n.a. | Placebo | Primary: Withdrawal Severity (Marijuana Withdrawal Checklist) Secondary: Relapse Cannabis Use Craving (VAS) | ↓ Withdrawal Severity (all combinations) ↓ Relapse Cannabis Use (Lofexidine, THC + Lofexidine) ↓ Craving (Lofexidine, THC + Lofexidine) |
| Dronabinol | ||||||||||
| Levin et. al., 2011 [54] | RCT | 156 | Cannabis Dependence (DSM-IV), Treatment Seeking | Dronabinol, Oral Capsules, 2 × 20 mg | MET, Relapse Prevention Therapy | 9 weeks | n.a. | Placebo | Primary: Abstinence (2 weeks, EOT) Secondary: Cannabis Use (Self-Reported) Withdrawal Severity (Withdrawal Discomfort Score) | =Abstinence =Cannabis Use ↓ Withdrawal Severity |
| Vandrey et. al., 2013 [55] | Placebo Controlled, Within Subject Study | 13 | Cannabis Dependence (DSM-IV), Non-Treatment Seeking | Dronabinol, Oral Capsules, 30 vs. 60 vs. 120 mg | n/a | 5 days | n.a. | Placebo | Primary: Withdrawal Severity (Marijuana Withdrawal Checklist) | ↓ Withdrawal Severity (dose-dependent) |
| Levin et. al., 2016 [56] | RCT | 122 | Cannabis Dependence (DSM-IV), Treatment Seeking | Dronabinol(3 × 20 mg) +Lofexidine(3 × 0.6 mg) | MET, Relapse Prevention Therapy | 10 weeks | n.a. | Placebo | Primary: Abstinence (3 weeks, EOT) Secondary: Withdrawal Severity | =Abstinence = Withdrawal Severity |
| Cannabidiol (CBD) | ||||||||||
| Freeman et. al., 2020 [57] | Phase 2a, double-blind, placebo-controlled, randomized, adaptive Bayesian trial | 48 | CUD (DSM-V),Treatment Seeking | CBD, Oral Capsules, 200 vs. 400 vs. 800 mg | Motivational Interviewing | 4 weeks | n.a. | Placebo | Primary: Cannabis Use (urinary THC-COOH: creatinine conc) Secondary: Withdrawal Severity (Cannabis Withdrawal Scale) | ↓ Cannabis Use (400 mg, 800 mg) ↓ Withdrawal Severity (800 mg) |
| Fatty Acid Amide Hydrolase (FAAH) Inhibitor | ||||||||||
| D’Souza et. al., 2019 [58] | Phase 2a, double-blind, placebo-controlled, randomized trial | 46 | Cannabis Dependence (DSM-IV), Treatment Seeking | PF-04457845,Oral Capsules, 4 mg | n/a | 4 weeks | n.a. | Placebo | Primary: Cannabis Withdrawal Severity Secondary: Cannabis Use (Urine + Self-Reported) | ↓ Cannabis Withdrawal ↓ Cannabis Use |
| Study | Type | N | Population | Intervention | Adjunct Intervention | Duration | Follow Up | Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Dronabinol | ||||||||||
| Bisaga et. al., 2015 [44] | RCT | 60 | Opioid Dependence (DSM-IV), Treatment Seeking | Dronabinol, Oral Capsule30 mg | MET, CBT, Relapse Prevention Therapy | 8 weeks. | 3 weeks. | Placebo | Primary Withdrawal Severity (SOWS) Naltrexone Treatment Retention | ↓ Withdrawal Severity = Naltrexone Treatment Retention |
| Jicha et. al., 2015 [59] | Within Subject RCT | 12 | Opioid Dependence (DSM-IV), Non-Treatment Seeking | Dronabinol, Oral Capsule5 vs. 10 vs. 20 vs. 30 mg (40 mg discontinued) | n/a | Single Dose | n.a. | Placebo, Oxycodone 30 vs. 60 mg | Physiological Tolerability | ↑ Heart Rate (>=20 mg) = Physiological Parameters (<20 mg) |
| Lofwall et. al., 2016 [60] | Within Subject RCT | 12 | Opioid Dependence (DSM-IV), Non-Treatment Seeking | Dronabinol, Oral Capsule5 vs. 10 vs. 20 vs. 30 mg (40 mg discontinued) | n/a | Single Dose | n.a. | Placebo, Oxycodone 30 vs. 60 mg | Withdrawal Severity (SOWS) Psychomotor/Cognitive Effects | ↓Withdrawal Severity (>=20 mg) ↑Psychomotor/Cognitive Effects |
| Cannabidiol (CBD) | ||||||||||
| Hurd et. al., 2019 [61] | RCT | 42 | Opioid Dependence (DSM-IV) | Cannabidiol, Oral Solution, 400,800 mg | n/a | 3 days | 7 days | Placebo | Primary Cue Induced Craving (HCQ) Anxiety (VAS-A) Secondary Cognition Affect Physiological Markers (Heart Rate, Cortisol) | ↓ Cue Induced Craving3 ↓ Anxiety =Cognition =Affect ↓Physiological Markers (Heart Rate, Cortisol) |
| Study | Type | N | Population | Intervention | Adjunct Intervention | Duration | Follow Up | Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Rimonabant | ||||||||||
| Soyka et. al., 2008 [45] | Phase 2a RCT | 258 | Alcohol Dependence (DSM-IV), Recently Detoxified | Rimonabant, Oral Capsule, 2 × 10 mg | n/a | 12 weeks | n.a. | Placebo | Primary Relapse to First Drink Relapse to Heavy Drinking Secondary Alcohol Consumption | =Relapse to First Drink =Relapse to Heavy Drinking =Alcohol Consumption |
| George et. al., 2009 [62] | Phase I/II RCT | 49 | Alcohol Dependence/Abuse (DSM-IV), Non-Treatment Seeking | Rimonabant, Oral Capsule, 20 mg | n/a | 2 weeks | n.a. | Placebo | Primary Alcohol Consumption | =Alcohol Consumption |
| Study | Type | N | Population | Intervention | Adjunct Intervention | Duration | Follow Up | Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Cannabidiol (CBD) | ||||||||||
| Meneses-Gaya et. al., 2021 [46] | RCT | 31 | Crack-Cocaine Dependence (DSM-IV) | CBD, Oral Solution, 300 mg | n/a | 10 days | n.a. | Placebo | Primary Cue Induced Craving Severity | =Cue Induced Craving Severity |
| Mongeau-Pérusse et. al., 2021 [47] | Phase II RCT | 50 | Cocaine Use Disorder (DSM-V | CBD, Oral Solution, 800 mg | Group Therapy | 12 weeks | n.a. | Placebo | Primary Cue Induced Craving Severity Secondary Time to Relapse | =Cue Induced Craving Severity =Time to Relapse |
| Study | Type | N | Population | Intervention | Adjunct Intervention | Duration | Follow Up | Comparator | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Rimonabant | ||||||||||
| Rigotti et. al., 2009 [63] | RCT | 755 | Nicotine Dependence (DSM-IV), Treatment Seeking | Rimonabant 20 mg + Nicotine Patch | Smoking Counselling | 10 weeks | 13 weeks | Rimonabant 20 mg + Placebo Patch | Primary Abstinence (EOT, 4 Week Continuous) Secondary Point Prevalence Abstinence (weeks 9,24) Sustained Abstinence (weeks 6-24) Weight Change | ↑ Abstinence (all measures) =Weight Change |
| STRATUS-WW 2005 [64] | Double-blind placebo-controlled parallel-assignment RCT | 5055 | Smokers (>10cpd), Treatment Seeking | Rimonabant5 vs. 20 mg | Behavioural Counselling | Phase 1: 10 weeks Phase 2: 42 weeks | 104 weeks | Placebo | Primary Relapse Prevention Rate Secondary Weight Change | ↑ Relapse Prevention Rate (20 mg) ↓ Weight Gain (20 mg) |
| STRATUS-EU 2006 [64,65] | Double-blind placebo-controlled parallel-assignment RCT | 783 | Smokers (>10cpd), Treatment Seeking | Rimonabant5 vs. 20 mg | Behavioural Counselling | 10 weeks | 48 weeks | Placebo | Primary Abstinence at EOT (10 weeks) and Prolonged (48 weeks) Secondary Weight Gain Adverse Events (GI Disturbance, Anxiety) | ↑ Abstinence (EOT & Prolonged) (20 mg) ↓ Weight Gain (20 mg) ↑ Adverse Events (20 mg) |
| STRATUS-US 2006 [64,65] | Double-blind placebo-controlled parallel-assignment RCT | 784 | Smokers (>10cpd), Treatment Seeking | Rimonabant5 vs. 20 mg | Behavioural Counselling | 10 weeks | 48 weeks | Placebo | Primary Abstinence at EOT (10 weeks) and Prolonged (48 weeks) Secondary Weight Gain Adverse Events (GI Disturbance, Anxiety) | ↑ Abstinence (EOT & Prolonged) (20 mg) ↓ Weight Gain (20 mg) ↑ Adverse Events (20 mg) |
| STRATUS-META 2006 [65] | Double-blind placebo-controlled parallel-assignment RCT | 530 | Smokers (>10cpd), Treatment Seeking | Rimonabant20mg | Behavioural Counselling | 10 weeks | n.a. | Placebo | Primary Abstinence at EOT (10 weeks) Secondary Weight Gain Adverse Events (GI Disturbance, Anxiety) | ↑ Abstinence (EOT) ↓ Weight Gain ↑ Adverse Events |
| Surinabant | ||||||||||
| Tonstad & Aubin, 2012 [66] | Double-blind placebo-controlled parallel-assignment RCT | 810 | Smokers (>10cpd | Surinabant 2.5 vs. 5 vs. 10 mg | Smoking Cessation Counselling | 8 weeks | 6 weeks | Placebo | Primary Abstinence (EOT, 4 weeks continuous) Secondary Weight Gain Neuropsychiatric SE | =Abstinence ↓ Weight Gain =Neuropsychiatric SE |
| Taranabant | ||||||||||
| Morrison et. al., 2010 [67] | RCT | 317 | Dependent Cigarette Smokers | Taranabant, Oral Capsules, 2 vs. 4 vs. 8 mg + Counselling | Smoking Cessation Counselling | 8 weeks | 6 weeks | Placebo | Primary Abstinence (EOT, 4 weeks continuous) Secondary Weight Gain Neuropsychiatric SE (Depression) Gastrointestinal SE | =Abstinence ↓ Weight Gain ↑ Neuropsychiatric SE ↑Gastrointestinal SE |
| Cannabidiol (CBD) | ||||||||||
| Morgan et. al., 2013 [68] | RCT | 24 | Dependent Cigarette Smokers, Non-Treatment Seeking | CBD, Inhaler, Ad Hoc Use | Smoking Cessation Counselling | 1 week | 2 weeks | Placebo | Primary Cigarette Usage Secondary Craving Mood Side Effects (Sedation, Depression, Anxiety) | ↓ Cigarette Usage =Craving =Mood Side Effects |
| Hindocha et. al., 2018a [69] | RCT Double Blind Cross-Over Design | 30 | Dependent Cigarette Smokers, Non Treatment Seeking | CBD, 800 mg | n/a | Single Dose | n.a. | Placebo | Primary Attentional Bias to Cigarette Cues during Abstinence Pleasantness of Cigarette Stimuli during Abstinence Craving Withdrawal Side Effects | ↓ Attentional Bias to Cigarette Cues during Abstinence ↓Pleasantness of Cigarette Stimuli during Abstinence =Craving =Withdrawal =Side Effects |
| Hindocha et. al., 2018b [70] | RCT Double Blind Cross-Over Design | 30 | Dependent Cigarette Smokers, Non Treatment Seeking | CBD, 800 mg | n/a | Single Dose | n.a. | Placebo | Verbal and Spatial Working Memory Impulsivity | =Verbal and Spatial Working Memory =Impulsivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharbi, K.A.; Bonomo, Y.A.; Hallinan, C.M. Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach. Int. J. Environ. Res. Public Health 2023, 20, 4087. https://doi.org/10.3390/ijerph20054087
Gharbi KA, Bonomo YA, Hallinan CM. Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach. International Journal of Environmental Research and Public Health. 2023; 20(5):4087. https://doi.org/10.3390/ijerph20054087
Chicago/Turabian StyleGharbi, Kayvan Ali, Yvonne Ann Bonomo, and Christine Mary Hallinan. 2023. "Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach" International Journal of Environmental Research and Public Health 20, no. 5: 4087. https://doi.org/10.3390/ijerph20054087
APA StyleGharbi, K. A., Bonomo, Y. A., & Hallinan, C. M. (2023). Evidence from Human Studies for Utilising Cannabinoids for the Treatment of Substance-Use Disorders: A Scoping Review with a Systematic Approach. International Journal of Environmental Research and Public Health, 20(5), 4087. https://doi.org/10.3390/ijerph20054087





