Abstract
Multimorbidity is increasing and poses a challenge to the clinical management of patients with multiple conditions and drug prescriptions. The objectives of this work are to evaluate if multimorbidity patterns are associated with quality indicators of medication: potentially inappropriate prescribing (PIP) or adverse drug reactions (ADRs). A multicentre prospective cohort study was conducted including 740 older (≥65 years) patients hospitalised due to chronic pathology exacerbation. Sociodemographic, clinical and medication related variables (polypharmacy, PIP according to STOPP/START criteria, ADRs) were collected. Bivariate analyses were performed comparing previously identified multimorbidity clusters (osteoarticular, psychogeriatric, minor chronic disease, cardiorespiratory) to presence, number or specific types of PIP or ADRs. Significant associations were found in all clusters. The osteoarticular cluster presented the highest prevalence of PIP (94.9%) and ADRs (48.2%), mostly related to anxiolytics and antihypertensives, followed by the minor chronic disease cluster, associated with ADRs caused by antihypertensives and insulin. The psychogeriatric cluster presented PIP and ADRs of neuroleptics and the cardiorespiratory cluster indicators were better overall. In conclusion, the associations that were found reinforce the existence of multimorbidity patterns and support specific medication review actions according to each patient profile. Thus, determining the relationship between multimorbidity profiles and quality indicators of medication could help optimise healthcare processes. Trial registration number: NCT02830425.
1. Introduction
The clinical management of older patients with multiple conditions and pharmaceutical treatments poses a big challenge for healthcare professionals and systems. On top of the ageing population, a high prevalence of multimorbidity (classically defined as the presence of two or more coexisting chronic conditions) has been described worldwide and is expected to continue increasing [1,2]. Therefore, conducting research on how to improve the care of multimorbid patients in healthcare services that have traditionally focused on single diseases should be deemed of the utmost priority [3,4].
Various definitions and research methodologies have been developed in order to shed light on the concept of multimorbidity, and there is accumulating evidence suggesting that chronic conditions give rise to association patterns [5,6,7]. Although there is no standard yet, many publications have successfully identified multimorbidity patterns [8,9], some of which are repeatedly found among different studies [10]. Furthermore, some patterns have been associated with outcomes such as lower function, higher healthcare utilisation, poor prognosis or higher mortality [11,12,13,14,15,16]. Therefore, identifying multimorbidity patterns could help design new strategies and guidelines focusing on the most appropriate practices according to each patient profile.
This is remarkably important in older patients who, in addition to multimorbidity, present polypharmacy and age-related factors that can influence and hinder pharmacological prescribing. Some examples include physiological changes in pharmacokinetics and pharmacodynamics, cognitive impairment, functional difficulties or geriatric syndromes [17,18,19,20]. All in all, prescribing while balancing the benefits and risks becomes an arduous task.
In this scenario, a lot of attention has been brought to potentially inappropriate prescribing (PIP), which may occur in situations such as prescribing medications with potential benefits that do not outweigh the harms (particularly given the existence of safer alternatives); prescribing an inappropriate dose or duration or a duplicate drug; or omitting potentially beneficial medications. Several tools have been developed to identify PIP, such as the STOPP/START (Screening Tool of Older Person’s potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment) criteria, the most used and validated in European older adults [21]. These criteria include both potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs). While PPOs can prevent patients from taking essential medication, leading to risks and negative outcomes [22,23], PIMs are a well-known risk factor for adverse drug reactions (ADRs) [24,25,26]. ADRs are highly frequent in older patients and have been estimated to be responsible for 10–30% of hospital admissions [27,28] as well as to occur in 16% of already hospitalised older patients [29].
Taking into account all these considerations, it is plausible that certain multimorbidity patterns may present an association with specific PIP and/or ADRs. Importantly, there are no publications to date studying this inter-relationship in detail. Identifying associations could pave the way to optimising and focusing medication review actions and improve healthcare in these complex patients, reducing undesired outcomes. The present analyses are part of the MoPIM multicentre cohort study [30], which has various objectives regarding multimorbidity, PIP and ADRs in older patients hospitalised due to chronic condition exacerbation. A set of four multimorbidity patterns were identified in a previous publication [31]; thus, the objectives of this work are to evaluate if any of these previously identified multimorbidity patterns are associated with the presence, number or specific types of PIP or ADRs.
2. Methodology
2.1. Design and Setting
A multicentre prospective cohort study including older patients hospitalised at the internal medicine or geriatric services at five general teaching hospitals in three different regions of Spain between September 2016 and December 2018 was conducted. The detailed protocol was previously published [30]. For the purposes of the study, older patients (≥65 years old) admitted as a result of the exacerbation of their chronic pathology were included. Patients referred to home hospitalisation, admitted because of an acute process unrelated to any chronic disease or with a fatal outcome expected at the time of admission, were not included. No written informed consent was deemed necessary for this study, according to the independent ethics committee.
2.2. Data Acquisition and Variables
The following sociodemographic and clinical data were retrieved from the electronic health records by the clinical team responsible for the patient: patient’s code, date of birth, sex, functional status just before admission (Barthel Index) [32], household (alone, with relatives or other people, in a nursing home) and existence of any contact with healthcare services in the 3 months prior to hospitalisation due to exacerbation of any chronic disease and destination at discharge from the present episode of hospitalisation (home, transfer to another hospital, transfer to a nursing home, death). Chronic active conditions were recorded from a consensual list of 64 conditions containing all chronic diseases of the Charlson Comorbidity Index [33] and including some risk factors as well. Geriatric syndromes and risk factors were also recorded from a list of 15 (see protocol [30]).
The number of chronic medications in the electronic prescription at the time of admission and the STOPP/START criteria (version 2) [21] detected upon admission, with the active principle involved, were collected by the pharmacist of the team. The 2nd version of STOPP/START criteria consists of a list of 114 medication indications, developed using a Delphi method by experts from different disciplines, who carried out a literature review. The criteria are directed to prevalent diseases in older patients, are ordered by physiological systems and are easy to relate to active diagnoses. This medication review process was part of the usual patient care routine in all participating centres. Medication was only considered chronic if prescribed at least 3 months before admission, and creams, ointments, healing materials and over-the-counter medicines were not considered. Active principles were considered individually when registering STOPP/START criteria, regardless of the administered drug combinations.
Finally, ADRs were identified by the clinical team both at admission and during the course of stay. ADRs were considered according to the WHO and the European Medicines Agency criteria [34,35,36]. The active principle involved and whether the ADR occurred at admission or during hospitalisation were collected. Consequences in terms of health (death, life-threatening, lengthening of hospitalisation, other important consequences under medical criteria) were registered if the ADR appeared during hospital stay.
2.3. Sampling and Analysis
Patients included were proportionally distributed to the annual volume of hospitalisations at the internal medicine and/or geriatric services of each centre.
The Updated Charlson Comorbidity Index [37] was calculated, adjusted by age and categorised by tertiles (2–6, 7–8, 9–14).
Multimorbidity patterns were identified using a soft clustering algorithm, as thoroughly described [31]. Firstly, some chronic conditions were grouped according to clinical criteria and then filtered by <2% prevalence, resulting in a list of 40 chronic conditions and 15 geriatric syndromes. Chronic conditions were weighted according to the required clinical management. Then, transformation and dimensionality reduction for the dataset were carried out with the PCAmix algorithm [38], and cluster analysis was performed with the fuzzy c-means algorithm [39]. This technique allowed for obtaining clusters of patients based on their chronic conditions and geriatric syndromes with a membership probability to every cluster. After computing several validation indexes [31], a range of statistically significant possibilities were obtained, and, after clinical revision and discussion among the research team, an eventual set of four clusters was established. These clusters were named ‘osteoarticular’, ‘psychogeriatric’, ‘minor chronic disease’ and ‘cardiorespiratory’. Patients were assigned to the cluster where their membership probability was highest.
All STOPP/START criteria were assessed, except for START criteria I (vaccines) due to difficulties of some centres in accessing the information. Regarding the implicit criterion STOPP A1 and given its high frequency, it was divided into the following categories according to the active principle involved: proton pump inhibitors, hypolipidemics, analgesics, acetylsalicylic acid, antihypertensives and others [40].
Active principles involved in ADRs were categorised in the following drug families: analgesic, angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker, antiarrhythmic, antibiotic, anticoagulant, antidepressant, antiepileptic, antiplatelet, antipsychotic, antivitamin K, benzodiazepine, beta blocker, bronchodilator, corticoid, loop diuretic, neuroleptic, insulin, opioid, oral anticoagulant, oral antidiabetic, potassium sparing diuretic, proton pump inhibitor, statin, thiazide diuretic and others. Equivalence with ATC (Anatomical Therapeutic Chemical) codes can be found in Table S1.
Binary variables were created to describe the presence of any STOPP/START PIP, any STOPP PIM, any START PPO, any ADR, any ADR at admission and any ADR during hospitalisation. This was performed similarly with numerical variables for the number of STOPP/START PIP (excluding implicit criteria STOPP A1, A2, A3), number of STOPP PIM (excluding STOPP A1, A2, A3), number of START PPO, number of ADR, number of ADR at admission and number of ADR during hospitalisation.
Descriptive analyses were performed for all variables. Bivariate analyses were conducted to assess possible associations between multimorbidity clusters and the presence or type of PIP or ADR by the Fisher’s exact test. Most frequent PIP criteria were selected with the aim of analysing at least the top 10 criteria for PIMs and PPOs, resulting in a cut-off of 5% of patients of a cluster for STOPP criteria and 3% of patients of a cluster for START criteria. ADRs were only analysed if present in at least 5 patients of a cluster. Post hoc pairwise Fisher’s exact tests were conducted for those previously significant tests (p < 0.05), and p-values were corrected for multiple hypothesis testing using Benjamini–Hochberg false discovery rate (FDR) method [41] at a 5% cut-off.
Comparisons between number of PIP or ADR were performed using the Kruskal–Wallis test. Pairwise comparisons between distributions of the number of PIP or ADR among the different clusters were performed using the Kolmogorov–Smirnov test. These comparisons were performed for the following variables: number of STOPP/START PIP (excluding implicit criteria, i.e., STOPP A), number of STOPP PIMs (excluding implicit criteria, i.e., STOPP A), number of START PPOs, number of ADRs, number of ADRs at the time of admission and number of ADRs during hospitalisation.
All analyses were performed with R (R Foundation for Statistical Computing, Vienna, Austria) [42].
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Cohort
A total of 740 patients were included; 53.2% were females, and 98.7% were diagnosed with two or more chronic conditions. The mean age was 84.1 (SD 7.0) years, and the mean Barthel Index was 65 (median 75). The cardiorespiratory cluster contained most patients (n = 325, 43.9%), followed by the psychogeriatric (n = 151, 20.4%), osteoarticular (n = 137, 18.5%) and minor chronic disease (n = 127, 17.2%) clusters. Sociodemographic and clinical variables are summarised in Table 1, according to the assigned multimorbidity cluster. The prevalences of chronic conditions and geriatric syndromes are described according to multimorbidity cluster in Table S2. Among all the detected STOPP/START criteria, the most prevalent STOPP criteria were A1: Drugs prescribed without an evidence-based clinical indication (n = 310, 25.7%), D5: Benzodiazepines for ≥4 weeks (n = 247, 20.5%) and K1: Benzodiazepines (n = 131, 10.9%), from a total of 1206 criteria detected. The most prevalent START criteria were E5: Vitamin D supplement in older people who are housebound or experiencing falls or with osteopenia (n = 76, 21.5%), H2: Laxatives in patients receiving opioids regularly (n = 50, 14.1%) and A8: Appropriate beta-blocker with stable systolic heart failure (n = 39, 11.0%), from a total of 353 criteria detected.

Table 1.
Sociodemographic and clinical variables of the cohort according to the assigned multimorbidity clusters.
3.2. Relationship between Multimorbidity Clusters and Potentially Inappropriate Prescribing
A bivariate analysis was performed to test the association of belonging to a cluster and presenting any STOPP/START PIP, STOPP PIMs or START PPOs. Table 2 shows that a significant association was found in all these three variables. Pairwise comparisons (Table S3) showed that the osteoarticular cluster was significantly different from all others regarding the presence of any PIP or any PIMs, and together with the minor chronic disease were significantly different in presence of PPOs from the other two.

Table 2.
Presence of any PIP, PIMs or PPOs according to STOPP/START criteria in relation to the assigned multimorbidity clusters.
Next, we compared the number of PIP, PIMs and PPOs between clusters taking into account only explicit criteria. Differences were found between clusters in all three variables (Kruskal–Wallis test: p < 0.001 in number of PIP, p < 0.001 in number of PIMs, p = 0.001 in number of PPOs). Pairwise comparisons between distributions showed that the osteoarticular cluster in both the number of STOPP/START PIP and STOPP PIMs was different from the other clusters (Kolmogorov–Smirnov test: p < 0.001 and p < 0.005, respectively), meaning that patients of this cluster tend to present a larger number of PIP and PIMs (Figure S1). No significant differences were found in the distribution of the number of START PPOs.
Then, a bivariate analysis was performed focusing on certain specific criteria, selecting the most frequent ones. Significant associations were found in the STOPP PIM criteria related to benzodiazepines (STOPP D5, G5, K1) or ACE inhibitors/angiotensin receptor blockers (STOPP B11), which were significantly higher in the osteoarticular cluster. PIMs related to proton pump inhibitors (extracted from STOPP A1) were significantly lower in the cardiorespiratory cluster than the rest. PIMs involving neuroleptic drugs (STOPP K2) were most prevalent in patients of the psychogeriatric cluster and least prevalent in those of the cardiorespiratory cluster (Figure 1A, Table S4).
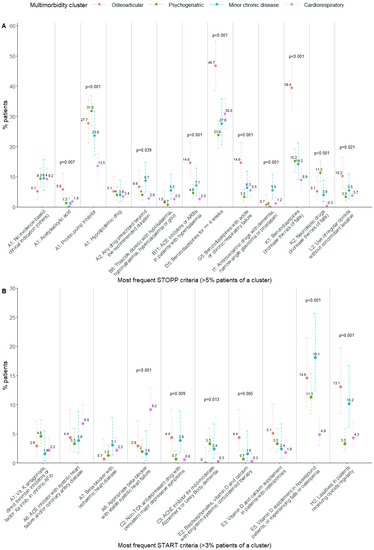
Figure 1.
(A): Percentage of patients per cluster having the most frequent STOPP PIM criteria. (B): Percentage of patients per cluster having the most frequent START PPO criteria. Fisher’s exact test p-value: p-values are shown in the figure when p < 0.05. Error bars show 95% confidence interval for the estimated proportion. ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; AFib: atrial fibrillation; TCA: tricyclic antidepressant; AChE: acetylcholinesterase.
Regarding START PPO analysis, the most frequent criteria were selected and compared between clusters as well. PPOs involving beta blockers (START A8) were positively associated with the cardiorespiratory cluster, while the lack of vitamin D prescribing (START E5) was negatively associated with it. Prescribing the omission of laxatives (START H2) was significantly higher in the osteoarticular and minor chronic disease clusters with respect to the others (Figure 1B, Table S5).
3.3. Relationship between Multimorbidity Clusters and Adverse Drug Reactions
A total of 376 ADRs were reported in 245 patients (33.1%), and 59.6% of those were detected at the time of admission in 153 patients (20.7%). Having an ADR was significantly associated with belonging to particular multimorbidity clusters. Almost half of the patients in the osteoarticular and minor chronic disease clusters suffered at least one ADR, and both clusters were significantly different from the psychogeriatric and cardiorespiratory. When separating ADRs into those detected at the time admission and those occurred during hospital stay, these two clusters also showed a significantly higher percentage of patients (Table 3 and Table S6).

Table 3.
Presence of any ADR in relation to the assigned multimorbidity clusters.
With respect to the number of ADRs between different clusters, significant differences were found when considering all ADRs, those detected at admission and those occurred during hospitalisation (Kruskal–Wallis test: p < 0.001 in all cases). Afterwards, a pairwise comparison of the distributions was performed too, which showed that the osteoarticular and the minor chronic disease clusters presented a different distribution from the psychogeriatric and cardiorespiratory clusters both regarding the total number of ADRs and those detected at the time of admission (Figure S2). No significant differences were found in the distribution of the number of ADRs occurred during hospital stay.
To determine the association of multimorbidity clusters to specific types of ADRs, a bivariate analysis was performed, similarly to the one involving the STOPP/START criteria. Figure 2 shows the percentage of patients that suffered an ADR of a certain drug family according to their assigned multimorbidity cluster, only considering those ADRs detected in at least five patients in a cluster. Patients belonging to the osteoarticular cluster suffered ADRs involving ACE inhibitors more frequently, as well as patients in the minor chronic disease cluster, which more frequently experienced ADRs related to angiotensin receptor blockers or insulin, with respect to the psychogeriatric or cardiorespiratory clusters. Furthermore, ADRs to neuroleptic drugs were more frequently suffered in psychogeriatric patients, and those involving diuretics were also associated with multimorbidity cluster belonging; however, no pairwise differences could be found (Table S7).
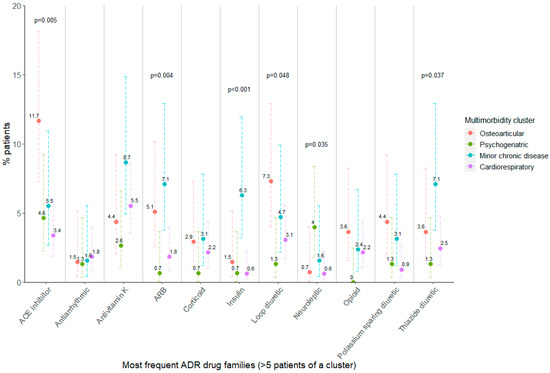
Figure 2.
Percentage of patients per cluster having at least one ADR registered in the most frequent drug families. Fisher’s exact test p-value is shown when p < 0.05. ADR: adverse drug reaction; ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker. Error bars show 95% confidence interval for the estimated proportion.
Finally, the relationship between multimorbidity cluster and the consequences of ADRs during admission was also tested, and a significant association was found (Figure 3; Fisher’s Exact Test: p-value = 0.02). For example, 18.8% of patients in the osteoarticular cluster who suffered an ADR during admission faced a life-threatening situation, whereas this did not happen to any patients in the psychogeriatric cluster. Nevertheless, in the psychogeriatric cluster, most ADRs caused a lengthening of hospital stay. None of the ADRs were fatal.
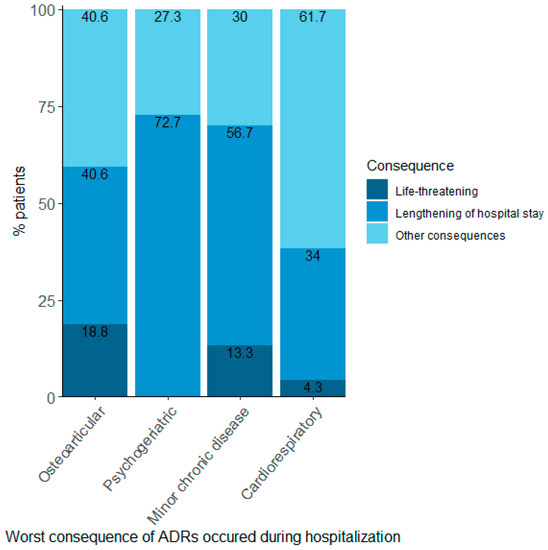
Figure 3.
Proportions of patients per cluster according to the worst consequence suffered in those patients with an ADR during hospitalisation. ADR: adverse drug reaction. Fisher’s Exact Test: p-value = 0.02.
4. Discussion
4.1. Main Important Results and Novelty
Our study successfully detected significant associations between multimorbidity patterns and specific PIMs, PPOs and ADRs, showing that patients in each multimorbidity cluster tend to present comparable health problems and, therefore, identifying patients with similar needs. The osteoarticular cluster displayed the worst situation regarding PIP and ADRs, particularly related to anxiolytics and antihypertensives. The psychogeriatric cluster, despite having the lowest number of chronic prescriptions, presented PIP of proton pump inhibitors and neuroleptics, with the latter also causing ADRs. The minor chronic disease cluster was associated with ADRs caused by antihypertensives and insulin, and the cardiorespiratory cluster showed fewer PIP and ADRs overall. Altogether, our results support the prioritisation of medication review in patients from the osteoarticular cluster, which accounted for the largest proportion of patients with PIP or ADRs, along with the most severe consequences of ADRs, followed by the minor chronic disease cluster.
This is, to our knowledge, the first study to consider and analyse the relationship of multimorbidity patterns with quality indicators of medication, such as PIP and ADRs. Our approach includes a novel methodology of defining multimorbidity in conjunction with an extensive set of explicit and implicit criteria (STOPP/START) regarding PIMs and PPOs and together with an exhaustive registration of ADRs, providing a unique dataset integrating this information. Significant associations were found between clusters when considering presence, number or specific PIMs, PPOs and ADRs, suggesting that these situations should be differently managed according to each particular patient profile.
4.2. Clinical Implications
The osteoarticular cluster not only presented the highest percentage of patients with at least a PIP or PIM but also a larger number of them than the other clusters. This could be partially explained due to a higher number of chronic prescriptions and is also consistent with the high prevalence found in the three benzodiazepine-related criteria (STOPP D5, G5, K1), frequently coexisting in the same patients. We certainly expected an overall high prescription of benzodiazepines [43,44], especially in this cluster that has the highest prevalence of depression and anxiety (61.3%). This could even be a result of excessive medicalisation in a female-predominant cluster. It is well-known that benzodiazepine prescribing is excessive [45], and this situation becomes particularly concerning in this patient profile due to its association with negative outcomes such as falls, fractures, dependence and cognitive decline [46]. The almost-ubiquitous prevalence of chronic pain (92.7%), frailty (83.9%) and degenerative arthropathy (81.0%) stress the need for benzodiazepine deprescribing. Nonetheless, we acknowledge the complexity of this process.
Furthermore, we found that side effects in prescribing for pain management might not be properly addressed in the osteoarticular cluster. A significant association with STOPP L2 and START H2 criteria was found, both referring to the requirement of laxative prescription in patients under opioid therapy. This was also found in the minor chronic disease cluster, consistent with its high prevalence of patients with chronic pain (69.3%). Nevertheless, it is plausible that patients in both clusters, which are already taking a large number of medications, could be using some herbal or over-the-counter products. Besides, these results did not correlate well with the prevalence of constipation in the minor chronic disease cluster, which accounted for the lowest proportion (32.3%). This could be explained by differences in the involved opioids, with patients prescribed transdermal fentanyl having less risk of constipation than those on oxycodone or morphine [47,48].
Additionally, the use of antihypertensive and diuretic drugs also posed a challenge in patients from both the osteoarticular and the minor chronic disease clusters (with many registered ADRs) compared to the others. Although it was not always possible to establish significant pairwise comparisons due to a low number of cases, there was a significant association overall. In the specific case of ACE inhibitors, they were found to be significantly different in the osteoarticular cluster as well as detected as PIMs in STOPP criteria B11. Conversely, angiotensin receptor blockers caused ADRs in a higher proportion of patients in the minor chronic disease cluster but were not labelled as inappropriately prescribed. Our results, therefore, suggest that, although side effects of antihypertensive drugs are well-known [49,50,51], decompensations particularly occur in these patients and may lead to life-threatening situations, which were consistently found to be higher in both clusters. However, these situations may be harder to address, as they might not always be identified as PIMs.
Remarkably, some of the ADRs detected were not caused by a previously identified PIM, revealing one of the limitations of PIP/PPO detection tools. This was especially evident in the minor chronic disease cluster, which unexpectedly presented a high number of ADRs. This cluster appears to be the most heterogeneous of all, and it is possible that this situation may have occurred due to a single disease prescribing approach, where various medications are accumulatively prescribed by different professionals. Moreover, it is also plausible that medication review in these patients, who are the least dependent and are mostly living with other people or alone, was not prioritised.
This situation contrasts with the findings regarding the psychogeriatric cluster, with the lowest number of chronic prescriptions, low number of PIP and ADRs and no in-hospital life-threatening ADRs. This could be explained by an increased effort in medication review and comprehensive clinical management. However, two situations stood out: PIMs of proton pump inhibitors were especially high, and neuroleptics were detected as PIMs and also caused ADRs. These results could be expected, yet problematic, as both are related to a variety of adverse outcomes [52,53,54,55,56,57], which could cause a high burden in already very frail patients. Therefore, there would still be room for deprescribing.
Lastly, patients in the cardiorespiratory cluster, containing almost half of the cohort, were undoubtedly in the most preferable situation: no remarkable PIMs or ADRs. Only one PPO criterion stood out: lack of a beta blocker prescription, which could be explained by the opposite effect of beta blockers to beta agonists, usually administered in patients with chronic obstructive pulmonary disease (COPD). However, the current literature recommends beta blocker prescribing in patients with heart disease and COPD [58]. All in all, these patients were minimally dependent, with a low number of chronic conditions and geriatric syndromes. Thus, it is plausible that although they had a chronic pathology exacerbation that led to hospital admission, their health status was overall better and only restricted to cardiorespiratory problems. Therefore, our results suggest these patients might be easier to handle.
Taken together, our results show how multimorbidity profiles built according to chronic conditions and geriatric syndromes have a significant association with PIP and ADRs. On the one hand, these results support the existing evidence on the concept of multimorbidity forming association patterns. The associations found on each profile to some extent validate our methodological approach, which successfully allocates patients with similar situations and needs. On the other hand, these results suggest that appropriate actions according to each patient profile could be taken in hospitals but also in primary care settings, which could be a useful approach to optimise and offer better health care. It is essential to effectively direct efforts in these complex patients, and, in this case, patient categorisation strategies together with multidisciplinary teams could be helpful to address the situation. Thus, further studies are needed to incorporate multimorbidity approaches in all levels of healthcare.
4.3. Comparison to Other Studies
Although there are previous studies on PIP and ADRs in different types of cohorts of multimorbid patients studying the risk factors, outcomes and interventions, the vast majority define multimorbidity with the presence of two or more chronic conditions or consider those from a short list [59,60,61,62,63]. These definitions, although easily determined, become too simplistic in a setting of older hospitalised patients, where almost all are multimorbid (98.7% in our cohort). Therefore, new analytical strategies need to be explored that consider a more comprehensive definition of multimorbidity, as there are currently no publications directly comparable to ours.
The most similar study is the one carried out by Teh et al., in 2018 [14], comparing multimorbidity patterns to the presence of any PIM or any PPO, but without considering explicit criteria nor ADRs. Multimorbidity profiles are built with a similar methodology and agreed upon by consensus among a multidisciplinary team involving clinicians as well. However, the cohort is comprised of community patients over 80 years old, considering a list of 14 conditions and using the first version of STOPP/START criteria, which disallows comparisons. Nonetheless, the cluster with the highest proportion of patients with any PIMs or PPOs is called ‘depression and arthritis’ and presents the highest prevalence of osteoporosis and osteoarthritis, suggesting that this cluster could be similar to the osteoarticular cluster from our cohort, which also presents depression and anxiety. In this article, the authors conclude that profiles of conditions may carry stronger associations with cross-sectional outcomes than the sum of those conditions.
4.4. Strengths and Limitations
This study presents multiple strengths. As a multicentre study, it presents increased external validity. Its prospective design ensures high data quality by an accurate and thorough registering of variables that may commonly be underreported, such as PIP and ADRs. In this sense, it is important that a multidisciplinary team composed of pharmacists and physicians work together in the medication-review process.
Furthermore, the approach of multimorbidity cluster analysis is novel and methodologically robust, incorporating both chronic conditions and geriatric syndromes and allowing for work with patient profiles instead of single conditions. Moreover, this study has been carried out with a well-defined cohort of hospitalised patients admitted due to chronic condition exacerbation. This constitutes a particularly vulnerable and complex group of patients, who may largely benefit from a reduction in negative outcomes. Finally, the unique approach of the study, considering multimorbidity patterns with specific PIP and ADRs all together, allows for obtaining a reliable picture of a complex situation that needs to be explored and addressed.
Nonetheless, some limitations of this study need to be taken into account. Firstly, methodological approaches to tackle multimorbidity are highly variable, and there is no consensus on the best practices to determine multimorbidity patterns. The results of this study are conditioned to the predefined multimorbidity patterns, which could be questioned, although they were comprehensive, considering a large list of conditions, and consensually selected by a research team including clinicians. Secondly, there may be differences in the reporting of PIP or ADRs between centres or healthcare professionals, despite the large efforts made to homogenise criteria. Regarding ADRs, active principles alone could not be analysed due to low occurrence so had to be collapsed into higher-level categories. Finally, the direct relationship between PIP and ADRs was not addressed, as this was far from the objectives of these analyses.
5. Conclusions
In older patients admitted to hospital because of chronic conditions’ exacerbation, it is possible to define multimorbidity clusters that are associated with quality indicators of medication prescribing such as the presence, number or specific types of PIP and ADRs. These associations validate and support the existence of such clusters and point to specific prescriptions that could be primarily reviewed and made adequate for each patient profile. Thus, determining the relationship between multimorbidity profiles and the quality indicators of medication could be key in remodelling and optimising healthcare processes in order to tackle the increasing prevalence of older patients with multimorbidity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192315902/s1, Figure S1: (A): Distribution of the number of STOPP/START PIP according to the assigned multimorbidity cluster. Pairwise comparisons be-tween cluster distributions performed with the Kolmogorov-Smirnov test showed significant differences between the osteoar-ticular cluster and the rest (p < 0.001). PIP: Potentially inappropriate prescribing. (B): Distribution of the number of STOPP PIM according to the assigned multimorbidity cluster. Pairwise comparisons between cluster distributions performed with the Kolmogorov-Smirnov test showed significant differences between the osteoarticular cluster and the rest (p < 0.005). PIM: potentially inappropriate medication. (C): Distribution of the number of START PPO according to the assigned multimorbidity cluster. Pairwise comparisons between cluster distributions performed with the Kolmogorov-Smirnov test showed no significant differences (p > 0.05). PPO: potential prescribing omission; Figure S2: (A): Distribution of the number of ADRs according to the assigned multimorbidity cluster. Pairwise comparisons between cluster distributions performed with the Kolmogorov-Smirnov test showed significant differences between the osteoarticular and the minor chronic disease clusters and the rest (p < 0.001). (B): Distribution of the number of ADRs at admission according to the assigned multimorbidity cluster. Pairwise comparisons between cluster distributions performed with the Kolmogorov-Smirnov test showed significant differences between the osteoarticular cluster and the minor chronic disease clusters and the rest (p < 0.05). (C): Distribution of the number of ADRs during admission according to the assigned multimorbidity cluster. Pairwise comparisons between cluster distributions performed with the Kolmogorov-Smirnov test showed no significant differences (p > 0.05). ADR: adverse drug reaction; Table S1: Equivalences between names used in ADRs classification and ATC codes; Table S2: Number and percentage of patients with each chronic condition or geriatric syndrome registered according to the assigned multimorbidity cluster; Table S3: p-values of post-hoc pairwise; Table S4: p-values of post-hoc pairwise; Table S5: p-values of post-hoc pairwise; Table S6: p-values of post-hoc pairwise; Table S7: p-values of post-hoc pairwise.
Author Contributions
Conceptualisation, M.B.; methodology, M.L., M.B., S.O., R.J., S.H. and M.Q.G.; validation, M.L. and M.B.; formal analysis, M.L.; investigation, S.O., D.S.-S., R.J., S.H., M.E.-F., M.A., M.d.A., G.J.N., R.H.-L. and MoPIM study group.; resources, R.J., S.H., M.E.-F., M.A. and R.H.-L.; data curation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, M.L. and M.B.; visualisation, M.L.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Instituto de Salud Carlos III-FEDER (PI15/00552) and from the Network for Research into Healthcare in Chronic Diseases, REDISSEC (RD16/0001/0002). These funding bodies did not have a role in the design of the study, in the collection, analysis, and interpretation of the data or in the writing of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the institutional review board (or ethics committee) of each centre: Comité Ético de investigación Clínica del Parc Taulí (ID: 20166570), Comitè Ètic d’Investigació Clínica Osona per a la Recerca i Educació Sanitàries (FORES) (ID: 2016922-PR153), Comité de Ètica de la Investigación con Medicamentos (CEIm)-Parc de Salut MAR (ID: 2016/6830/I), Comité Ético de Investigación Clínica de Euskadi (ID: PI2016060) and Comité de Ética de Investigación del Hospital Universitario de Canarias (ID: MBM-MOD-2016-01 (2016-56)). No written informed consent was deemed necessary for this study.
Informed Consent Statement
Written patient consent was waived considering that they were often very old patients, in an acute process with intellectual impairment or delirium, and sometimes living alone or in a nursing home and that it would have been difficult to explain patient consent and make sure it was understood. In this observational study, we considered it important to include patients who were representative of all complex clinical conditions and to elude a possible selection bias (especially frequent in older patients) that would have invalidated the results. On the other hand, data (not included in these analyses) about chronic medications, PIP and intention to modify the treatment, during the first days related to possible PIP, had to be gathered at the beginning of the hospitalisation period and could not be delayed.
Data Availability Statement
The data presented in this study are openly available in Zenodo at DOI 10.5281/zenodo.7371151.
Acknowledgments
Marina Lleal is a doctoral candidate in the Methodology of Biomedical Research and Public Health programme at the Universitat Autònoma de Barcelona (UAB), Barcelona, Spain (European Higher Education Area Doctoral Programme in Methodology of Biomedical Research and Public Health in Department of Paediatrics, Obstetrics and Gynaecology, Preventive Medicine and Public Health, Universitat Autònoma de Barcelona (UAB), Bellaterra, Barcelona, Spain). The authors acknowledge the dedication and support of the entire MoPIM research group, listed by institution: Parc Taulí Hospital Universitari. Institut d’Investigació i Innovació Parc Taulí (I3PT): Marisa Baré (Institutional Committee for the Improvement of Clinical Practice Adequacy, Clinical Epidemiology and Cancer Screening Department; CRiSP research group; REDISSEC; RICAPPS), Susana Herranz (Acute care Geriatric Unit; CRiSP research group, REDISSEC), Rosa Jordana (Internal Medicine Department, CRiSP research group), Maria Queralt Gorgas (Pharmacy Department; REDISSEC), Sara Ortonobes (Pharmacy Department) and Marina Lleal (Institutional Committee for the Improvement of Clinical Practice Adequacy, Clinical Epidemiology and Cancer Screening Department; CRiSP research group; Universitat Autònoma de Barcelona); University Hospital of Vic: Pere Roura-Poch (Epidemiology Unit; REDISSEC), Daniel Sevilla-Sánchez (Pharmacy Department), Núria Solà (Pharmacy Department), Javier González (Pharmacy Department), Núria Molist (Geriatrics Department-C3RG research group) Mariona Espaulella-Ferrer (Geriatrics Department-C3RG research group) and Oscar Mascaró (Internal Medicine Department); Hospital del Mar Medical Research Institute-IMIM: Elisabet de Jaime (Geriatrics Department), Olivia Ferrandez (Pharmacy Department), Maria Sala (Department of Epidemiology and Evaluation; REDISSEC), Miguel Ángel Márquez (Geriatrics Department), Marta Arellano (Geriatrics Department), Carlos Clemente (Geriatrics Department), Olga Sabartés (Geriatrics Department), Núria Carballo (Pharmacy Department) and Marta de Antonio (Pharmacy Department); Hospital de Galdakao: Rafael Estrada (Internal Medicine Department) and Maria Olatz Ibarra (Pharmacy Department); Complejo Hospitalario Universitario de Canarias: Candelaria Martin (Internal Medicine Department), Gloria Julia Nazco (Pharmacy Department) and Rubén Hernández-Luis (Internal Medicine Department).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fortin, M.; Stewart, M.; Poitras, M.-E.; Almirall, J.; Maddocks, H. A Systematic Review of Prevalence Studies on Multimorbidity: Toward a More Uniform Methodology. Ann. Fam. Med. 2012, 10, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- The Academy of Medical Sciences. Multimorbidity: A Priority For Global Health Research; The Academy of Medical Sciences: London, UK, 2018; Available online: https://acmedsci.ac.uk/file-download/82222577 (accessed on 8 August 2022).
- Rijken, M.; Struckmann, V.; Van Der Heide, I.; Hujala, A.; Barbabella, F.; Van Ginneken, E.; Schellevis, F. How to Improve Care For People with Multimorbidity in Europe? European Observatory on Health Systems and Policies: Brussels, Belgium, 2016; Available online: https://www.euro.who.int/en/about-us/partners/observatory/publications/policy-briefs-and-summaries/how-to-improve-care-for-people-with-multimorbidity-in-europe (accessed on 8 August 2022).
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef]
- Diederichs, C.; Berger, K.; Bartels, D.B. The Measurement of Multiple Chronic Diseases—A Systematic Review on Existing Multimorbidity Indices. J. Gerontol. Ser. A 2011, 66, 301–311. [Google Scholar] [CrossRef]
- Violán, C.; Foguet-Boreu, Q.; Fernández-Bertolín, S.; Guisado-Clavero, M.; Cabrera-Bean, M.; Formiga, F.; Valderas, J.M.; Roso-Llorach, A. Soft clustering using real-world data for the identification of multimorbidity patterns in an elderly population: Cross-sectional study in a Mediterranean population. BMJ Open 2019, 9, e029594. [Google Scholar] [CrossRef]
- Zacarías-Pons, L.; Vilalta-Franch, J.; Turró-Garriga, O.; Saez, M.; Garre-Olmo, J. Multimorbidity patterns and their related characteristics in European older adults: A longitudinal perspective. Arch. Gerontol. Geriatr. 2021, 95, 104428. [Google Scholar] [CrossRef]
- Ng, S.K.; Tawiah, R.; Sawyer, M.; Scuffham, P. Patterns of multimorbid health conditions: A systematic review of analytical methods and comparison analysis. Int. J. Epidemiol. 2018, 47, 1687–1704. [Google Scholar] [CrossRef]
- Juul-Larsen, H.G.; Andersen, O.; Bandholm, T.; Bodilsen, A.C.; Kallemose, T.; Jørgensen, L.M.; Klausen, H.H.; Gilkes, H.; Petersen, J. Differences in function and recovery profiles between patterns of multimorbidity among older medical patients the first year after an acute admission—An exploratory latent class analysis. Arch. Gerontol. Geriatr. 2019, 86, 103956. [Google Scholar] [CrossRef]
- Lleal, M.; Corral-Vázquez, C.; Baré, M.; Comet, R.; Herranz, S.; Baigorri, F.; Gimeno-Miguel, A.; Raurich, M.; Fortià, C.; Navarro, M.; et al. Multimorbidity patterns in COVID-19 patients and their relationship to infection severity: MRisk-COVID study. PLoS ONE 2022. under review. [Google Scholar]
- Zheng, D.D.; Loewenstein, D.A.; Christ, S.L.; Feaster, D.J.; Lam, B.L.; McCollister, K.E.; Curiel-Cid, R.E.; Lee, D.J. Multimorbidity patterns and their relationship to mortality in the US older adult population. PLoS ONE 2021, 16, e0245053. [Google Scholar] [CrossRef] [PubMed]
- Teh, R.O.; Menzies, O.H.; Connolly, M.J.; Doughty, R.N.; Wilkinson, T.J.; Pillai, A.; Lumley, T.; Ryan, C.; Rolleston, A.; Broad, J.; et al. Patterns of multi-morbidity and prediction of hospitalisation and all-cause mortality in advanced age. Age Ageing 2018, 47, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Juul-Larsen, H.G.; Christensen, L.D.; Bandholm, T.; Andersen, O.; Kallemose, T.; Jørgensen, L.M.; Petersen, J. Patterns of Multimorbidity and Differences in Healthcare Utilization and Complexity Among Acutely Hospitalized Medical Patients (≥65 Years)–A Latent Class Approach. Clin. Epidemiol. 2020, 2020, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Pírez, J.; Ioakeim-Skoufa, I.; Gimeno-Miguel, A.; Poblador-Plou, B.; González-Rubio, F.; Muñoyerro-Muñiz, D.; Rodríguez-Herrera, J.; Goicoechea-Salazar, J.A.; Prados-Torres, A.; Villegas-Portero, R. Multimorbidity Profiles and Infection Severity in COVID-19 Population Using Network Analysis in the Andalusian Health Population Database. Int. J. Environ. Res. Public Health 2022, 19, 3808. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jackson, S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2003, 57, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Motter, F.R.; Fritzen, J.S.; Hilmer, S.N.; Paniz, É.V.; Paniz, V.M.V. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 2018, 74, 679–700. [Google Scholar] [CrossRef] [PubMed]
- Corsonello, A.; Pedone, C.; Incalzi, R.A. Age-Related Pharmacokinetic and Pharmacodynamic Changes and Related Risk of Adverse Drug Reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Boparai, M.K.; Korc-Grodzicki, B. Prescribing for Older Adults. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2011, 78, 613–626. [Google Scholar] [CrossRef]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2014, 44, 213–218. [Google Scholar] [CrossRef]
- Cadogan, C.A.; Ryan, C.; Hughes, C.M. Appropriate Polypharmacy and Medicine Safety: When Many is not Too Many. Drug Saf. 2016, 39, 109–116. [Google Scholar] [CrossRef]
- Cherubini, A.; Corsonello, A.; Lattanzio, F. Underprescription of Beneficial Medicines in Older People: Causes, Consequences and Prevention. Drugs Aging 2012, 29, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Regueiro, R.; Fonseca-Aizpuru, E.; López-Colina, G.; Álvarez-Uría, A.; Rodríguez-Ávila, E.; Morís-De-La-Tassa, J. Prescripción inadecuada y efectos adversos a medicamentos en pacientes de edad avanzada. Rev. Clín. Esp. 2011, 211, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, H.; Gallagher, P.; Ryan, C.; Byrne, S.; O’Mahony, D. Potentially Inappropriate Medications Defined by STOPP Criteria and the Risk of Adverse Drug Events in Older Hospitalized Patients. Arch. Intern. Med. 2011, 171, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; Redley, B.; de Courten, B.; Manias, E. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 4150–4172. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Lizaraso, F.; Carvajal, A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 759–770. [Google Scholar] [CrossRef]
- Cabré, M.; Elias, L.; Garcia, M.; Palomera, E.; Serra-Prat, M. Avoidable hospitalizations due to adverse drug reactions in an acute geriatric unit. Analysis of 3,292 patients. Med. Clín. 2018, 150, 209–214. [Google Scholar] [CrossRef]
- Jennings, E.L.M.; Murphy, K.D.; Gallagher, P.; O’Mahony, D. In-hospital adverse drug reactions in older adults; prevalence, presentation and associated drugs—A systematic review and meta-analysis. Age Ageing 2020, 49, 948–958. [Google Scholar] [CrossRef]
- Baré, M.; Herranz, S.; Jordana, R.; Gorgas, M.Q.; Ortonobes, S.; Sevilla, D.; De Jaime, E.; Ibarra, O.; Martín, C. Multimorbidity patterns in chronic older patients, potentially inappropriate prescribing and adverse drug reactions: Protocol of the multicentre prospective cohort study MoPIM. BMJ Open 2020, 10, e033322. [Google Scholar] [CrossRef]
- Baré, M.; Herranz, S.; Roso-Llorach, A.; Jordana, R.; Violán, C.; Lleal, M.; Roura-Poch, P.; Arellano, M.; Estrada, R.; Nazco, G.J. Multimorbidity patterns of chronic conditions and geriatric syndromes in older patients from the MoPIM multicentre cohort study. BMJ Open 2021, 11, e049334. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- WHO Centre for Health Development. A Glossary of Terms for Community Health Care and Services for Older Persons; WHO Kobe Centre for Health Development Ageing and Health Technical Report; World Health Organization: Geneva, Switzerland, 2004; Available online: http://apps.who.int/iris/bitstream/handle/10665/68896/WHO_WKC_Tech.Ser._04.2.pdf (accessed on 27 October 2022).
- European Medicines Agency; Heads of Medicines Agencies. Guideline on Good Pharmacovigilance Practices (GVP) Module VI–Management and Reporting of Adverse Reactions to Medicinal Products (Rev 1); European Medicines Agency: Amsterdam, The Netherlands, 2014.
- Mascolo, A.; Scavone, C.; Sessa, M.; di Mauro, G.; Cimmaruta, D.; Orlando, V.; Rossi, F.; Sportiello, L.; Capuano, A. Can causality assessment fulfill the new European definition of adverse drug reaction? A review of methods used in spontaneous reporting. Pharmacol. Res. 2017, 123, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Chavent, M.; Kuentz-Simonet, V.; Labenne, A.; Saracco, J. Multivariate analysis of mixed data: The R package PCAmixdata. arXiv 2014, arXiv:1411.4911. [Google Scholar]
- Bezdek, J.C.; Ehrlich, R.; Full, W. FCM: The fuzzy c-means clustering algorithm. Comput. Geosci. 1984, 10, 191–203. [Google Scholar] [CrossRef]
- Baré, M.; Lleal, M.; Ortonobes, S.; Gorgas, M.Q.; Sevilla-Sánchez, D.; Carballo, N.; De Jaime, E.; Herranz, S. Factors associated to potentially inappropriate prescribing in older patients according to STOPP/START criteria: MoPIM multicentre cohort study. BMC Geriatr. 2022, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 20 April 2022).
- Torres-Bondia, F.; De Batlle, J.; Galván, L.; Buti, M.; Barbé, F.; Piñol-Ripoll, G. Trends in the consumption rates of benzodiazepines and benzodiazepine-related drugs in the health region of Lleida from 2002 to 2015. BMC Public Health 2020, 20, 818. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Utilización de medicamentos ansiolíticos e hipnóticos en España [Use of anxiolytic and hypnotic drugs in Spain]. 2021. Available online: https://www.aemps.gob.es/medicamentos-de-uso-humano/observatorio-de-uso-de-medicamentos/informes-ansioliticos-hipnoticos/ (accessed on 6 October 2022).
- Gerlach, L.B.; Wiechers, I.R.; Maust, D.T. Prescription Benzodiazepine Use Among Older Adults: A Critical Review. Harv. Rev. Psychiatry 2018, 26, 264–273. [Google Scholar] [CrossRef]
- Markota, M.; Rummans, T.A.; Bostwick, J.M.; Lapid, M.I. Benzodiazepine Use in Older Adults: Dangers, Management, and Alternative Therapies. Mayo Clin. Proc. 2016, 91, 1632–1639. [Google Scholar] [CrossRef]
- Hadley, G.; Derry, S.; Moore, R.A.; Wiffen, P.J. Transdermal fentanyl for cancer pain. Cochrane Database Syst. Rev. 2013, 2018, CD010270. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Knight, T.; Schein, J.; Carter, C.; Staats, P. Risk of Constipation in Patients Prescribed Fentanyl Transdermal System or Oxycodone Hydrochloride Controlled-Release in a California Medicaid Population. Consult. Pharm. 2004, 19, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2019, 43, 99–107. [Google Scholar] [CrossRef]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R. Major Side Effects of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers. UpToDate. Available online: https://www.uptodate.com/contents/major-side-effects-of-angiotensin-converting-enzyme-inhibitors-and-angiotensin-ii-receptor-blockers (accessed on 13 October 2022).
- Jaynes, M.; Kumar, A.B. The risks of long-term use of proton pump inhibitors: A critical review. Ther. Adv. Drug Saf. 2018, 10, 2042098618809927. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Maddukuri, G.; Xie, Y. Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe. Am. J. Kidney Dis. 2020, 75, 497–507. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hsu, W.-T.; Lai, C.-C.; Esmaily-Fard, A.; Tsai, Y.-W.; Chiu, C.-C.; Wang, J.; Chang, S.-S.; Lee, C. Use of antipsychotics increases the risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 1167–1178. [Google Scholar] [CrossRef]
- Nosè, M.; Recla, E.; Trifirò, G.; Barbui, C. Antipsychotic drug exposure and risk of pneumonia: A systematic review and meta-analysis of observational studies. Pharmacoepidemiol. Drug Saf. 2015, 24, 812–820. [Google Scholar] [CrossRef]
- Zhai, Y.; Yin, S.; Zhang, D. Association between Antipsychotic Drugs and Mortality in Older Persons with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2016, 52, 631–639. [Google Scholar] [CrossRef]
- Atti, A.; Gozzi, B.F.; Zuliani, G.; Bernabei, V.; Scudellari, P.; Berardi, D.; De Ronchi, D.; Tarricone, I.; Menchetti, M. A systematic review of metabolic side effects related to the use of antipsychotic drugs in dementia. Int. Psychogeriatr. 2013, 26, 19–37. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Xiang, Z.-J.; Yang, J.-H.; Wang, W.-J.; Xu, Z.-C.; Xiang, R.-L. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Jungo, K.T.; Streit, S.; Lauffenburger, J.C. Patient factors associated with new prescribing of potentially inappropriate medications in multimorbid US older adults using multiple medications. BMC Geriatr. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Q.; Ying, K.; Lun, P.; Tan, K.T.; Ang, W.; Munro, Y.; Ding, Y.Y. Intervention elements to reduce inappropriate prescribing for older adults with multimorbidity receiving outpatient care: A scoping review. BMJ Open 2020, 10, e039543. [Google Scholar] [CrossRef]
- O’Mahony, D.; Gallagher, P.F.; Lavan, A.H. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin. Interv. Aging 2016, 11, 857–866. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; O’Connor, M.N.; Eustace, J.; Byrne, S.; Petrovic, M.; Gallagher, P. The adverse drug reaction risk in older persons (ADRROP) prediction scale: Derivation and prospective validation of an ADR risk assessment tool in older multi-morbid patients. Eur. Geriatr. Med. 2018, 9, 191–199. [Google Scholar] [CrossRef]
- Laatikainen, O.; Sneck, S.; Turpeinen, M. Medication-related adverse events in health care—What have we learned? A narrative overview of the current knowledge. Eur. J. Clin. Pharmacol. 2021, 78, 159–170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).