Sex Differences in Multimorbidity, Inappropriate Medication and Adverse Outcomes of Inpatient Care: MoPIM Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Data Acquisition and Variables
2.3. Sampling and Analysis
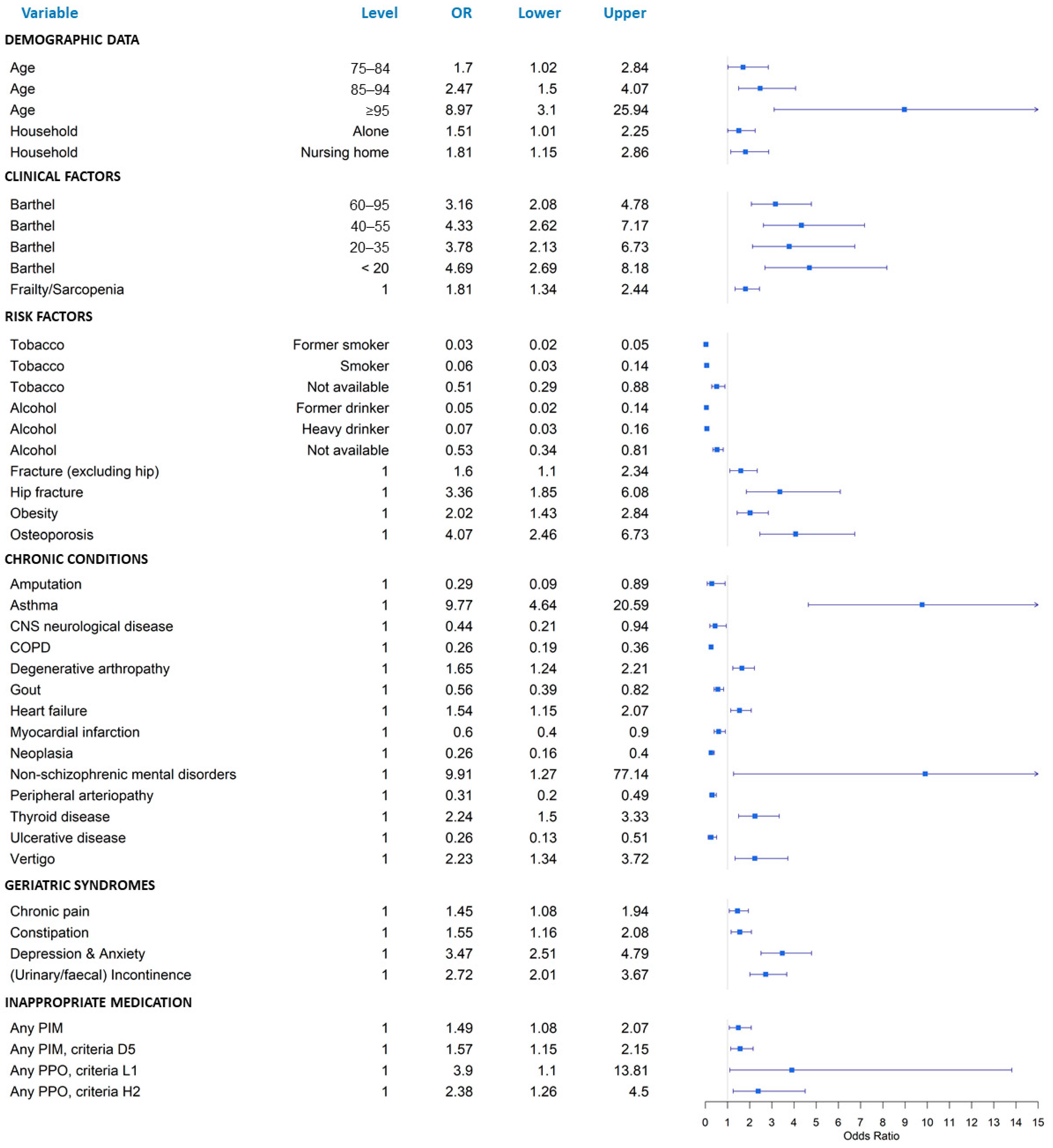
3. Results
4. Discussion
4.1. Main Important Results and Novelty
4.2. Comparing with Other Studies
4.3. Clinical Interpretation of Results
4.4. Possible Clinical Implications
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat Statistics. European Commission. Available online: https://ec.europa.eu/eurostat/databrowser/view/tps00028/default/table?lang=en (accessed on 12 July 2022).
- Bolte, G.; Jacke, K.; Groth, K.; Kraus, U.; Dandolo, L.; Fiedel, L.; Debiak, M.; Kolossa-Gehring, M.; Schneider, A.; Palm, K. Integrating Sex/Gender into Environmental Health Research: Development of a Conceptual Framework. Int. J. Environ. Res. Public Health 2021, 18, 12118. [Google Scholar] [CrossRef]
- Greaves, L.; Ritz, S.A. Sex, Gender and Health: Mapping the Landscape of Research and Policy. Int. J. Environ. Res. Public Health 2022, 19, 2563. [Google Scholar] [CrossRef] [PubMed]
- Avoidable Mortality (Preventable and Treatable). Health at a Glance 2019: OECD Indicators. Available online: https://www.oecd-ilibrary.org/sites/3b4fdbf2-en/index.html?itemId=/content/component/3b4fdbf2-en (accessed on 27 January 2023).
- Ahrenfeldt, L.J.; Möller, S.; Thinggaard, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex Differences in Comorbidity and Frailty in Europe. Int. J. Public Health 2019, 64, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, D.; Moseley, P.; Sørup, F.K.H.; Baldi, P.; Brunak, S. Population-wide analysis of differences in disease progression patterns in men and women. Nat. Commun. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Baré, M.; Herranz, S.; Roso-Llorach, A.; Jordana, R.; Violán, C.; Lleal, M.; Roura-Poch, P.; Arellano, M.; Estrada, R.; Nazco, G.J.; et al. Multimorbidity patterns of chronic conditions and geriatric syndromes in older patients from the MoPIM multicentre cohort study. BMJ Open 2021, 11, e049334. [Google Scholar] [CrossRef]
- Zacarías-Pons, L.; Vilalta-Franch, J.; Turró-Garriga, O.; Saez, M.; Garre-Olmo, J. Multimorbidity patterns and their related characteristics in European older adults: A longitudinal perspective. Arch. Gerontol. Geriatr. 2021, 95, 104428. [Google Scholar] [CrossRef] [PubMed]
- Beil, M.; Flaatten, H.; Guidet, B.; Sviri, S.; Jung, C.; de Lange, D.; Leaver, S.; Fjølner, J.; Szczeklik, W.; van Heerden, P.V. The management of multi-morbidity in elderly patients: Ready yet for precision medicine in intensive care? Crit. Care 2021, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.J.; Carrillo, I.; Pérez-Pérez, P.; Astier-Peña, M.P.; Caro-Mendivelso, J.; Olivera, G.; Silvestre, C.; Nuín, M.A.; Aranaz-Andrés, J.M.; on behalf the SOBRINA Research Team. Avoidable Adverse Events Related to Ignoring the Do-Not-Do Recommendations: A Retrospective Cohort Study Conducted in the Spanish Primary Care Setting. J. Patient Saf. 2021, 17, e858–e865. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.J.; Mondor, L.; Koné, A.J.P.; Hogan, D.B.; Wodchis, W.P. Sex differences in multimorbidity and polypharmacy trends: A repeated cross-sectional study of older adults in Ontario, Canada. PLoS ONE 2021, 16, e0250567. [Google Scholar] [CrossRef]
- Almagro, P.; Ponce, A.; Komal, S.; Villaverde, M.D.L.A.; Castrillo, C.; Grau, G.; Simon, L.; De La Sierra, A. Multimorbidity gender patterns in hospitalized elderly patients. PLoS ONE 2020, 15, e0227252. [Google Scholar] [CrossRef] [PubMed]
- Agur, K.; McLean, G.; Hunt, K.; Guthrie, B.; Mercer, S.W. How Does Sex Influence Multimorbidity? Secondary Analysis of a Large Nationally Representative Dataset. Int. J. Environ. Res. Public Health 2016, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.G.; Weymann, D.; Pratt, B.; Smolina, K.; Gladstone, E.J.; Raymond, C.; Mintzes, B. Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age Ageing 2016, 45, 535–542. [Google Scholar] [CrossRef] [PubMed]
- A Rochon, P.; Mason, R.; Gurwitz, J.H. Increasing the visibility of older women in clinical research. Lancet 2020, 395, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- NICE. Multimorbidity: Clinical Assessment and Management. Guideline; NICE 2016; NICE: London, UK, 2016; ISBN 978-1-4731-2065-5. Available online: www.nice.org.uk/guidance/ng56 (accessed on 10 July 2022).
- Onder, G.; Vetrano, D.L.; Palmer, K.; Trevisan, C.; Amato, L.; Berti, F.; Campomori, A.; Catalano, L.; Corsonello, A.; Kruger, P.; et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin. Exp. Res. 2022, 34, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Muth, C.; Blom, J.W.; Smith, S.M.; Johnell, K.; Gonzalez-Gonzalez, A.I.; Nguyen, T.S.; Brueckle, M.-S.; Cesari, M.; Tinetti, M.E.; Valderas, J.M. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: A systematic guideline review and expert consensus. J. Intern. Med. 2019, 285, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wallace, E.; O’Dowd, T.; Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 2021, 2021, CD006560. [Google Scholar] [CrossRef] [PubMed]
- Baré, M.; Herranz, S.; Jordana, R.; Gorgas, M.Q.; Ortonobes, S.; Sevilla, D.; De Jaime, E.; Ibarra, O.; Martín, C.; MoPIM study group. Multimorbidity patterns in chronic older patients, potentially inappropriate prescribing and adverse drug reactions: Protocol of the multicentre prospective cohort study MoPIM. BMJ Open 2020, 10, e033322. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Amblàs-Novellas, J.; Martori, J.C.; Espaulella, J.; Oller, R.; Molist-Brunet, N.; Inzitari, M.; Romero-Ortuno, R. Frail-VIG index: A concise frailty evaluation tool for rapid geriatric assessment. BMC Geriatr. 2018, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, C.; Johnson, L.; Purdy, S.; Valderas, J.M.; A Montgomery, A. Epidemiology and impact of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2011, 61, e12–e21. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Baré, M.; Lleal, M.; Ortonobes, S.; Gorgas, M.Q.; Sevilla-Sánchez, D.; Carballo, N.; De Jaime, E.; Herranz, S.; MoPIM study group. Factors associated to potentially inappropriate prescribing in older patients according to STOPP/START criteria: MoPIM multicentre cohort study. BMC Geriatr. 2022, 22, 44. [Google Scholar] [CrossRef]
- WHO Centre for Health Development (Kobe, Japan). A Glossary of Terms for Community Health Care and Services for Older Persons; WHO Centre for Health Development: Kobe, Japan, 2004; Available online: https://apps.who.int/iris/handle/10665/68896 (accessed on 6 July 2022).
- European Medicines Agency. Note for Guidance on Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. EMEA, 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf (accessed on 26 September 2022).
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.; Barthel, D. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a Continuous Graph Layout Algorithm for Handy Network Visualization Designed for the Gephi Software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009; Available online: https://ojs.aaai.org/index.php/ICWSM/article/view/13937 (accessed on 12 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 10 November 2021).
- Ho, I.S.-S.; Azcoaga-Lorenzo, A.; Akbari, A.; Davies, J.; Hodgins, P.; Khunti, K.; Kadam, U.; Lyons, R.; McCowan, C.; Mercer, S.W.; et al. Variation in the estimated prevalence of multimorbidity: Systematic review and meta-analysis of 193 international studies. BMJ Open 2022, 12, e057017. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Mair, F.S.; Fortin, M.; Guthrie, B.; Nunes, B.P.; Miranda, J.J.; Boyd, C.M.; Pati, S.; Mtenga, S.; Smith, S.M. Multimorbidity. Nat. Rev. Dis. Prim. 2022, 8, 48. [Google Scholar] [CrossRef]
- Robertson, L.; Vieira, R.; Butler, J.; Johnston, M.; Sawhney, S.; Black, C. Identifying multimorbidity clusters in an unselected population of hospitalised patients. Sci. Rep. 2022, 12, 5134. [Google Scholar] [CrossRef]
- Toepfer, S.; Bolbrinker, J.; König, M.; Steinhagen-Thiessen, E.; Kreutz, R.; DeMuth, I. Potentially inappropriate medication in older participants of the Berlin Aging Study II (BASE-II)—Sex differences and associations with morbidity and medication use. PLoS ONE 2019, 14, e0226511. [Google Scholar] [CrossRef]
- Toepfer, S.; König, M.; Spira, D.; Drewelies, J.; Kreutz, R.; Bolbrinker, J.; Demuth, I. Sex Differences in Characteristics Associated with Potentially Inappropriate Medication Use and Associations with Functional Capacity in Older Participants of the Berlin Aging Study II. Gerontology 2021, 68, 664–672. [Google Scholar] [CrossRef]
- Delara, M.; Murray, L.; Jafari, B.; Bahji, A.; Goodarzi, Z.; Kirkham, J.; Chowdhury, M.; Seitz, D.P. Prevalence and factors associated with polypharmacy: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 601. [Google Scholar] [CrossRef]
- Muhlack, D.C.; Hoppe, L.K.; Stock, C.; Haefeli, W.E.; Brenner, H.; Schöttker, B. The associations of geriatric syndromes and other patient characteristics with the current and future use of potentially inappropriate medications in a large cohort study. Eur. J. Clin. Pharmacol. 2018, 74, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Johnell, K.; Weitoft, G.R.; Fastbom, J. Sex Differences in Inappropriate Drug Use: A Register-Based Study of Over 600,000 Older People. Ann. Pharmacother. 2009, 43, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Tazzeo, C.; Rizzuto, D.; Calderón-Larrañaga, A.; Roso-Llorach, A.; Marengoni, A.; Welmer, A.-K.; Onder, G.; Trevisan, C.; Vetrano, D.L. Multimorbidity patterns and risk of frailty in older community-dwelling adults: A population-based cohort study. Age Ageing 2021, 50, 2183–2191. [Google Scholar] [CrossRef]
- Díaz-Gutiérrez, M.J.; Martínez-Cengotitabengoa, M.; de Adana, E.S.; Cano, A.I.; Martínez-Cengotitabengoa, M.T.; Besga, A.; Segarra, R.; González-Pinto, A. Relationship between the use of benzodiazepines and falls in older adults: A systematic review. Maturitas 2017, 101, 17–22. [Google Scholar] [CrossRef]
- Alhawassi, T.M.; Krass, I.; Bajorek, B.V.; Pont, L.G. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 2014, 9, 2079–2086. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Madla, C.M.; Gavins, F.K.; Merchant, H.A.; Orlu, M.; Murdan, S.; Basit, A.W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv. Rev. 2021, 175, 113804. [Google Scholar] [CrossRef]
- Ng, S.K.; Tawiah, R.; Sawyer, M.; Scuffham, P. Patterns of multimorbid health conditions: A systematic review of analytical methods and comparison analysis. Leuk. Res. 2018, 47, 1687–1704. [Google Scholar] [CrossRef]
- Jennings, E.L.M.; Murphy, K.D.; Gallagher, P.; O’Mahony, D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs—A systematic review and meta-analysis. Age Ageing 2020, 49, 948–958. [Google Scholar] [CrossRef]
- Robinson, E.G.; Hedna, K.; Hakkarainen, K.M.; Gyllensten, H. Healthcare costs of adverse drug reactions and potentially inappropriate prescribing in older adults: A population-based study. BMJ Open 2022, 12, e062589. [Google Scholar] [CrossRef] [PubMed]

| Variable | Level | Total Cohort | 65–84 Years | ≥85 Years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p-Value | Female | Male | p-Value | Female | Male | p-Value | ||
| Total | 394 | 346 | 157 | 187 | 237 | 159 | ||||
| SOCIODEMOGRAPHIC | ||||||||||
| Age | <75 | 29 (7.4) | 52 (15.0) | <0.001 | 29 (18.5) | 52 (27.8) | - | - | - | - |
| 75–84 | 128 (32.5) | 135 (39.0) | 128 (81.5) | 135 (72.2) | - | - | ||||
| 85–94 | 212 (53.8) | 154 (44.5) | - | - | 212 (89.5) | 154 (96.9) | ||||
| ≥95 | 25 (6.4) | 5 (1.5) | - | - | 25 (10.6) | 5 (3.1) | ||||
| Household | Alone | 73 (18.5) | 49 (14.2) | 0.009 | 35 (22.3) | 29 (15.5) | 0.272 | 38 (16.0) | 20 (12.6) | 0.019 |
| Nursing home | 61 (15.5) | 34 (9.8) | 12 (7.6) | 16 (8.6) | 49 (20.7) | 18 (11.3) | ||||
| With relatives/other people | 260 (66.0) | 263 (76.0) | 110 (70.1) | 142 (75.9) | 150 (63.3) | 121 (76.1) | ||||
| CLINICAL AND RISK FACTORS | ||||||||||
| Barthel | <20 | 59 (15.0) | 31 (9.0) | <0.001 | 18 (11.5) | 16 (8.6) | 0.001 | 41 (17.3) | 15 (9.4) | <0.001 |
| 20–35 | 46 (11.7) | 30 (8.7) | 10 (6.4) | 14 (7.5) | 36 (15.2) | 16 (10.1) | ||||
| 40–55 | 79 (20.1) | 45 (13.0) | 30 (19.1) | 22 (11.8) | 49 (20.7) | 23 (14.5) | ||||
| 60–95 | 165 (41.9) | 129 (37.3) | 66 (42.0) | 56 (30.0) | 99 (41.8) | 73 (45.9) | ||||
| 100 | 45 (11.4) | 111 (32.1) | 33 (21.0) | 79 (42.3) | 12 (5.1) | 32 (20.1) | ||||
| Frailty | No | 125 (31.7) | 158 (45.7) | <0.001 | 59 (37.6) | 98 (52.4) | 0.008 | 66 (27.9) | 60 (37.7) | 0.05 |
| Yes | 269 (68.3) | 188 (54.3) | 98 (62.4) | 89 (47.6) | 171 (72.2) | 99 (62.3) | ||||
| Charlson | 2–5 | 86 (21.8) | 62 (17.9) | 0.271 | 42 (26.8) | 44 (23.5) | 0.781 | 44 (18.6) | 18 (11.3) | 0.089 |
| 6–8 | 219 (55.6) | 192 (55.5) | 79 (50.3) | 97 (51.9) | 140 (59.1) | 95 (59.8) | ||||
| 9–14 | 89 (22.6) | 92 (26.6) | 36 (22.9) | 46 (24.6) | 53 (22.4) | 46 (28.9) | ||||
| Tobacco | Non-smoker | 325 (82.5) | 92 (26.6) | <0.001 | 133 (84.7) | 37 (19.8) | <0.001 | 192 (81.0) | 55 (34.6) | <0.001 |
| Former smoker | 19 (4.8) | 197 (56.9) | 9 (5.7) | 114 (61.0) | 10 (4.2) | 83 (52.2) | ||||
| Smoker | 7 (1.8) | 33 (9.5) | 5 (3.2) | 25 (13.4) | 2 (0.8) | 8 (5.0) | ||||
| Not available | 43 (10.9) | 24 (6.9) | 10 (6.4) | 11 (5.9) | 33 (13.9) | 13 (8.2) | ||||
| Alcohol | Non-drinker | 338 (85.8) | 197 (56.9) | <0.001 | 137 (87.3) | 87 (46.5) | <0.001 | 201 (84.8) | 110 (69.2) | <0.001 |
| Former drinker | 4 (1.0) | 47 (13.6) | 2 (1.3) | 35 (18.7) | 2 (0.8) | 12 (7.6) | ||||
| Heavy drinker | 6 (1.5) | 51 (14.7) | 5 (3.2) | 41 (21.9) | 1 (0.4) | 10 (6.3) | ||||
| Not available | 46 (11.7) | 51 (14.7) | 13 (8.3) | 24 (12.8) | 33 (13.9) | 27 (17.0) | ||||
| Prior exacerbation | No | 126 (32.0) | 99 (28.6) | 0.361 | 40 (25.5) | 50 (26.7) | 0.887 | 86 (36.3) | 49 (30.8) | 0.309 |
| Yes (total) | 268 (68.0) | 247 (71.4) | 117 (74.5) | 137 (73.3) | 151 (63.7) | 110 (69.2) | ||||
| INAPPROPRIATE MEDICATION | ||||||||||
| Polypharmacy | Oligopharmacy (0–4) | 28 (7.1) | 18 (5.2) | 0.393 | 5 (3.2) | 9 (4.8) | 0.27 | 23 (9.7) | 9 (5.7) | 0.265 |
| Moderate polypharmacy (5–9) | 142 (36.0) | 117 (33.8) | 52 (33.1) | 48 (25.7) | 90 (38.0) | 69 (43.4) | ||||
| Excessive polypharmacy (≥10) | 224 (56.9) | 211 (61.0) | 100 (63.7) | 130 (69.5) | 124 (52.3) | 81 (50.9) | ||||
| Any PIM | No | 91 (23.1) | 107 (30.9) | 0.021 | 38 (24.2) | 58 (31.0) | 0.2 | 53 (22.4) | 49 (30.8) | 0.077 |
| Yes | 303 (76.9) | 239 (69.1) | 119 (75.8) | 129 (69.0) | 184 (77.6) | 110 (69.2) | ||||
| Any PPO (no vaccines) | No | 252 (64.0) | 225 (65.0) | 0.821 | 104 (66.2) | 131 (70.1) | 0.522 | 148 (62.5) | 94 (59.1) | 0.575 |
| Yes | 142 (36.0) | 121 (35.0) | 53 (33.8) | 56 (30.0) | 89 (37.6) | 65 (40.9) | ||||
| ADVERSE OUTCOMES | ||||||||||
| Length of stay | - | 11 (7–17) | 11.5 (8–17) | 0.146 | 13 (8–20) | 13 (9–20.5) | 0.763 | 9 (7–15) | 10 (7–14.5) | 0.642 |
| Nursing home as destination at discharge * | No | 294 (82.6) | 275 (86.5) | 0.199 | 123 (89.1) | 146 (85.9) | 0.496 | 171 (78.4) | 129 (87.2) | 0.046 |
| Yes | 62 (17.4) | 43 (13.5) | 15 (10.9) | 24 (14.1) | 47 (21.6) | 19 (12.8) | ||||
| In-hospital mortality | No | 356 (90.4) | 318 (91.9) | 0.542 | 138 (87.9) | 170 (90.9) | 0.464 | 218 (92.0) | 148 (93.1) | 0.832 |
| Yes | 38 (9.6) | 28 (8.1) | 19 (12.1) | 17 (9.1) | 19 (8.0) | 11 (6.9) | ||||
| Cause of in-hospital mortality ** | Treatment complication | 0 (0) | 1 (3.9) | 0.388 | 0 (0) | 1 (6.7) | 0.501 | 0 (0) | 0 (0) | 1.000 |
| Diseaseexacerbation | 32 (86.5) | 23 (88.5) | 16 (88.9) | 13 (86.7) | 16 (84.2) | 10 (90.9) | ||||
| Other cause | 5 (13.5) | 2 (7.7) | 2 (11.1) | 1 (6.7) | 3 (15.8) | 1 (9.1) | ||||
| Any adverse drug reaction (ADR) | No | 262 (66.5) | 233 (67.3) | 0.869 | 98 (62.4) | 122 (65.2) | 0.667 | 164 (69.2) | 111 (69.8) | 0.985 |
| Yes | 132 (33.5) | 113 (32.7) | 59 (37.6) | 65 (34.8) | 73 (30.8) | 48 (30.2) | ||||
| ADR at admission (n = 153) | No | 47 (35.6) | 45 (39.8) | 0.511 | 20 (33.9) | 28 (43.1) | 0.357 | 27 (37.0) | 17 (35.4) | 1.000 |
| Yes | 85 (64.4) | 68 (60.2) | 39 (66.1) | 37 (56.9) | 46 (63.0) | 31 (64.6) | ||||
| New ADR during hospitalization (n = 120) | No | 69 (52.3) | 56 (49.6) | 0.702 | 32 (54.2) | 30 (46.2) | 0.472 | 37 (50.7) | 26 (54.2) | 0.715 |
| Yes | 63 (47.7) | 57 (50.4) | 27 (45.7) | 35 (53.9) | 36 (49.3) | 22 (45.8) | ||||
| Worst consequence of ADR during hospitalization | Life-threatening | 9 (14.3) | 3 (5.3) | 0.240 | 5 (18.5) | 2 (5.7) | 0.214 | 4 (11.1) | 1 (4.5) | 0.340 |
| Lengthening of stay | 26 (41.3) | 28 (49.1) | 13 (48.1) | 16 (45.7) | 13 (36.1) | 12 (54.6) | ||||
| Other clinically imp. | 28 (44.4) | 26 (45.6) | 9 (33.3) | 17 (48.6) | 19 (52.8) | 9 (40.9) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baré, M.; Lleal, M.; Sevilla-Sánchez, D.; Ortonobes, S.; Herranz, S.; Ferrandez, O.; Corral-Vázquez, C.; Molist, N.; Nazco, G.J.; Martín-González, C.; et al. Sex Differences in Multimorbidity, Inappropriate Medication and Adverse Outcomes of Inpatient Care: MoPIM Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3639. https://doi.org/10.3390/ijerph20043639
Baré M, Lleal M, Sevilla-Sánchez D, Ortonobes S, Herranz S, Ferrandez O, Corral-Vázquez C, Molist N, Nazco GJ, Martín-González C, et al. Sex Differences in Multimorbidity, Inappropriate Medication and Adverse Outcomes of Inpatient Care: MoPIM Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3639. https://doi.org/10.3390/ijerph20043639
Chicago/Turabian StyleBaré, Marisa, Marina Lleal, Daniel Sevilla-Sánchez, Sara Ortonobes, Susana Herranz, Olivia Ferrandez, Celia Corral-Vázquez, Núria Molist, Gloria Julia Nazco, Candelaria Martín-González, and et al. 2023. "Sex Differences in Multimorbidity, Inappropriate Medication and Adverse Outcomes of Inpatient Care: MoPIM Cohort Study" International Journal of Environmental Research and Public Health 20, no. 4: 3639. https://doi.org/10.3390/ijerph20043639
APA StyleBaré, M., Lleal, M., Sevilla-Sánchez, D., Ortonobes, S., Herranz, S., Ferrandez, O., Corral-Vázquez, C., Molist, N., Nazco, G. J., Martín-González, C., Márquez, M. Á., & on behalf of the MoPIM Study Group. (2023). Sex Differences in Multimorbidity, Inappropriate Medication and Adverse Outcomes of Inpatient Care: MoPIM Cohort Study. International Journal of Environmental Research and Public Health, 20(4), 3639. https://doi.org/10.3390/ijerph20043639








