Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Initial Assessment
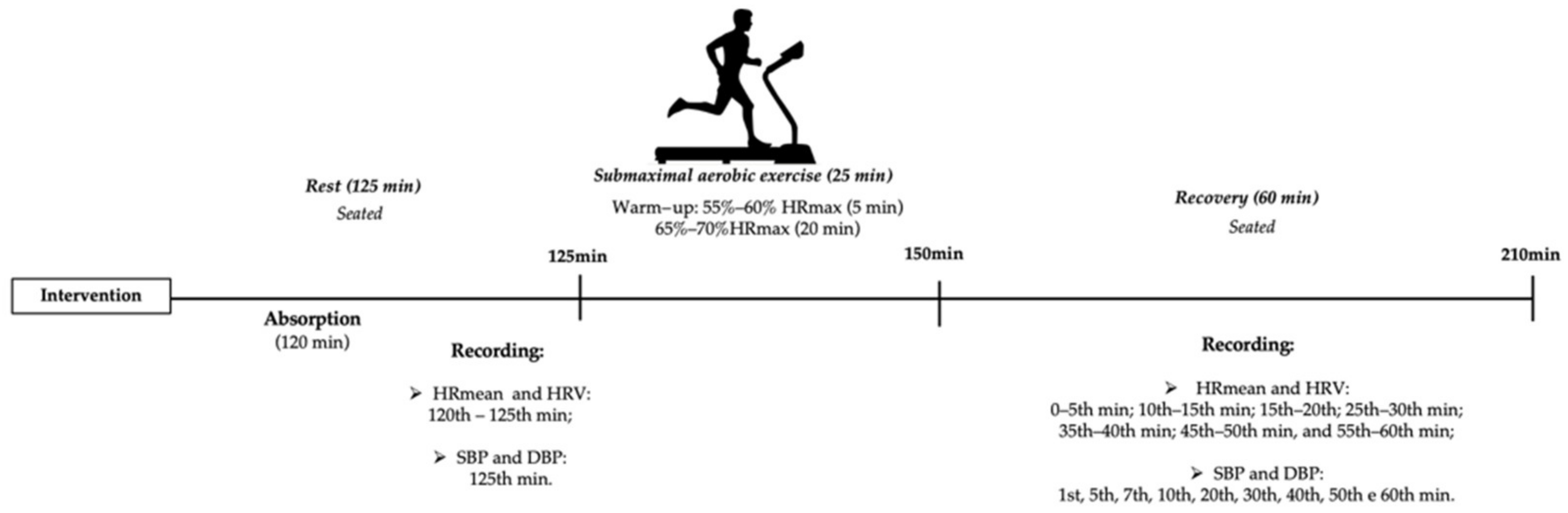
2.3. Interventions
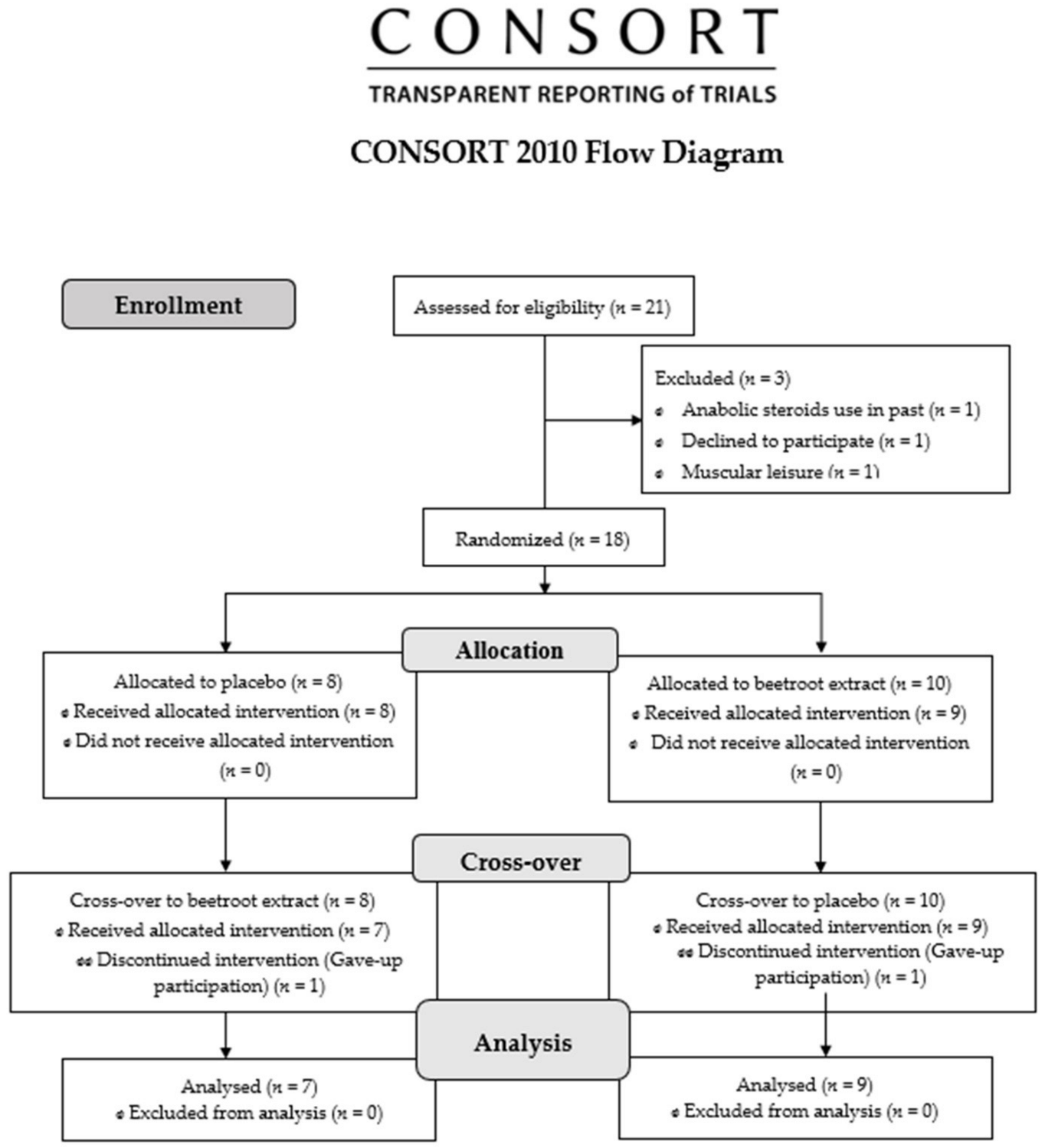
2.4. Blinding and Randomization
2.5. Outcomes
Blood Pressure
2.6. HR and HRV Analysis
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Sample Profile
3.2. Blood Pressure following from Exercise
3.3. HR and HRV Recovery after Exercise
4. Discussion
- (a)
- In the beetroot protocol immediately after exercise the means of the HR, SBP and DBP values were reduced more quickly.
- (b)
- HF indices (typical of vagal modulation) recovered earlier after exercise cessation in the beetroot vs. placebo protocol.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Wong, M.; Jirangrat, W.; Teh, K.H.; Ali, A. Acute Supplementation with Nitrate-Rich Beetroot Juice Causes a Greater Increase in Plasma Nitrite and Reduction in Blood Pressure of Older Compared to Younger Adults. Nutrients 2019, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The Nitrate-Independent Blood Pressure-Lowering Effect of Beetroot Juice: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Benjamim, C.J.R.; Porto, A.A.; Valenti, V.E.; Sobrinho, A.C.D.S.; Garner, D.M.; Gualano, B.; Bueno Júnior, C.R. Nitrate Derived from Beetroot Juice Lowers Blood Pressure in Patients with Arterial Hypertension: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 823039. [Google Scholar] [CrossRef]
- Remington, J.; Winters, K. Effectiveness of dietary inorganic nitrate for lowering blood pressure in hypertensive adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 365–389. [Google Scholar] [CrossRef]
- Silva, K.V.C.; Costa, B.D.; Gomes, A.C.; Saunders, B.; Mota, J.F. Factors that Moderate the Effect of Nitrate Ingestion on Exercise Performance in Adults: A Systematic Review with Meta-Analyses and Meta-Regressions. Adv. Nutr. 2022, 13, 1866–1881. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Jakubowski, H. Pathophysiological consequences of homocysteine excess. J. Nutr. 2006, 136 (Suppl. 6), 1741S–1749S. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Notay, K.; Incognito, A.V.; Millar, P.J. Acute beetroot juice supplementation on sympathetic nerve activity: A randomized, double-blind, placebo-controlled proof-of-concept study. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H59–H65. [Google Scholar] [CrossRef] [PubMed]
- Bond, V.; Curry, B.H.; Adams, R.G.; Asadi, M.S.; Stancil, K.A.; Millis, R.M.; Haddad, G.E. Effects of Nitrate Supplementation on Cardiovascular and Autonomic Reactivity in African-American Females. ISRN Physiol. 2014, 2014, 676235. [Google Scholar] [CrossRef]
- Vanderlei, L.C.; Pastre, C.M.; Hoshi, R.A.; Carvalho, T.D.; Godoy, M.F. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Kliszczewicz, B.; Garner, D.M.; Cavalcante, T.C.F.; da Silva, A.A.M.; Santana, M.D.R.; Valenti, V.E. Is Caffeine Recommended Before Exercise? A Systematic Review to Investigate Its Impact on Cardiac Autonomic Control via Heart Rate and Its Variability. J. Am. Coll. Nutr. 2020, 39, 563–573. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Monteiro, L.R.L.; Pontes, Y.M.M.; Silva, A.A.M.D.; Souza, T.K.M.; Valenti, V.E.; Garner, D.M.; Cavalcante, T.C.F. Caffeine slows heart rate autonomic recovery following strength exercise in healthy subjects. Rev. Port. Cardiol. 2021, 40, 399–406. [Google Scholar] [CrossRef]
- Porto, A.A.; Valenti, V.E.; Tonon do Amaral, J.A.; Benjamim, C.J.R.; Garner, D.M.; Ferreira, C. Energy Drink before Exercise did not Affect Autonomic Recovery Following Moderate Aerobic Exercise: A Crossover, Randomized and Controlled Trial. J. Am. Coll. Nutr. 2021, 40, 280–286. [Google Scholar] [CrossRef]
- Porto, A.A.; Benjamim, C.J.R.; Gonzaga, L.A.; Luciano de Almeida, M.; Bueno Júnior, C.R.; Garner, D.M.; Valenti, V.E. Caffeine intake and its influences on heart rate variability recovery in healthy active adults after exercise: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1071–1082. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; SJúnior, F.W.; de Figueirêdo, M.Í.L.S.; Benjamim, C.J.R.; Cavalcante, T.C.F.; da Silva, A.A.M.; Monteiro, L.R.L.; Santana, M.D.R.; Garner, D.M.; Valenti, V.E. Beetroot (Beta vulgaris L.) Extract Acutely Improves Heart Rate Variability Recovery Following Strength Exercise: A Randomized, Double-Blind, Placebo-Controlled Crossover Trial-Pilot Study. J. Am. Coll. Nutr. 2021, 40, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; De Cocker, K.; Teychenne, M.J.; Brown, W.J.; Biddle, S.J.H. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Rzewnicki, R.; Vanden Auweele, Y.; De Bourdeaudhuij, I. Addressing overreporting on the International Physical Activity Questionnaire (IPAQ) telephone survey with a population sample. Public Health Nutr. 2003, 6, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Bonnemeier, H.; Richardt, G.; Potratz, J.; Wiegand, U.K.; Brandes, A.; Kluge, N.; Katus, H.A. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Griesenbeck, J.S.; Steck, M.D.; Huber, J.C., Jr.; Sharkey, J.R.; Rene, A.A.; Brender, J.D. Development of estimates of dietary nitrates, nitrites, and nitrosamines for use with the Short Willet Food Frequency Questionnaire. Nutr. J. 2009, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Jakubcik, E.M.; Rutherfurd-Markwick, K.; Chabert, M.; Wong, M.; Ali, A. Pharmacokinetics of Nitrate and Nitrite Following Beetroot Juice Drink Consumption. Nutrients 2021, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065.
- Benjamim, C.J.R.; Júnior, F.W.S.; Porto, A.A.; Rocha, É.M.B.; Santana, M.D.; Garner, D.M.; Valenti, V.E.; Bueno Júnior, C.R. Bitter Orange (Citrus aurantium L.) Intake Before Submaximal Aerobic Exercise Is Safe for Cardiovascular and Autonomic Systems in Healthy Males: A Randomized Trial. Front. Nutr. 2022, 9, 890388. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV--heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research-Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Quintana, D.S. Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiology 2017, 54, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Hupin, D.; Sarajlic, P.; Venkateshvaran, A.; Fridén, C.; Nordgren, B.; Opava, C.H.; Lundberg, I.E.; Bäck, M. Cardiovascular Autonomic Function Changes and Predictors During a 2-Year Physical Activity Program in Rheumatoid Arthritis: A PARA 2010 Substudy. Front. Med. 2021, 8, 788243. [Google Scholar] [CrossRef] [PubMed]
- Carrijo, V.H.V.; Amaral, A.L.; Mariano, I.M.; de Souza, T.C.F.; Batista, J.P.; de Oliveira, E.P.; Puga, G.M. Beetroot juice intake with different amounts of nitrate does not change aerobic exercise-mediated responses in heart rate variability in hypertensive postmenopausal women: A randomized, crossover and double-blind study. J. Exerc. Sci. Fit. 2021, 19, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. Acute cardiovascular responses to interval exercise: A systematic review and meta-analysis. J. Sport. Sci. 2020, 38, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Sirvent, P.; Perrey, S.; Raynaud, E.; Mercier, J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J. Appl. Physiol. 2004, 97, 2132–2138. [Google Scholar] [CrossRef]
- Peçanha, T.; de Brito, L.C.; Fecchio, R.Y.; de Sousa, P.N.; da Silva Junior, N.D.; de Abreu, A.P.; da Silva, G.V.; Mion-Junior, D.; Forjaz, C.L. Metaboreflex activation delays heart rate recovery after aerobic exercise in never-treated hypertensive men. J. Physiol. 2016, 594, 6211–6223. [Google Scholar] [CrossRef]
- Sperling, M.P.; Simões, R.P.; Caruso, F.C.; Mendes, R.G.; Arena, R.; Borghi-Silva, A. Is heart rate variability a feasible method to determine anaerobic threshold in progressive resistance exercise in coronary artery disease? Braz. J. Phys. Ther. 2016, 20, 289–297. [Google Scholar] [CrossRef]
- Amaral, A.L.; Mariano, I.M.; Carrijo, V.H.V.; de Souza, T.C.F.; Batista, J.P.; Mendonça, A.M.; de Souza, A.V.; Caixeta, D.C.; Teixeira, R.R.; Espindola, F.S.; et al. A Single Dose of Beetroot Juice does not Change Blood Pressure Response Mediated by Acute Aerobic Exercise in Hypertensive Postmenopausal Women. Nutrients 2019, 11, 1327. [Google Scholar] [CrossRef]
- Strilchuk, L.; Cincione, R.I.; Fogacci, F.; Cicero, A.F.G. Dietary interventions in blood pressure lowering: Current evidence in 2020. Kardiol. Pol. 2020, 78, 659–666. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values |
|---|---|
| Age (years) | 21.31 ± 2.26 (18–27) |
| BMI (kg/m2) | 24.82 ± 1.99 (20.93–28.72) |
| Height (m) | 173.8 ± 0.06 (1.65–1.87) |
| Mass (kg) | 75.10 ± 7.43 (57–84) |
| Heart rate (bpm) | 74 ± 11 (51–91) |
| SBP (mmHg) | 117 ± 3(110–120) |
| DBP (mmHg) | 77 ± 3 (70–80) |
| Variables | Treatment | Rest | Rec (1 min) | Rec (5 min) | Rec (10 min) | Rec (20 min) | Rec (30 min) | Rec (40 min) | Rec (50 min) | Rec (60 min) |
|---|---|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | Beet | 119 ± 9 (114–124) | 132 ± 8 * (128–136) | 122 ± 7 (119–126) | 119 ± 7 (116–122) | 119 ± 7 (116–122) | 118 ± 6 (115–121) | 117 ± 7 (114–120) | 117 ± 7 (114–120) | 116 ± 6 (113–120) |
| Placebo | 119 ± 8 (115–123) | 134 ± 8 * (129–138) | 124 ± 6 * (121–127) | 121 ± 6 (117–123) | 118 ± 5 (115–120) | 118 ± 5 (115–120) | 118 ± 5 (115–120) | 118 ± 5 (115–120) | 118 ± 5 (115–120) | |
| DBP (mmHg) | Beet | 80 ± 6 (77–83) | 83 ± 7 (79–87) | 81 ± 6 (78–85) | 82 ± 8 (78–87) | 81 ± 7 (78–85) | 82 ± 7 (79–86) | 81 ± 7 (78–85) | 81 ± 7 (77–85) | 81 ± 7 (78–85) |
| Placebo | 80 ± 4 (78–82) | 84 ± 10 * (80––89) | 83 ± 4 (81–85) | 82 ± 4 (80–84) | 80 ± 4 (79–83) | 80 ± 4 (79– 83) | 80 ± 5 (78–82) | 80 ± 5 (78–82) | 81 ± 3 (80–82) | |
| MAP (mmHg) | Beet | 93 ± 6 (80–105) | 100 ± 7 * (87–113) | 95 ± 5 (81–109) | 94 ± 5 (80–108) | 94 ± 5 (80–108) | 94 ± 5 (80–108) | 93 ± 5 (79–108) | 93 ± 6 (80–106) | 94 ± 6 (80–106) |
| Placebo | 93 ± 4 (77–109) | 101 ± 8 * (89–113) | 96 ± 3 (78–114) | 95 ± 3 (77–113) | 93 ± 3 (75–111) | 93 ± 3 (75–111) | 92 ± 3 (74–110) | 92 ± 3 (74–110) | 93 ± 3 (71–115) | |
| PP (mmHg) | Beet | 39 ± 8 (28–5) | 48 ± 8 (37–59) | 40 ± 7 (28–52) | 36 ± 11 (27–46) | 37 ± 9 (27–47) | 36 ± 9 (25–46) | 35 ± 7 (23–47) | 36 ± 6 (23–49) | 35 ± 7 (23–46) |
| Placebo | 39 ± 6 (26–52) | 49 ± 11 (39–58) | 41 ± 7 (29–52) | 38 ± 7 (26–50) | 38 ± 7 (24–50) | 37 ± 6 (24–50) | 38 ± 7 (26–50) | 38 ± 6 (26–49) | 36 ± 6 (22–50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamim, C.J.R.; de Sousa Júnior, F.W.; Porto, A.A.; Andrade, C.V.G.; de Figueiredo, M.Í.L.S.; Benjamim, C.J.R.; da Silva Rodrigues, G.; Rocha, E.M.B.; Cavalcante, T.F.; Garner, D.M.; et al. Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial. Int. J. Environ. Res. Public Health 2023, 20, 4019. https://doi.org/10.3390/ijerph20054019
Benjamim CJR, de Sousa Júnior FW, Porto AA, Andrade CVG, de Figueiredo MÍLS, Benjamim CJR, da Silva Rodrigues G, Rocha EMB, Cavalcante TF, Garner DM, et al. Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial. International Journal of Environmental Research and Public Health. 2023; 20(5):4019. https://doi.org/10.3390/ijerph20054019
Chicago/Turabian StyleBenjamim, Cicero Jonas R., Francisco Welington de Sousa Júnior, Andrey Alves Porto, Camila Venancia Guerra Andrade, Maria Íris L. Saraiva de Figueiredo, Cicera Josilânia R. Benjamim, Guilherme da Silva Rodrigues, Elida M. Braga Rocha, Taisy Ferro Cavalcante, David M. Garner, and et al. 2023. "Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial" International Journal of Environmental Research and Public Health 20, no. 5: 4019. https://doi.org/10.3390/ijerph20054019
APA StyleBenjamim, C. J. R., de Sousa Júnior, F. W., Porto, A. A., Andrade, C. V. G., de Figueiredo, M. Í. L. S., Benjamim, C. J. R., da Silva Rodrigues, G., Rocha, E. M. B., Cavalcante, T. F., Garner, D. M., Valenti, V. E., & Bueno Júnior, C. R. (2023). Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial. International Journal of Environmental Research and Public Health, 20(5), 4019. https://doi.org/10.3390/ijerph20054019








