Multidisciplinary Intensive Rehabilitation Program for People with Parkinson’s Disease: Gaps between the Clinic and Real-World Mobility
Abstract
1. Introduction
2. Materials and Methods
2.1. The Multidisciplinary Intensive Outpatient Rehabilitation (MIOR) Program
2.2. Clinical Evaluation in the Lab
2.3. Assessment of Daily-Living Walking
2.4. Qualitative Measurements of Daily-Living Gait
2.5. Statistical Analyses
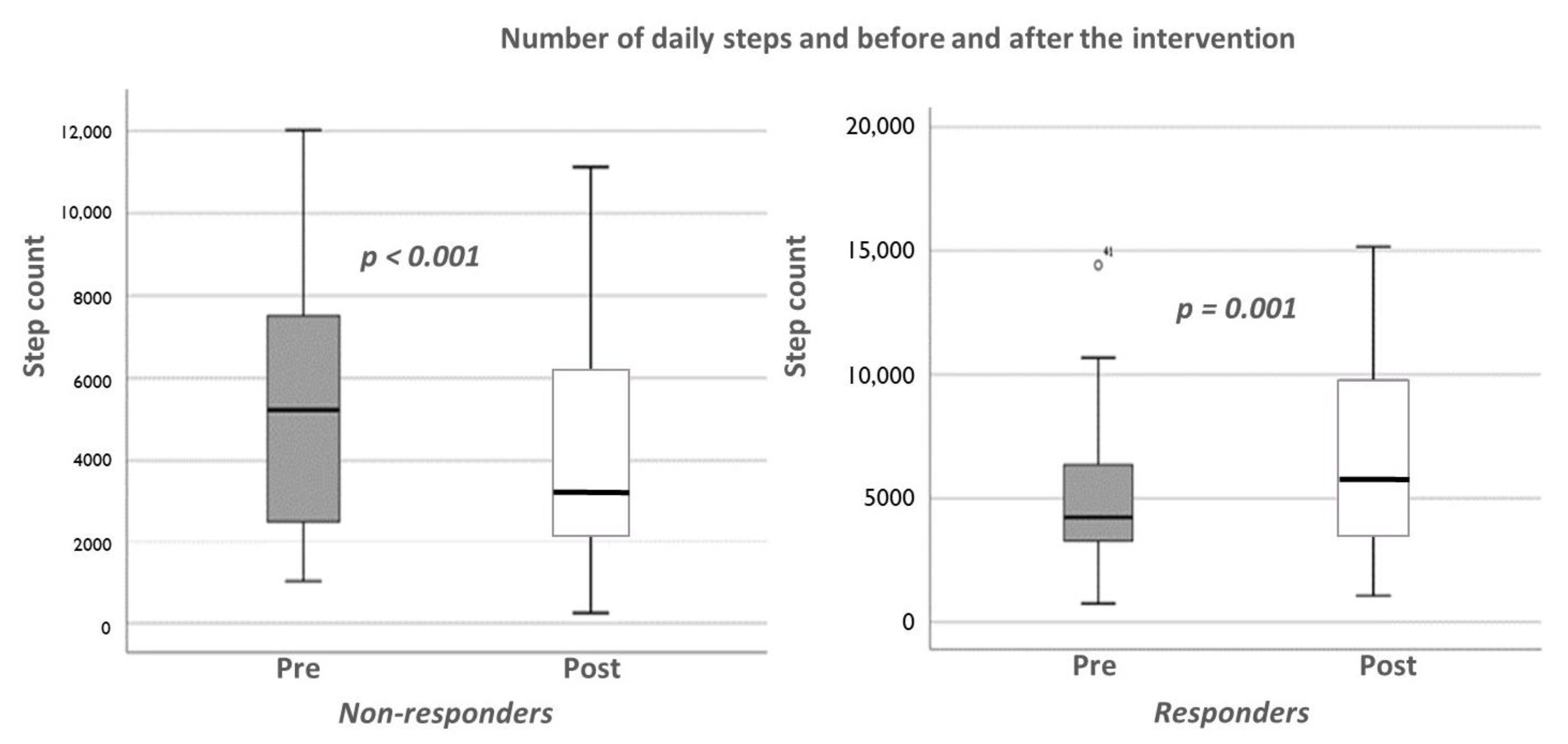
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frazzitta, G.; Maestri, R.; Bertotti, G.; Riboldazzi, G.; Boveri, N.; Perini, M.; Uccellini, D.; Turla, M.; Comi, C.; Pezzoli, G.; et al. Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil. Neural Repair 2015, 29, 123–131. [Google Scholar] [CrossRef]
- Fontanesi, C.; Kvint, S.; Frazzitta, G.; Bera, R.; Ferrazzoli, D.; Di, R.A.; Rebholz, H.; Friedman, E.; Pezzoli, G.; Quartarone, A.; et al. Intensive Rehabilitation Enhances Lymphocyte BDNF-TrkB Signaling in Patients With Parkinson’s Disease. Neurorehabil. Neural Repair 2016, 30, 411–418. [Google Scholar] [CrossRef]
- Frazzitta, G.; Bertotti, G.; Riboldazzi, G.; Turla, M.; Uccellini, D.; Boveri, N.; Guaglio, G.; Perini, M.; Comi, C.; Balbi, P.; et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: A randomized controlled trial with 1-year follow-up. Neurorehabil. Neural Repair 2012, 26, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Manor, Y.; Green, Y.; Tahel, G.; Badichi, I.; Ben-Or, G.; Shtainshlaifer, N.; Shiffer, A.; Gabso-Rajuan, M.; Kurtzman, H.; et al. Multidisciplinary intensive outpatient rehabilitation program for patients with moderate-to-advanced Parkinson’s disease. NeuroRehabilitation 2021, 49, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.; Berking, S.; Astalosch, M.; Grunheid, R.; Kuhn, A.A. Motor and non-motor improvements following short-term multidisciplinary day-clinic care in Parkinson’s disease. J. Neural Transm. 2022, 129, 1419–1426. [Google Scholar] [CrossRef]
- Strand, K.L.; Cherup, N.P.; Totillo, M.C.; Castillo, D.C.; Gabor, N.J.; Signorile, J.F. Periodized Resistance Training With and Without Functional Training Improves Functional Capacity, Balance, and Strength in Parkinson’s Disease. J. Strength Cond. Res. 2021, 35, 1611–1619. [Google Scholar] [CrossRef]
- Braz de Oliveira, M.P.; Maria Dos, R.L.; Pereira, N.D. Effect of Resistance Exercise on Body Structure and Function, Activity, and Participation in Individuals With Parkinson Disease: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.P.B.; Lobato, D.F.M.; Smaili, S.M.; Carvalho, C.; Borges, J.B.C. Effect of aerobic exercise on functional capacity and quality of life in individuals with Parkinson’s disease: A systematic review of randomized controlled trials. Arch. Gerontol. Geriatr. 2021, 95, 104422. [Google Scholar] [CrossRef] [PubMed]
- Ekker, M.S.; Janssen, S.; Nonnekes, J.; Bloem, B.R.; de Vries, N.M. Neurorehabilitation for Parkinson’s disease: Future perspectives for behavioural adaptation. Park. Relat. Disord. 2016, 22 (Suppl. 1), S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.Y.; Wong-Yu, I.S.K. Six-Month Community-Based Brisk Walking and Balance Exercise Alleviates Motor Symptoms and Promotes Functions in People with Parkinson’s Disease: A Randomized Controlled Trial. J. Parkinsons Dis. 2021, 11, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Cuignet, T.; Perchoux, C.; Caruso, G.; Klein, O.; Klein, S.; Chaix, B.; Kestens, Y.; Philippe, G. Mobility among older adults: Deconstructing the effects of motility and movement on wellbeing. Urban Stud. 2020, 57, 383–401. [Google Scholar] [CrossRef]
- Fillekes, M.P.; Giannouli, E.; Kim, E.K.; Zijlstra, W.; Weibel, R. Towards a comprehensive set of GPS-based indicators reflecting the multidimensional nature of daily mobility for applications in health and aging research. Int. J. Health Geogr. 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Atrsaei, A.; Hansen, C.; Elshehabi, M.; Solbrig, S.; Berg, D.; Liepelt-Scarfone, I.; Maetzler, W.; Aminian, K. Effect of Fear of Falling on Mobility Measured During Lab and Daily Activity Assessments in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 722830. [Google Scholar] [CrossRef] [PubMed]
- Del, D.S.; Galna, B.; Godfrey, A.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Mirelman, A.; Hausdorff, J.M.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 500–506. [Google Scholar] [CrossRef]
- Del, D.S.; Kirk, C.; Yarnall, A.J.; Rochester, L.; Hausdorff, J.M. Body-Worn Sensors for Remote Monitoring of Parkinson’s Disease Motor Symptoms: Vision, State of the Art, and Challenges Ahead. J. Parkinsons Dis. 2021, 11, S35–S47. [Google Scholar] [CrossRef]
- Elshehabi, M.; Del, D.S.; Hobert, M.A.; Warmerdam, E.; Sunkel, U.; Schmitz-Hubsch, T.; Behncke, L.M.; Heinzel, S.; Brockmann, K.; Metzger, F.G.; et al. Walking parameters of older adults from a lower back inertial measurement unit, a 6-year longitudinal observational study. Front. Aging Neurosci. 2022, 14, 789220. [Google Scholar] [CrossRef] [PubMed]
- Galperin, I.; Herman, T.; Assad, M.; Ganz, N.; Mirelman, A.; Giladi, N.; Hausdorff, J.M. Sensor-Based and Patient-Based Assessment of Daily-Living Physical Activity in People with Parkinson’s Disease: Do Motor Subtypes Play a Role? Sensors 2020, 20, 7015. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Hillel, I.; Shustak, S.; Del, D.S.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Rochester, L.; Mirelman, A. Everyday Stepping Quantity and Quality Among Older Adult Fallers With and Without Mild Cognitive Impairment: Initial Evidence for New Motor Markers of Cognitive Deficits? J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1078–1082. [Google Scholar] [CrossRef]
- Mirelman, A.; Giladi, N.; Hausdorff, J.M. Body-Fixed Sensors for Parkinson Disease. JAMA 2015, 314, 873–874. [Google Scholar] [CrossRef]
- Del, D.S.; Galna, B.; Lord, S.; Nieuwboer, A.; Bekkers, E.M.J.; Pelosin, E.; Avanzino, L.; Bloem, B.R.; Olde Rikkert, M.G.M.; Nieuwhof, F.; et al. Falls Risk in Relation to Activity Exposure in High-Risk Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1198–1205. [Google Scholar] [CrossRef]
- Manor, B.; Dagan, M.; Herman, T.; Gouskova, N.A.; Vanderhorst, V.G.; Giladi, N.; Travison, T.G.; Pascual-Leone, A.; Lipsitz, L.A.; Hausdorff, J.M. Multitarget Transcranial Electrical Stimulation for Freezing of Gait: A Randomized Controlled Trial. Mov. Disord. 2021, 36, 2693–2698. [Google Scholar] [CrossRef]
- Giannouli, E.; Bock, O.; Mellone, S.; Zijlstra, W. Mobility in Old Age: Capacity Is Not Performance. Biomed. Res. Int. 2016, 2016, 3261567. [Google Scholar] [CrossRef]
- Hillel, I.; Gazit, E.; Nieuwboer, A.; Avanzino, L.; Rochester, L.; Cereatti, A.; Croce, U.D.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur. Rev. Aging Phys. Act. 2019, 16, 6. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Hillel, I.; Mirelman, A.; Regev, K.; Hsieh, K.L.; Karni, A.; Devos, H.; Sosnoff, J.J.; Hausdorff, J.M. A wearable sensor identifies alterations in community ambulation in multiple sclerosis: Contributors to real-world gait quality and physical activity. J. Neurol. 2020, 267, 1912–1921. [Google Scholar] [CrossRef]
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Atrsaei, A.; Corra, M.F.; Dadashi, F.; Vila-Cha, N.; Maia, L.; Mariani, B.; Maetzler, W.; Aminian, K. Gait speed in clinical and daily living assessments in Parkinson’s disease patients: Performance versus capacity. NPJ Parkinsons Dis. 2021, 7, 24. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Lei, H.; Parvaneh, S.; Sherman, S.; Najafi, B. Motor Performance Assessment in Parkinson’s Disease: Association between Objective In-Clinic, Objective In-Home, and Subjective/Semi-Objective Measures. PLoS ONE 2015, 10, e0124763. [Google Scholar] [CrossRef] [PubMed]
- Ramig, L.O.; Sapir, S.; Countryman, S.; Pawlas, A.A.; O'Brien, C.; Hoehn, M.; Thompson, L.L. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: A 2 year follow up. J. Neurol. Neurosurg. Psychiatry 2001, 71, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Salomon, A.; Galperin, I.; Buzaglo, D.; Mirelman, A.; Regev, K.; Karni, A.; Schmitz-Hubsch, T.; Paul, F.; Devos, H.; Sosnoff, J.J.; et al. Fragmentation, circadian amplitude, and fractal pattern of daily-living physical activity in people with multiple sclerosis: Is there relevant information beyond the total amount of physical activity? Mult. Scler. Relat. Disord. 2022, 681, 04108. [Google Scholar] [CrossRef] [PubMed]
- Oveisgharan, S.; Dawe, R.J.; Leurgans, S.E.; Yu, L.; Schneider, J.A.; Bennett, D.A.; Buchman, A.S. Total daily physical activity, brain pathologies, and parkinsonism in older adults. PLoS ONE 2020, 15, e0232404. [Google Scholar] [CrossRef]
- Galperin, I.; Hillel, I.; Del, D.S.; Bekkers, E.M.J.; Nieuwboer, A.; Abbruzzese, G.; Avanzino, L.; Nieuwhof, F.; Bloem, B.R.; Rochester, L.; et al. Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson’s disease. Park. Relat. Disord. 2019, 62, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Slaght, J.; Senechal, M.; Hrubeniuk, T.J.; Mayo, A.; Bouchard, D.R. Walking Cadence to Exercise at Moderate Intensity for Adults: A Systematic Review. J. Sport. Med. 2017, 2017, 4641203. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Jansen, C.P.; Toosizadeh, N.; Mohler, M.J.; Najafi, B.; Wendel, C.; Schwenk, M. The association between motor capacity and mobility performance: Frailty as a moderator. Eur. Rev. Aging Phys. Act. 2019, 16, 16. [Google Scholar] [CrossRef]
- Roth, N.; Wieland, G.P.; Kuderle, A.; Ullrich, M.; Gladow, T.; Marxreiter, F.; Klucken, J.; Eskofier, B.M.; Kluge, F. Do We Walk Differently at Home? A Context-Aware Gait Analysis System in Continuous Real-World Environments. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 1932–1935. [Google Scholar] [PubMed]
- Weiss, A.; Sharifi, S.; Plotnik, M.; van Vugt, J.P.; Giladi, N.; Hausdorff, J.M. Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil. Neural Repair 2011, 25, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Tuijt, R.; Read, J.; Pigott, J.; Davies, N.; Manthorpe, J.; Frost, R.; Schrag, A.; Walters, K. Health care professionals' perspectives on self-management for people with Parkinson’s: Qualitative findings from a UK study. BMC Geriatr. 2021, 21, 706. [Google Scholar] [CrossRef]
- Tuijt, R.; Tan, A.; Armstrong, M.; Pigott, J.; Read, J.; Davies, N.; Walters, K.; Schrag, A. Self-Management Components as Experienced by People with Parkinson’s Disease and Their Carers: A Systematic Review and Synthesis of the Qualitative Literature. Park. Dis. 2020, 2020, 8857385. [Google Scholar] [CrossRef]
- Shah, R.; Read, J.; Davies, N.; Nimmons, D.; Pigott, J.; Schrag, A.; Walters, K.; Armstrong, M. People with Parkinson’s perspectives and experiences of self-management: Qualitative findings from a UK study. PLoS ONE 2022, 17, e0273428. [Google Scholar] [CrossRef]
- Weiss, A.; Brozgol, M.; Dorfman, M.; Herman, T.; Shema, S.; Giladi, N.; Hausdorff, J.M. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recording. Neurorehabil. Neural Repair 2013, 27, 742–752. [Google Scholar] [CrossRef] [PubMed]
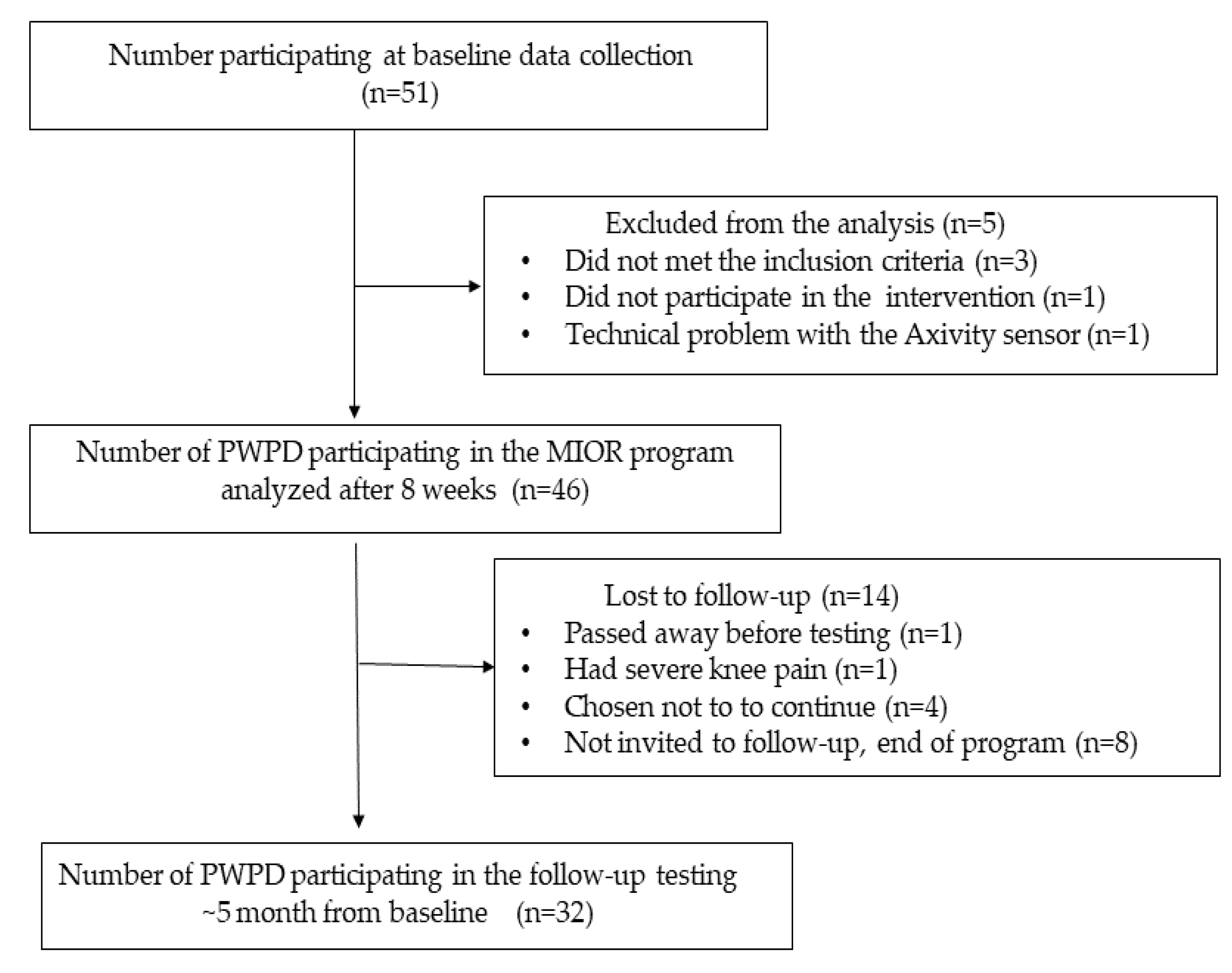
| Variable | n = 46 |
|---|---|
| Age (years) | 70.1 ± 7.8 |
| Gender (n, % male) | 31 (67.4) |
| Height (cm) | 168.8 ± 8.1 |
| Weight (kg) | 73.83 ± 13.99 |
| BMI (kg/m2) | 25.8 ± 3.9 |
| Disease duration (years) | 9.2 ± 6.2 |
| Hoen and Yahr stage number (%) Stage 2 27 (58.7) Stage 2.5 5 (10.9) Stage 3 12 (26.1) Stage 4 1 (2.2) |
| Pre-Intervention | Post-Intervention | p-Value | |
|---|---|---|---|
| PD Related Symptoms | |||
| MDS-UPDRS III | 29.76 (±13.37) | 29.07 (±12.19) | 0.500 |
| LEDD (Mg/d) | 602.71 (±303.39) | 605.87 (±301.44) | 0.622 |
| NFOG-Q (total) | 10.50 [0.00–20.25] | 6.00 [0.00–17.00] | 0.007 |
| Gait and Balance | |||
| Gait Speed (m/s) | 1.14 (±3.2) | 1.21 (±2.8) | 0.101 |
| Gait Speed DT (m/s) | 0.91 (±2.8) | 0.99 (±2.4) | 0.016 |
| 10MWT (sec) | 8.67 [7.78–10.62] | 8.45 [6.95–10.05] | 0.034 |
| 10MWT_DT (sec) | 10.65 [9.10–13.60] | 10.28 [8.70–12.00] | 0.040 |
| MiniBEST | 19.00 [15.00–21.25] | 21.00 [17.00–24.00] | <0.001 |
| TUG (sec) | 11.54 [8.82–15.00] | 10.30 [7.84–12.45] | 0.002 |
| TUG_DT (sec) | 16.87 [11.84–24.28] | 14.27 [11.13–18.98] | 0.020 |
| 30 Seconds Sit To Stand (Repetitions) | 9.50 [7.00–11.25] | 11.00 [7.75–12.25] | 0.006 |
| 6MWT (Meters) | 361.22 (±120.42) | 406.83 (±11.58) | <0.001 |
| Falls (Number/Month) | 0.00 [0.00–0.20] | 0.00 [0.00–0.00] | 0.134 |
| Cognitive and mental health measurements | |||
| MoCA | 22.20 (±3.78) | 23.80 (±3.83) | <0.001 |
| FES-I | 24.50 [21.00–35.00] | 25.00 [19.75–32.25] | 0.159 |
| GDS | 4.50 [1.00–5.00] | 2.00 [0.00–14.00] | 0.005 |
| SF-12 (health) | 13.70 (±2.46) | 14.33 (±2.21) | 0.065 |
| SF-12 (mental) | 20.00 [17.00–23.00] | 20.00 [17.00–22.25] | 0.552 |
| SF-12 (total) | 33.39 (±5.37) | 34.20 (±5.13) | 0.185 |
| Variable | Pre-Intervention | At 3-Months Follow-Up | p-Value |
|---|---|---|---|
| NFOG-Q (total) | 17.50 [0.00–21.75] | 8.50 [0.0–19.75] | 0.014 |
| Dynamic balance and walking endurance | |||
| Gait Speed DT (m/s) ** | 0.92 (±2.8) | 1.05 (±2.3) | 0.004 |
| 10MWT (sec) * | 8.67 [7.66–10.95] | 8.12 [6.60–9.59] | 0.016 |
| 10MWT_DT (sec) ** | 10.55 [9.02–13.60] | 9.56 [8.10–11.80] | 0.007 |
| MiniBEST * | 18.26 (±4.70) | 20.03 (±4.26) | 0.018 |
| TUG (sec) * | 12.70 [9.16–15.00] | 9.87 [8.40–12.20] | 0.001 |
| TUG_DT (sec) * | 18.10 [12.60–24.43] | 15.30 [9.79–19.22] | 0.004 |
| 30 Seconds Sit To Stand (Repetitions) | 8.94 (±3.43) | 9.72 (±4.66) | 0.170 |
| 6MWT (Meters) | 340.50 (±117.51) | 387.25 (±133.39) | 0.012 |
| General cognition and mental health | |||
| MoCA *** | 22.58 (±3.51) | 22.96 (±3.95) | 0.563 |
| Geriatric Depression Scale | 5.00 [1.25–8.00] | 3.50 [2.00–6.00] | 0.212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, M.; Herman, T.; Ganz, N.; Badichi, I.; Gurevich, T.; Hausdorff, J.M. Multidisciplinary Intensive Rehabilitation Program for People with Parkinson’s Disease: Gaps between the Clinic and Real-World Mobility. Int. J. Environ. Res. Public Health 2023, 20, 3806. https://doi.org/10.3390/ijerph20053806
Cohen M, Herman T, Ganz N, Badichi I, Gurevich T, Hausdorff JM. Multidisciplinary Intensive Rehabilitation Program for People with Parkinson’s Disease: Gaps between the Clinic and Real-World Mobility. International Journal of Environmental Research and Public Health. 2023; 20(5):3806. https://doi.org/10.3390/ijerph20053806
Chicago/Turabian StyleCohen, Moriya, Talia Herman, Natalie Ganz, Inbal Badichi, Tanya Gurevich, and Jeffrey M. Hausdorff. 2023. "Multidisciplinary Intensive Rehabilitation Program for People with Parkinson’s Disease: Gaps between the Clinic and Real-World Mobility" International Journal of Environmental Research and Public Health 20, no. 5: 3806. https://doi.org/10.3390/ijerph20053806
APA StyleCohen, M., Herman, T., Ganz, N., Badichi, I., Gurevich, T., & Hausdorff, J. M. (2023). Multidisciplinary Intensive Rehabilitation Program for People with Parkinson’s Disease: Gaps between the Clinic and Real-World Mobility. International Journal of Environmental Research and Public Health, 20(5), 3806. https://doi.org/10.3390/ijerph20053806







