Selenium Species and Fractions in the Rock–Soil–Plant Interface of Maize (Zea mays L.) Grown in a Natural Ultra-Rich Se Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Geological Settings of the Study Area
2.2. Collection and Preparation of Rock, Soil, and Plant Samples
2.3. Reagents and Standards
2.4. Total Selenium (tSe) Determination in Rock, Soil, and Plant Samples
2.5. Chemical Fractions of Se in Rock and Soil Samples
2.6. Enzyme-Soluble Selenium Species Extraction from Plant Organs
2.7. Statistical Analysis
3. Results and Discussion
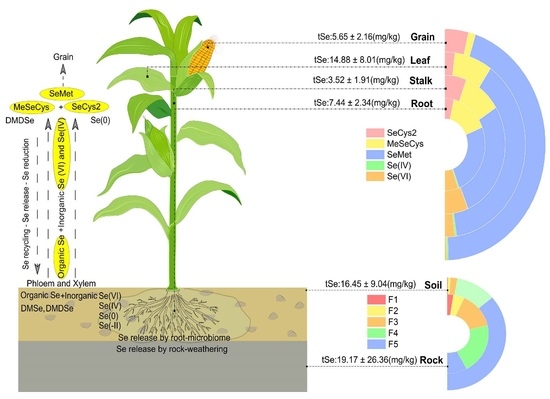
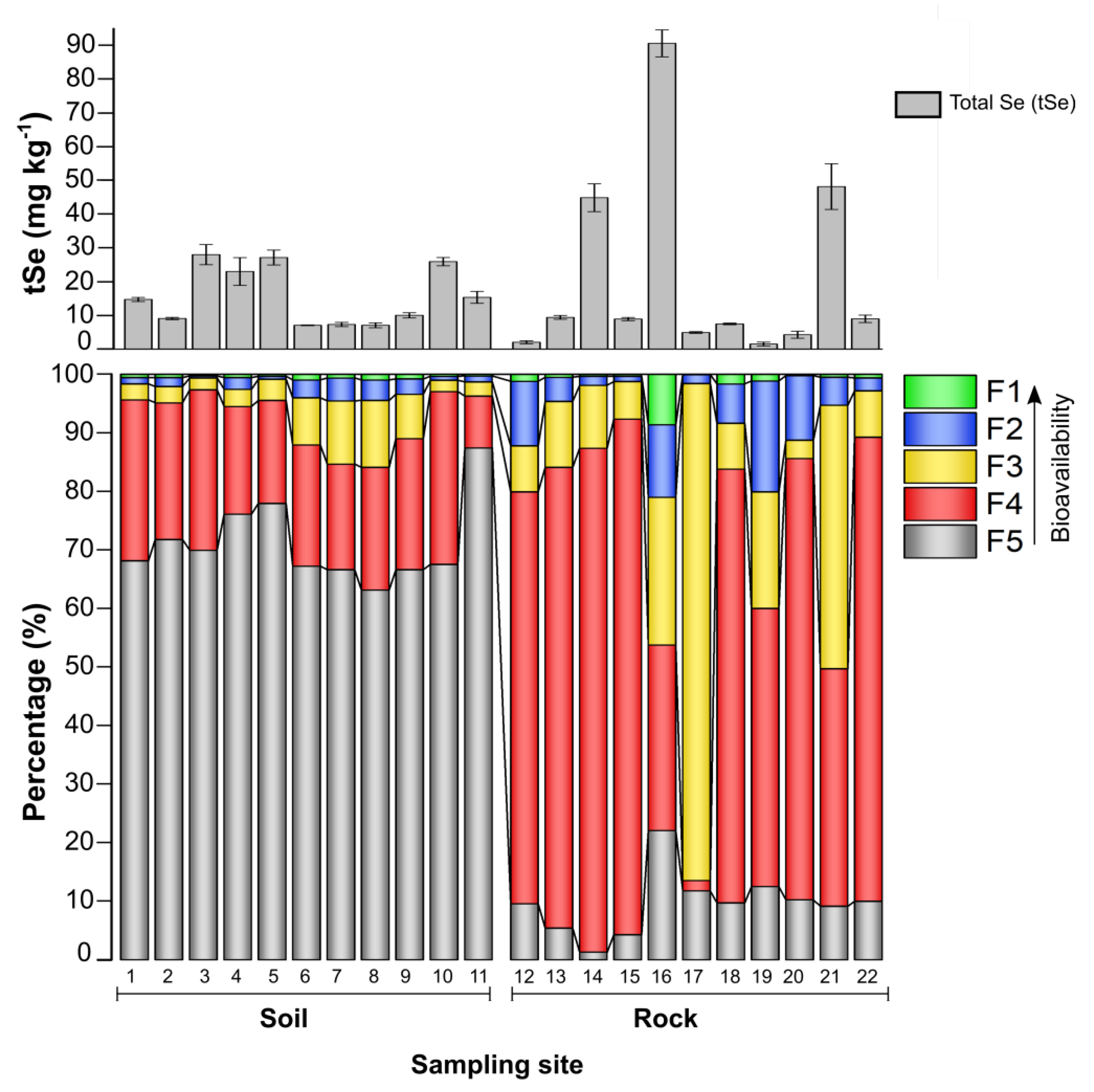
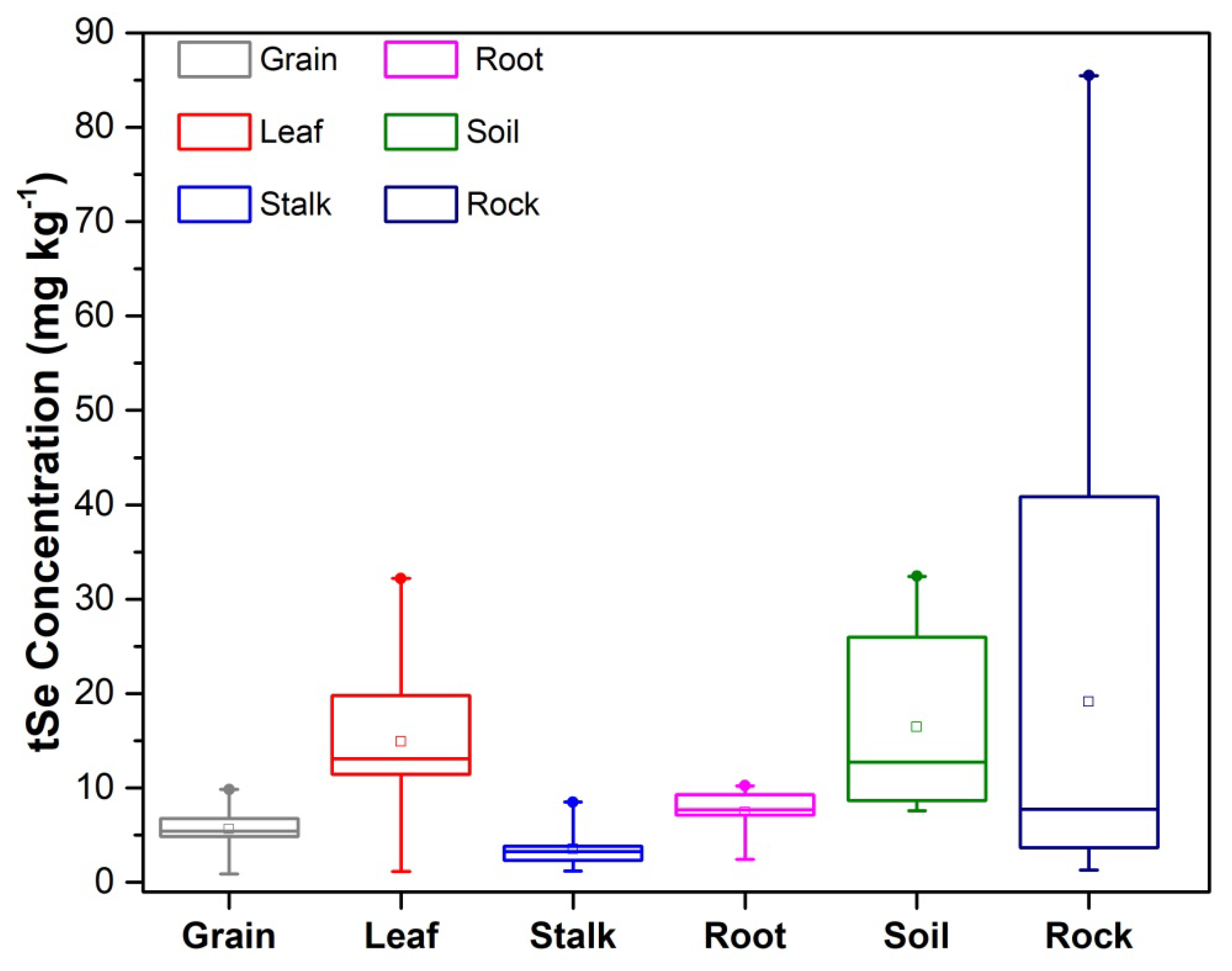
3.1. Selenium Fractions in Rock–Soils Interface and Translocation Potential
3.2. Selenium Accumulation in Maize
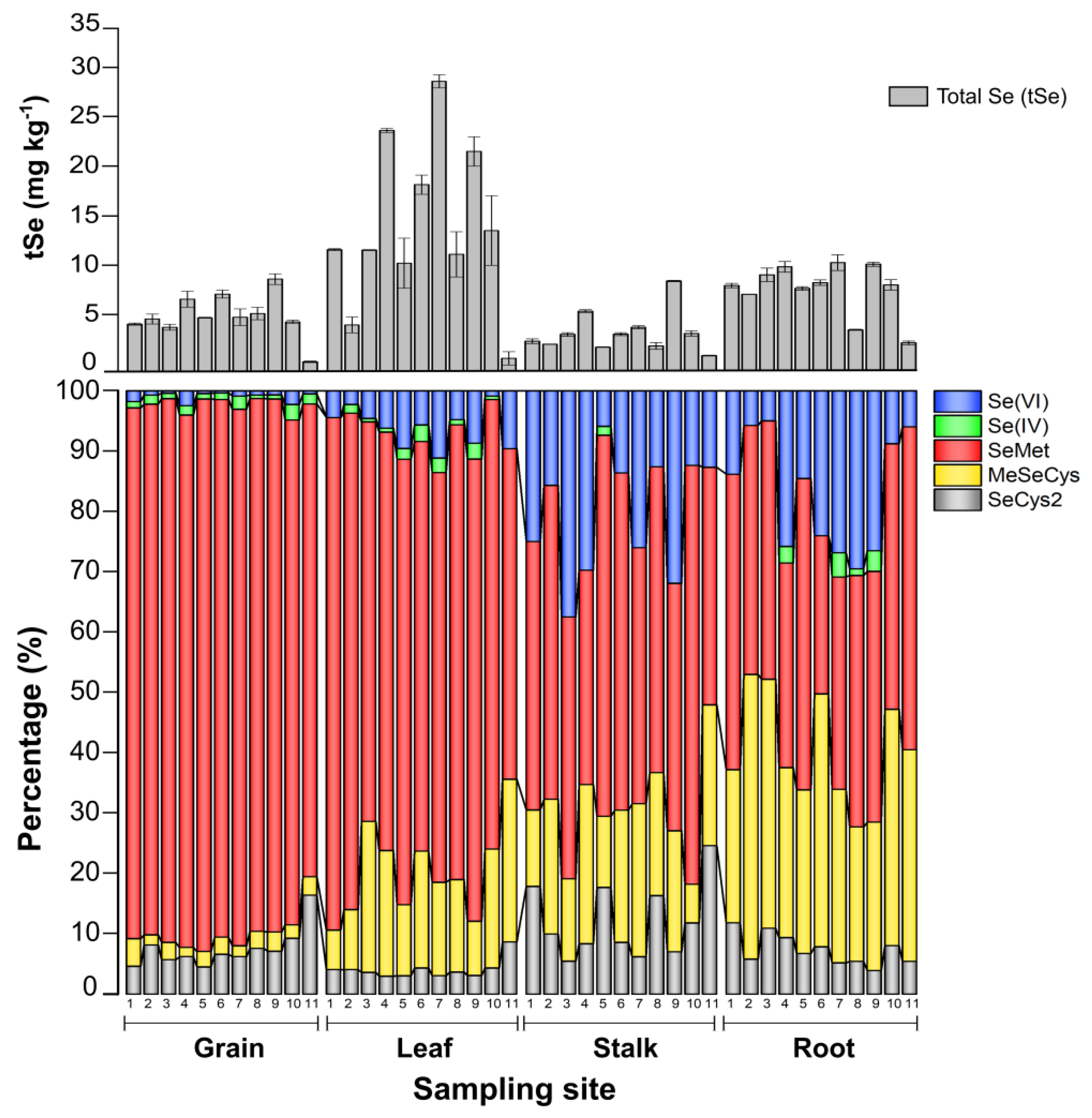
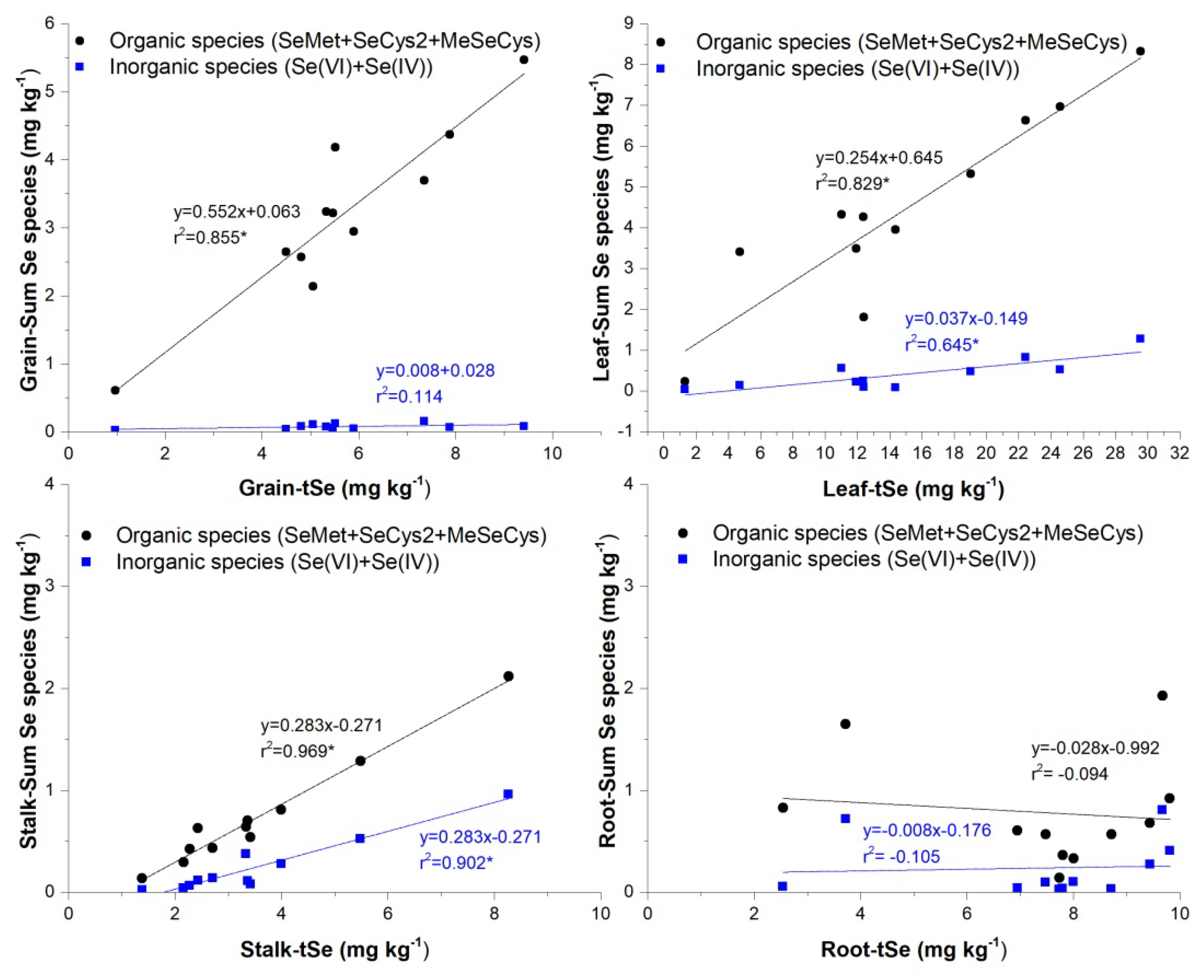
3.3. Selenium Organ-Specific Species Distribution in Maize
3.4. Current and Future Prospects of the Ultra-High Se Environment in Naore Village
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. The influence of Selenium on human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcón, M.; López-Martínez, M.C. Essentiality of Selenium in the human body: Relationship with different diseases. Sci. Total Environ. 2000, 249, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In The Crust, Elsevier-Pergamon; Rudnick, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–64. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium Geochemestry and health. Int. Symp. Med. Geol. 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Qin, H.B.; Zhu, J.M.; Liang, L.; Wang, M.S.; Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [Google Scholar] [CrossRef]
- Cui, Z.; Huang, J.; Peng, Q.; Yu, D.; Wang, S.; Liang, D. Risk assessment for human health in a seleniferous area, Shuang’an, China. Environ. Sci. Pollut. Res. 2017, 24, 17701–17710. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of Selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium-rich dissolved organic matter determines selenium uptake in wheat grown on Low-selenium arable land soils. Plant Soil. 2016, 408, 73–94. [Google Scholar] [CrossRef]
- Martens, A.; Suarez, L. Selenium speciation of soil/ediment determined with sequential extractions and hydride generation atomic absorption spectrophotometry. Environ. Sci. Technol. 1997, 31, 133–139. [Google Scholar] [CrossRef]
- Kulp, T.R.; Pratt, L.M. Speciation and weathering of Selenium in Upper Cretaceous chalk and shale from South Dakota and Wyoming, USA. Geochim. Cosmochim. Acta 2004, 68, 3687–3701. [Google Scholar] [CrossRef]
- Li, J.; Peng, Q.; Liang, D.; Liang, S.; Chen, J.; Sun, H.; Li, S.; Lei, P. Effects of aging on the fraction distribution and bioavailability of Selenium in three different soils. Chemosphere 2016, 144, 2351–2359. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Wang, D.; Wei, W.; Fu, D.; Lin, Z. Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ. 2012, 427–428, 159–164. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Z.; Xue, M.; Peng, Q.; Zhou, F.; Wang, D.; Dinh, Q.T.; Liu, Y.; Liang, D. Assessing the uptake of Selenium from naturally richsoils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ. 2019, 689, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Qi, S.; Yin, X. The threshold effect between the soil bioavailable molar Se:Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef]
- Zyambo, K.; Hodges, P.; Chandwe, K.; Chisenga, C.C.; Mayimbo, S.; Amadi, B.; Kelly, P.; Kayamba, V. Selenium status in adults and children in Lusaka, Zambia. Heliyon 2022, 8, e09782. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of Selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Sun, G.X.; Liu, X.; Williams, P.N.; Zhu, Y.G. Distribution and translocation of Selenium from soil to grain and its speciation in Paddy Rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef]
- Zakeri, N.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S. Selenium supplementation and oxidative stress: A review. Pharmanutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Gergely, V.; Kubachka, K.M.; Mounicou, S.; Fodor, P.; Caruso, J.A. Selenium speciation in Agaricus bisporus and Lentinula edodes mushroom proteins using multi-dimensional chromatography coupled to inductively coupled plasma mass spectrometry. J. Chromatogr. A 2006, 1101, 94–102. [Google Scholar] [CrossRef]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of Selenium during grain biofortification, processing and production of Se-rich food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. The Lujiaping Formation of Northern Daba Mountain. J. Stratigraphy. 2006, 30, 149–156, (In Chinese with English Abstract). [Google Scholar]
- Luo, K.; Xu, L.; Tan, J.; Wang, D.; Xiang, L. Selenium source in the selenosis area of the Daba region, South Qinling Mountain, China. Environ. Geol. 2004, 45, 426–432. [Google Scholar] [CrossRef]
- HJ/T 166-2004; Technical Specification for Soil Environmental Monitoring. Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC): Beijing, China, 2004; 44p. (In Chinese)
- DZ/T 0295-2016; Specification of Land Quality Geochemical Assessment. Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC): Beijing, China, 2016; 52p. (In Chinese)
- Food and Agriculture Organization of the United Nations (FAO). World reference base for soil resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Tang, M.; Lu, G.; Fan, B.; Xiang, W.; Bao, Z. Bioaccumulation and risk assessment of heavy metals in soil-crop systems in Liujiang karst area, Southwestern China. Environ. Sci. Pollut. Res. 2021, 28, 9657–9669. [Google Scholar] [CrossRef]
- ERM_Certificate. Available online: http://www.speciation.net/Database//ERMBC210a (accessed on 9 September 2022).
- Tian, H.; Xie, S.; Carranza, E.J.M.; Bao, Z.; Zhang, H.; Wu, S.; Wei, C.; Ma, Z. Distributions of Selenium and related elements in high pyrite and Se-rich rocks from Ziyang, Central China. J. Geochemical. Explor. 2020, 212, 106506. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Luo, K.; Li, H. Environmental behaviors of Selenium in soil of typical selenosis area, China. J. Environ. Sci. 2008, 20, 859–864. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Y.; Zeeshan, M.; Qin, S.; Ma, J.; Liu, L.; Yang, J.; Zhou, X.; Huang, J. Arbuscular mycorrhizal fungi reverse selenium stress in Zea mays seedlings by improving plant and soil characteristics. Ecotoxicol. Environ. Saf. 2021, 228, 113000. [Google Scholar] [CrossRef]
- Schilling, K.; Johnson, T.M.; Dhillon, K.S.; Mason, P.R.D. Fate of Selenium in soils at a seleniferous site recorded by high precision Se isotope measurements. Environ. Sci. Technol. 2015, 49, 9690–9698. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Huang, X.; Huang, C.; Wang, H.; Yin, H.; Shao, Y.; Li, P. Linking microbial community composition to farming pattern in selenium-rich region: Potential role of microorganisms on Se geochemistry. J. Environ. Sci. 2022, 112, 269–279. [Google Scholar] [CrossRef]
- Qin, H.B.; Zhu, J.M.; Su, H. Selenium fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 2011, 86, 626–633. [Google Scholar] [CrossRef]
- Qin, H.B.; Zhu, J.M.; Lin, Z.Q.; Xu, W.P.; Tan, D.C.; Zheng, L.R.; Takahashi, Y. Selenium speciation in seleniferous agricultural soils under different cropping systems using sequential extraction and X-ray absorption spectroscopy. Environ. Pollut. 2017, 225, 361–369. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Z.; Lv, Y.; Hou, Q.; Xia, X.; Feng, H.; Zhang, M.; Jin, L.; Kan, Z. The origin and geochemical cycle of soil selenium in a Se-rich area of China. J. Geochemical. Explor. 2014, 139, 97–108. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, N.; Li, S.; Li, L.; Su, H.; Liu, C. Distribution and transport of Selenium in Yutangba, China: Impact of human activities. Sci. Total Environ. 2008, 392, 252–261. [Google Scholar] [CrossRef]
- De Feudis, M.; D’Amato, R.; Businelli, D.; Guiducci, M. Fate of Selenium in soil: A case study in a maize (Zea mays L.) field under two irrigation regimes and fertilized with sodium selenite. Sci. Total Environ. 2019, 659, 131–139. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, B.; Wang, Z.; Xiao, H.; Mao, D.; Su, H. Distribution of Selenium in corn and its relationship with soil selenium in yutangba mini-landscape. Chin. J. Geochem. 2000, 20, 161–166. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Effect of Selenium on Salt Tolerance in Maize Plants. J. Environ. Sci. 2008, 49, 91–124. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Płaczek, A.; Patorczyk-Pytlik, B. The dynamics of selenium uptake by maize (Zea mays L.). Agronomy 2021, 11, 1305. [Google Scholar] [CrossRef]
- Lai, J.; Messing, J. Increasing maize seed methionine by mRNA stability. Plant J. 2002, 30, 395–402. [Google Scholar] [CrossRef]
- Beilstein, M.; Whanger, P.; Yang, G. Chemical forms of Selenium in corn and rice grown in a high selenium area of China. Biomed Environ. Sci. 1991, 4, 392. [Google Scholar] [PubMed]
- Duncan, E.G.; Maher, W.A.; Jagtap, R.; Krikowa, F.; Roper, M.M.; O’Sullivan, C.A. Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ. Geochem. Health. 2017, 39, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Pilon-smits, E.A.H.; Quinn, C.F. Selenium Metabolism in Plants. In Progress in Botany Cham; Springer: Cham, Switzerland, 2010; pp. 93–107. [Google Scholar] [CrossRef]
- Neuhierl, B.; Thanbichler, M.; Lottspeich, F.; Böck, A. A family of S-methylmethionine-dependent thiol/selenol methyltransferases. Role in selenium tolerance and evolutionary relation. J. Biol. Chem. 1999, 274, 5407–5414. [Google Scholar] [CrossRef]
- Ellis, D.R.; Salt, D.E. Plants, Selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Price, A.H.; Meharg, A.A. Grain accumulation of selenium species in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, L.; Yang, K.; Zhang, S. Influence of mycorrhizal inoculation on the accumulation and speciation of Selenium in maize growing in selenite and selenate spiked soils. Pedobiologia 2011, 54, 267–272. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Abiven, S.; Heim, A.; Schmidt, M. Lignin content and chemical characteristics in maize and wheat vary between plant organs and growth stages: Consequences for assessing lignin dynamics in soil. Plant Soil. 2011, 343, 369–378. [Google Scholar] [CrossRef]
- Smrkolj, P.; Stibilj, V.; Kreft, I.; Kapolna, E. Selenium species determination in selenium-rich pumpkin (Cucurbita pepo L.) seeds by HPLC-UV-HG-AFS. Anal. Sci. 2005, 21, 1501–1504. [Google Scholar] [CrossRef]
- Cuderman, P.; Stibilj, V. Stability of Se species in plant extracts rich in phenolic substances. Anal. Bioanal. Chem. 2001, 396, 1433–1439. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. Phenolic compounds in maize grains and its nixtamalized products. Phenolic Compd. -Nat. Sources Importance Appl. 2017, 8, 215–232. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese cooking methods on the content and speciation of Selenium in Selenium bio-fortified cereals and soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Muleya, M.; Young, S.D.; Reina, S.V.; Ligowe, I.S.; Broadley, M.R.; Joy, E.J.M.; Chopera, P.; Bailey, E.H. Selenium speciation and bioaccessibility in Se-fertilised crops of dietary importance in Malawi. J. Food Compos. Anal. 2021, 98, 103841. [Google Scholar] [CrossRef]
- SCF (Scientific Committee on Food). Opinion on the Scientific Committee on Food on the tolerable upper intake level of Selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef]
- Li, S.; Xiao, T.; Zheng, B. Medical geology of arsenic, Selenium and thallium in China. Sci. Total Environ. 2012, 421–422, 31–40. [Google Scholar] [CrossRef]
- Brown, K.M.; Arthur, J.R. Selenium, Selenoproteins and human health: A Review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Mintziori, G.; Mousiolis, A.; Duntas, L.H.; Goulis, D.G. Evidence for a manifold role of Selenium in infertility. Hormones 2020, 19, 55–59. [Google Scholar] [CrossRef]
- Lammi, M.; Qu, C. Selenium-Related Transcriptional regulation of gene expression. Int. J. Mol. Sci. 2018, 19, 2665. [Google Scholar] [CrossRef]
- Norton, R.L.; Hoffmann, P.R. Molecular aspects of medicine selenium and asthma. Mol. Aspects Med. 2012, 33, 98–106. [Google Scholar] [CrossRef]
- Wang, Y.; Rijntjes, E.; Wu, Q.; Lv, H.; Gao, C.; Shi, B.; Schomburg, L. Selenium deficiency is linearly associated with hypoglycemia in healthy adults. Redox Biol. 2020, 37, 101709. [Google Scholar] [CrossRef]
- Alcolea, V.; Pérez-Silanes, S. Selenium as an interesting option for the treatment of Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112673. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Kheyri, Z. The rationale for selenium supplementation in inflammatory bowel disease: A mechanism-based point of view. Nutrition 2021, 85, 111153. [Google Scholar] [CrossRef] [PubMed]
- GH/T 1135-2017; Selenium Rich Agricultural Products. GH-China Supply and Marketing Cooperation General Association: Beijing, China, 25 April 2017.
- Bañuelos, G.S.; Walse, S.S.; Yang, S.I.; Pickering, I.J.; Fakra, S.C.; Marcus, M.A.; Freeman, J.L. Quantification, Localization, and Speciation of Selenium in Seeds of Canola and Two Mustard Species Compared to Seed-Meals Produced by Hydraulic Press. Anal. Chem. 2012, 84, 6024–6030. [Google Scholar] [CrossRef] [PubMed]
- Amoako, P.O.; Kahakachchi, C.L.; Dodova, E.N.; Uden, P.C.; Tyson, J.F. Speciation, Quantification and Stability of Selenomethionine, S-(Methylseleno) Cysteine and Selenomethionine Se-Oxide in Yeast-Based Nutritional Supplements. J. Anal. At. Spectrom. 2007, 22, 938–946. [Google Scholar] [CrossRef]
- Shi, W.; Hou, Y.; Zhang, Z.; Yin, X.; Zhao, X.; Yuan, L. Determination of Selenium Speciation in High Se-Enriched Edible Fungus Ganoderma Lucidum Via Sequential Extraction. Horticulturae 2023, 9, 161. [Google Scholar] [CrossRef]

| Sample | Parameter | Plot 1 | Plot 2 | Plot 3 | Plot 4 | Plot 5 | Plot 6 | Plot 7 | Plot 8 | Plot 9 | Plot 10 | Plot 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock | No. | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | R22 |
| tSe (mg/kg) | 2.0 ± 0.4 | 10.0 ± 0.5 | 41.5 ±4.1 | 7.7 ±0.5 | 85.5 ± 4.0 | 3.8 ± 1.0 | 6.1 ± 0.2 | 1.3 ± 0.3 | 3.7 ± 0.6 | 40.9 ± 6.8 | 7.7 ± 1.1 | |
| pH | 9.3 | 9.0 | 8.5 | 8.7 | 8.0 | 8.3 | 9.1 | 9.3 | 9.3 | 6.5 | 8.8 | |
| TOC (%) | 2.1 | 2.0 | 1.2 | 8.4 | 13.1 | 3.4 | 3.1 | 8.1 | 3.8 | 15.0 | 0.2 | |
| Soil | No. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 |
| tSe (mg/kg) | 13.3 ± 0.6 | 8.4 ± 0.3 | 29.4 ± 3.0 | 22.0 ± 4.1 | 29.9 ± 2.2 | 7.8 ± 0.1 | 8.0 ± 0.6 | 8.8 ± 0.7 | 11.1 ± 0.8 | 27.8 ± 1.2 | 14.2 ± 1.8 | |
| pH | 7.5 | 7.7 | 7.9 | 8.7 | 7.7 | 7.6 | 7.7 | 7.3 | 6.5 | 7.5 | 7.6 | |
| TOC (%) | 4.5 | 3.3 | 4.9 | 5.7 | 7.0 | 2.2 | 5.0 | 3.2 | 5.2 | 4.2 | 5.1 | |
| Maize | No. | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 |
| tSe-grain (mg/kg) | 4.8 ± 0.1 | 5.3 ± 0.5 | 4.5 ± 0.3 | 7.3 ± 0.8 | 5.5 ± 0.05 | 7.9 ± 0.4 | 5.5 ± 0.8 | 5.9 ± 0.63 | 9.4 ± 0.5 | 5.0 ± 0.2 | 1.0 ± 0.08 | |
| tSe-leaf (mg/kg) | 12.4 ± 0.8 | 4.7 ± 0.05 | 12.4 ± 0.2 | 24.6 ± 2.5 | 11.0 ± 1.0 | 19.0 ± 0.7 | 29.6 ± 2.3 | 11.9 ± 1.5 | 22.4 ± 3.5 | 14.3 ± 0.7 | 1.3 ± 0.2 | |
| tSe-stalk (mg/kg) | 2.7 ± 0.2 | 2.4 ± 0.2 | 3.3 ± 0.1 | 5.5 ± 0.03 | 2.2 ± 0.13 | 3.4 ± 0.1 | 4.0 ± 0.3 | 2.3 ± 0.06 | 8.3 ± 0.2 | 3.4 ± 0.04 | 1.4 ± 0.2 | |
| tSe-root (mg/kg) | 7.7 ± 0.6 | 6.9 ± 0.3 | 8.7 ± 0.5 | 9.4 ± 0.2 | 7.5 ± 0.2 | 8.0 ± 0.7 | 9.8 ± 0.05 | 3.7 ± 0.2 | 9.7 ± 0.5 | 7.8 ± 0.2 | 2.5 ± 0.1 | |
| Biomass-grain (g) | 414.8 | 385.1 | 282.1 | 298.2 | 101.6 | 322.2 | 369.9 | 356.3 | 515.8 | 188.7 | 415.0 | |
| Biomass-leaf (g) | 94.9 | 106.3 | 88.9 | 63.2 | 100.1 | 146.3 | 128.7 | 125.8 | 145.1 | 84.5 | 143.6 | |
| Biomass-stalk (g) | 94.5 | 93.5 | 136.6 | 121.5 | 98.3 | 106.5 | 124.0 | 116.2 | 114.4 | 145.8 | 133.5 | |
| Biomass-root (g) | 63.0 | 109.62 | 34.2 | 33.8 | 39.3 | 99.5 | 35.8 | 49.0 | 56.2 | 85.5 | 96.2 |
| Maize Organ | tSe | SUM-Se Extracted | Organic Species | Inorganic Species | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SeMet | SeCys2 | MeSeCys | Se(IV) + Se(VI) | Extraction Efficiency | |||||||
| (mg/kg) | (mg/kg) | (mg/kg) | (%) | (mg/kg) | (%) | (mg/kg) | (%) | (mg/kg) | (%) | (%) | |
| Grain | 5.6 ± 2.2 | 5.6 ± 2.3 | 5.0 ± 2.1 | 86.7 ± 3.5 | 0.43 ± 0.17 | 7.4 ± 3.3 | 0.13 ± 0.1 | 2.62 ± 0.86 | 0.06 ± 0.03 | 2.3 ± 1.3 | 98.7 ± 9.8 |
| Leaf | 14.9 ± 8.4 | 8.0 ± 4.6 | 6.2 ± 3.5 | 80.0 ± 8.3 | 0.33 ± 0.15 | 4.0 ± 1.6 | 1.2 ± 0.82 | 16.1 ± 6.5 | 0.3 ± 0.28 | 7.3 ± 3.8 | 54.1 ± 15.5 |
| Stalk | 3.5 ± 1.9 | 1.5 ± 1.2 | 0.81 ± 0.57 | 48.1 ± 10.4 | 0.17 ± 0.1 | 12.0 ± 6.0 | 0.3 ± 0.31 | 18.3 ± 6.3 | 0.19 ± 0.21 | 20.1 ± 10.0 | 37.5 ± 10.6 |
| Root | 7.4 ± 2.3 | 1.5 ± 1.1 | 0.76 ± 0.58 | 41.8 ± 7.9 | 0.11 ± 0.07 | 7.3 ± 2.5 | 0.46 ± 0.29 | 32.7 ± 8.4 | 0.18 ± 0.21 | 17.9 ± 10.7 | 25.4 ± 25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinzon-Nuñez, D.A.; Wiche, O.; Bao, Z.; Xie, S.; Fan, B.; Zhang, W.; Tang, M.; Tian, H. Selenium Species and Fractions in the Rock–Soil–Plant Interface of Maize (Zea mays L.) Grown in a Natural Ultra-Rich Se Environment. Int. J. Environ. Res. Public Health 2023, 20, 4032. https://doi.org/10.3390/ijerph20054032
Pinzon-Nuñez DA, Wiche O, Bao Z, Xie S, Fan B, Zhang W, Tang M, Tian H. Selenium Species and Fractions in the Rock–Soil–Plant Interface of Maize (Zea mays L.) Grown in a Natural Ultra-Rich Se Environment. International Journal of Environmental Research and Public Health. 2023; 20(5):4032. https://doi.org/10.3390/ijerph20054032
Chicago/Turabian StylePinzon-Nuñez, Diego Armando, Oliver Wiche, Zhengyu Bao, Shuyun Xie, Bolun Fan, Wenkai Zhang, Molan Tang, and Huan Tian. 2023. "Selenium Species and Fractions in the Rock–Soil–Plant Interface of Maize (Zea mays L.) Grown in a Natural Ultra-Rich Se Environment" International Journal of Environmental Research and Public Health 20, no. 5: 4032. https://doi.org/10.3390/ijerph20054032
APA StylePinzon-Nuñez, D. A., Wiche, O., Bao, Z., Xie, S., Fan, B., Zhang, W., Tang, M., & Tian, H. (2023). Selenium Species and Fractions in the Rock–Soil–Plant Interface of Maize (Zea mays L.) Grown in a Natural Ultra-Rich Se Environment. International Journal of Environmental Research and Public Health, 20(5), 4032. https://doi.org/10.3390/ijerph20054032