Soil Organic Matter Molecular Composition Shifts Driven by Forest Regrowth or Pasture after Slash-and-Burn of Amazon Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Controlled Forest Burning
2.2. Soil and Litter Sampling and Characterization
2.3. Pyrolysis–Gas Chromatography–Mass Spectrometry (Py-GC/MS) Analysis
3. Results and Discussion
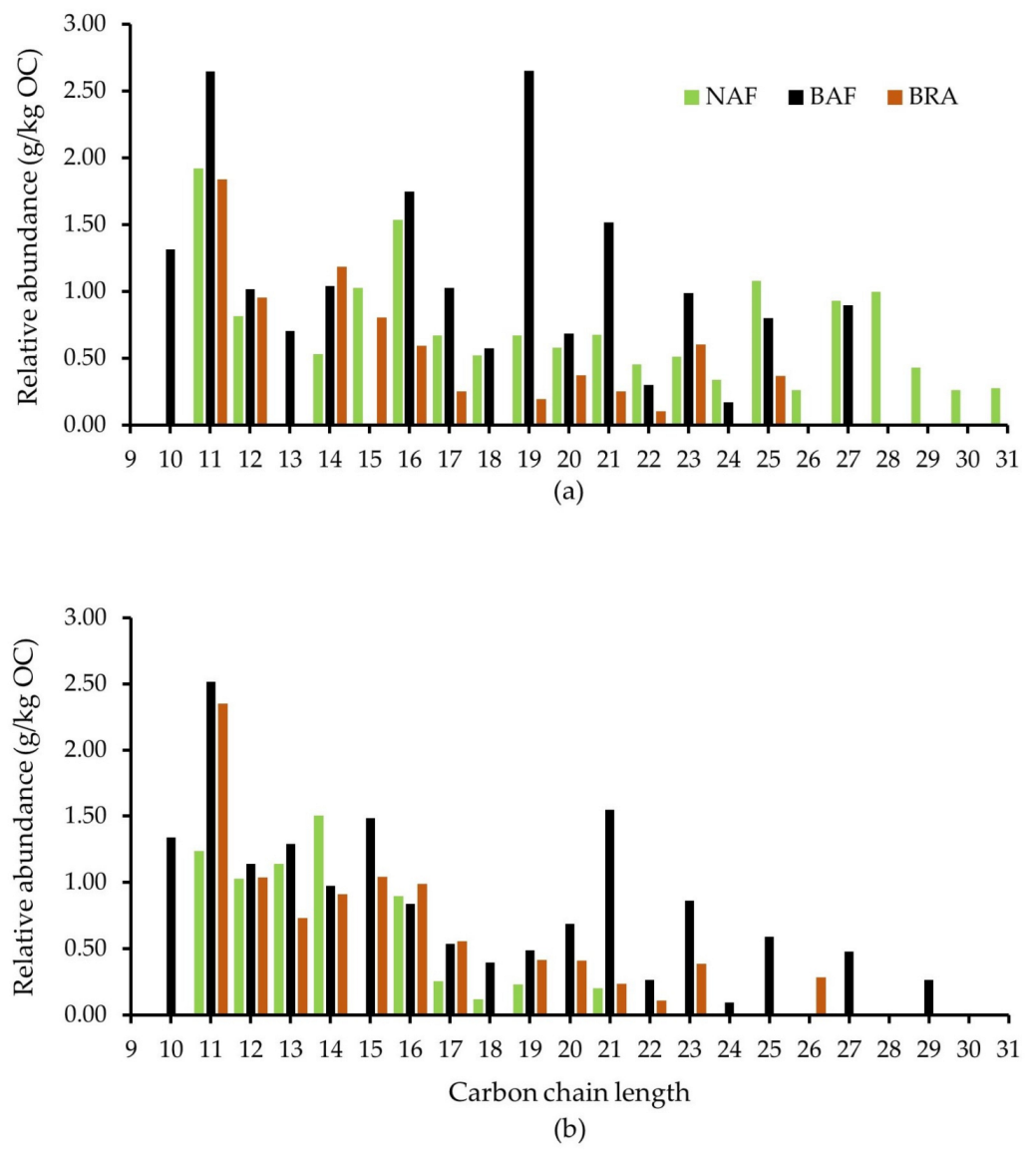
3.1. Molecular Composition of SOM Assessed by Py-GC/MS
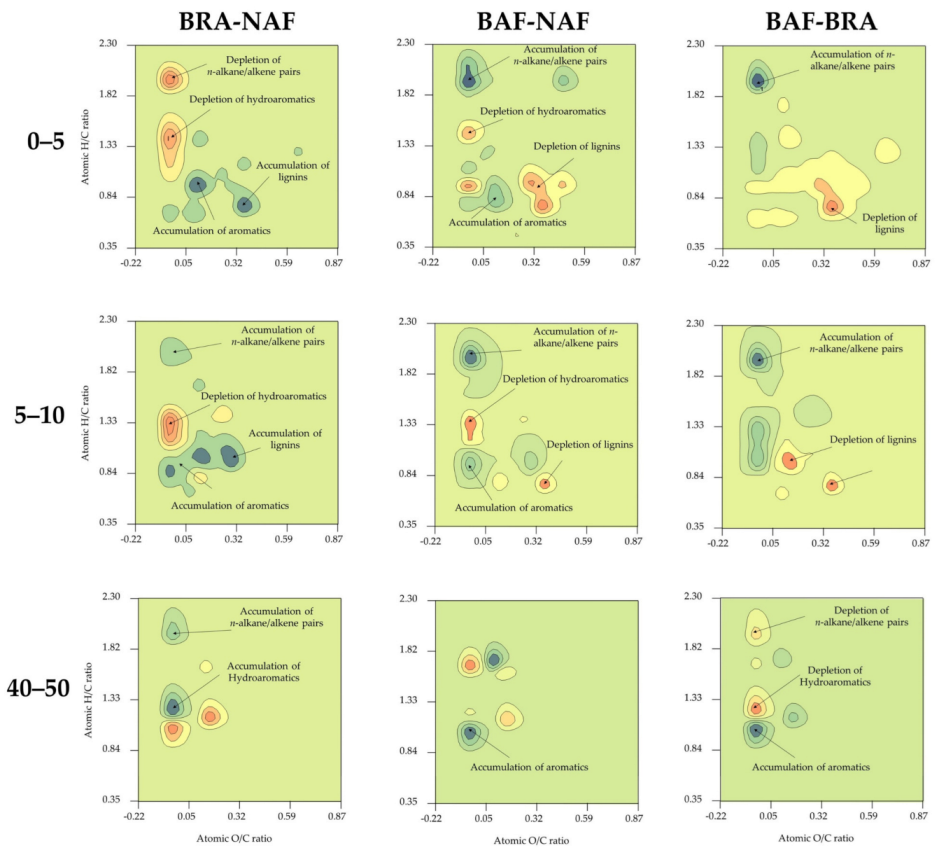
3.2. Shifts in SOM Composition Revealed by van Krevelen Subtraction Plots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brando, P.M.; Macedo, M.; Silvério, D.; Rattis, L.; Paolucci, L.; Alencar, A.; Coe, M.; Amorim, C. Amazon wildfires: Scenes from a foreseeable disaster. Flora 2020, 268, 151609. [Google Scholar] [CrossRef]
- Fujisaka, S.; White, D. Pasture or permanent crops after slash-and-burn cultivation? Land-use choice in three Amazon colonies. Agrofor. Syst. 1998, 42, 45–59. [Google Scholar] [CrossRef]
- Béliveau, A.; Davidson, R.; Lucotte, M.; Lopes, L.O.C.; Paquet, S.; Vasseur, C. Early effects of slash-and-burn cultivation on soil physicochemical properties of small-scale farms in the Tapajós region, Brazilian Amazon. J. Agric. Sci. 2015, 153, 205–221. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; Merloti, L.F.; Fonseca, M.C.; Cannavan, F.S.; Tsai, S.M. Forest-to-pasture conversion and recovery based on assessment of microbial communities in Eastern Amazon rainforest. FEMS Microbiol. Ecol. 2019, 95, fiy236. [Google Scholar] [CrossRef]
- Finer, M.; Villa, L. Amazon Fire Tracker 2021: Brazilian Amazon Fire Season Begins. MAAP. Available online: https://www.maaproject.org/2021/amazon-fire-tracker-1 (accessed on 15 July 2022).
- Lovejoy, T.E.; Nobre, C. Amazon tipping point: Last chance for action. Sci. Adv. 2019, 5, eaba2949. [Google Scholar] [CrossRef]
- Ometto, J.P.; Aguiar, A.P.D.; Martinelli, L.A. Amazon deforestation in Brazil: Effects, drivers and challenges. Carbon Manag. 2014, 2, 575–585. [Google Scholar] [CrossRef]
- Almeida, C.A.; Coutinho, A.C.; Esquerdo, J.C.D.M.; Adami, M.; Venturieri, A.; Diniz, C.G.; Dessay, N.; Durieux, L.; Gomes, A.R. High spatial resolution land use and land cover mapping of the Brazilian Legal Amazon in 2008 using Landsat-5/TM and MODIS. Acta Amazon. 2016, 46, 291–302. [Google Scholar] [CrossRef]
- Brando, P.M.; Paolucci, L.; Ummenhofer, C.C.; Ordway, E.M.; Hartmann, H.; Cattau, M.E.; Rattis, L.; Medjibe, V.; Coe, M.T.; Balch, J. Droughts, wildfires, and forest carbon cycling: A pantropical synthesis. Annu. Rev. Earth Planet. Sci. 2019, 47, 555–581. [Google Scholar] [CrossRef]
- Brando, P.M.; Soares-Filho, B.; Rodrigues, L.; Assunção, A.; Morton, D.; Tuchschneider, D.; Fernandes, E.C.M.; Macedo, M.N.; Oliveira, U.; Coe, M.T. The gathering firestorm in Southern Amazonia. Sci. Adv. 2020, 6, eaay1632. [Google Scholar] [CrossRef]
- Brando, P.M.; Silvério, D.; Maracahipes-Santos, L.; Oliveira-Santos, C.; Levick, S.R.; Coe, M.T.; Migliavacca, M.; Balch, J.K.; Macedo, M.N.; Nepstad, D.C.; et al. Prolonged tropical forest degradation due to compounding disturbances: Implications for CO2 and H2O fluxes. Glob. Chang. Biol. 2019, 25, 2855–2868. [Google Scholar] [CrossRef]
- Cerri, C.E.P.; Paustian, K.; Bernoux, M.; Victoria, R.L.; Melillos, J.M.; Cerri, C.C. Modeling changes in soil organic matter in Amazon forest to pasture conversion with the Century model. Glob. Chang. Biol. 2004, 10, 815–832. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Rocha, W.; Balch, J.K.; Brando, P.M.; Doughty, C.E.; Malhi, Y. Impacts of fire on sources of soil CO2 efflux in a dry Amazon rain forest. Glob. Chang. Biol. 2018, 24, 3629–3641. [Google Scholar] [CrossRef]
- Kroeger, E.M. Rainforest-to-pasture conversion stimulates soil methanogenesis across the Brazilian Amazon. ISME J. 2021, 15, 658–672. [Google Scholar] [CrossRef]
- Leal, O.A.; Dick, D.P.; Costa, F.S.; Knicker, H.; Carvalho Júnior, J.A.; Santos, J.C. Carbon in physical fractions and organic matter chemical composition of an Acrisol after Amazon forest burning and conversion into pasture. J. Braz. Chem. Soc. 2018, 30, 413–424. [Google Scholar] [CrossRef]
- Marques, J.D.O.; Luizão, F.J.; Teixeira, W.G.; Nogueira, E.M.; Fearnside, P.M.; Sarrazin, M. Soil Carbon Stocks under Amazonian Forest: Distribution in the Soil Fractions and Vulnerability to Emission. Open J. For. 2017, 7, 121–142. [Google Scholar] [CrossRef]
- Fujisaki, K.; Perrin, A.; Desjardins, T.; Bernoux, M.; Balbino, L.C.; Brossard, M. From forest to cropland and pasture systems: A critical review of soil organic carbon stocks changes in Amazonia. Glob. Chang. Biol. 2015, 21, 2773–2786. [Google Scholar] [CrossRef]
- Poorter, L.; Craven, D.; Jakovac, C.; Van der Sande, M.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A.; et al. Multidimensional tropical forest recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Alexis, M.A.; Rasse, D.P.; Knicker, H.; Anquetil, C.; Rumpel, C. Evolution of soil organic matter after prescribed fire: A 20-year chronosequence. Geoderma 2012, 189, 98–107. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Faria, S.R.; Varela, M.E.; Knicker, H.; González-Vila, F.J.; González-Pérez, J.A.; Keizer, J.J. Characterization of wildfire effects on soil organic matter using analytical pyrolysis. Geoderma 2012, 191, 24–30. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; Almendros, G.; Miller, A.Z.; Hatcher, P.G.; González-Pérez, J.A. Hydrophobicity of soils affected by fires: An assessment using molecular markers from ultra-high resolution mass spectrometry. Sci. Total Environ. 2022, 817, 152957. [Google Scholar] [CrossRef] [PubMed]
- Bárcenas-Moreno, G.; Jiménez-Compán, E.; San Emeterio, L.M.; Jiménez-Morillo, N.T.; González-Pérez, J.A. Soil pH and soluble organic matter shifts exerted by heating affect microbial response. Int. J. Environ. Res. Public Health 2022, 19, 15751. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H.; González-Vila, F.J.; González-Vázquez, R. Biodegradability of organic matter in fire-affected mineral soils of Southern Spain. Soil Biol. Biochem. 2013, 56, 31–39. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Desjardin, T.; Barros, E.; Sarrazin, M.; Girardin, C.; Mariotti, A. Effects of forest conversion to pasture on soil carbon content and dynamics in Brazilian Amazonia. Agric. Ecosyst. Environ. 2004, 103, 365–373. [Google Scholar] [CrossRef]
- Durigan, M.R.; Cherubin, M.R.; Camargo, P.B.; Ferreira, J.N.; Berenguer, E.; Gardner, T.A.; Barlow, J.; Dias, C.T.S.; Signor, D.; Júnior, R.C.O.; et al. Soil organic matter responses to anthropogenic forest disturbance and land use change in the eastern Brazilian Amazon. Sustainability 2017, 9, 379. [Google Scholar] [CrossRef]
- Knicker, H.; Almendros, G.; González-Vila, F.J.; González-Pérez, J.A.; Polvillo, O. Characteristic alterations of quantity and quality of soil organic matter caused by forest fires in continental Mediterranean ecosystems: A solid-state 13C NMR study. Eur. J. Soil Sci. 2006, 57, 558–569. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Bullock, J.M. Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132236. [Google Scholar] [CrossRef]
- Figueiredo, V.; Enrich-Prast, A.; Rütting, T. Evolution of nitrogen cycling in regrowing Amazonian rainforest. Sci. Rep. 2019, 9, 8538. [Google Scholar] [CrossRef]
- Poorter, L.; Rozendall, D.M.A.; Bongers, F.; Almeida, J.S.; Álvarez, F.S.; Andrade, J.L. Functional recovery of secondary tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2003405118. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; De la Rosa, J.M.; Waggoner, D.; Almendros, G.; González-Vila, F.J.; González-Pérez, J.A. Fire effects in the molecular structure of soil organic matter fractions under Quercus suber cover. Catena 2016, 145, 266–273. [Google Scholar] [CrossRef]
- Deus, M.; Miller, A.Z.; Jiménez-Morillo, N.T. Molecular Characterization of Burned Organic Matter at Different Soil Depths and Its Relationship with Soil Water Repellency: A Preliminary Result. Agronomy 2021, 11, 2560. [Google Scholar] [CrossRef]
- Almendros, G.; Tinoco, P.; De la Rosa, J.M.; Knicker, H.; González-Pérez, J.A.; González-Vila, F.J. Selective effects of forest fires on the structural domains of soil humic acids as shown by dipolar dephasing 13C NMR and graphical-statistical analysis of pyrolysis compounds. J. Soils Sediments 2016, 18, 1303–1313. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; Almendros, G.; De la Rosa, J.M.; Jordán, A.; Zavala, L.M.; Granged, A.J.P.; González-Pérez, J.A. Effect of a wildfire and of post-fire restoration actions in the organic matter structure in soil fractions. Sci. Total Environ. 2020, 728, 138715. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Knicker, H.; López-Capel, E.; Manning, D.A.C.; González-Pérez, J.A.; González-Vila, F.J. Direct detection of black carbon in soils by PyGC/MS, Carbon-13 NMR spectroscopy and thermogravimetric techniques. Soil Sci. Soc. Am. J. 2008, 72, 258–267. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Soil Resources Reports No. 106: World Reference Base for Soils Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária (Embrapa). Sistema Brasileiro de Classificação de Solos, 2nd ed.; Embrapa: Brasília, Brazil, 2006. [Google Scholar]
- Carvalho, J.A., Jr.; Amaral, S.S.; Costa, M.A.M.; Soares Neto, T.G.; Veras, C.A.G.; Costa, F.S.; van Leeuwen, T.T.; Krieger Filho, G.C.; Tourigny, E.; Forti, M.C.; et al. CO2 and CO emission rates from three forest fire controlled experiments in Western Amazonia. Atmos. Environ. 2016, 135, 73–83. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análise de Solo, Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul, Departamento de Solos: Porto Alegre, Brazil, 1995; p. 174. [Google Scholar]
- Almendros, G.; Hernández, Z.; Sanz, J.; Rodríguez-Sánchez, S.; Jiménez-González, M.A.; González-Pérez, J.A. Graphical statistical approach to soil organic matter resilience using analytical pyrolysis data. J. Chromatogr. A 2018, 1533, 164–173. [Google Scholar] [CrossRef]
- Girona-García, A.; Badía-Villas, D.; Jiménez-Morillo, N.T.; González-Pérez, J.A. Changes in soil organic matter composition after Scots pine afforestation in a native European beech forest revealed by analytical pyrolysis (Py-GC/MS). Sci. Total Environ. 2019, 691, 1155–1161. [Google Scholar] [CrossRef]
- Miller, A.Z.; Jiménez-Morillo, N.T.; Coutinho, M.L.; Gazquez, F.; Palma, V.; Sauro, F.; Pereira, M.F.C.; Rull, F.; Toulkeridis, T.; Caldeira, A.T.; et al. Organic geochemistry and mineralogy suggest anthropogenic impact in speleothem chemistry from volcanic show caves of the Galapagos. Iscience 2022, 25, 104556. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morillo, N.T.; González-Pérez, J.A.; Almendros, G.; De la Rosa, J.M.; Waggoner, D.C.; Jordán, A.; Zavala, L.M.; González-Vila, F.J.; Hatcher, P.G. Ultra-high resolution mass spectrometry of physical speciation patterns of organic matter in fire-affected soils. J. Environ. Manag. 2018, 225, 139–147. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Song, Q.; Wang, Y.; Yu, S. Phytotoxic effects of volatile organic compounds in soil water taken from a Eucalyptus urophylla plantation. Plant Soil 2014, 377, 203–215. [Google Scholar] [CrossRef]
- Gooré, S.G.; Ouattara, Z.A.; Yapi, A.T.; Békro, Y.-A.; Bighelli, A.; Paoli, M.; Tomi, F. Chemical composition of the leaf oil of Artabotrys jollyanus from Côte d’Ivoire. Rev. Bras. Farmacogn. 2017, 27, 414–418. [Google Scholar] [CrossRef]
- Bull, I.D.; Nott, C.J.; van Bergen, P.F.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted classical experiments—VI. The occurrence and source of organic acids in an experimental grassland soil. Soil Biol. Biochem. 2000, 32, 1367–1376. [Google Scholar] [CrossRef]
- Kellner, H.; Luis, P.; Pecyna, M.J.; Barbi, F.; Kapturska, D.; Krüger, D.; Zak, D.R.; Marmeisse, R.; Vandenbol, M.; Hofrichter, M. Widespread occurrence of expressed fungal secretory peroxidases in forest soils. PLoS ONE 2014, 9, e95557. [Google Scholar] [CrossRef]
- Waggoner, D.C.; Chen, H.; Willoughby, A.S.; Hatcher, P.G. Formation of black carbon-like and alicyclic aliphatic compounds by hydroxyl radical initiated degradation of lignin. Org. Geochem. 2015, 82, 69–76. [Google Scholar] [CrossRef]
- Almendros, G.; González-Vila, F.J. Wildfires, soil carbon balance and resilient organic matter in Mediterranean ecosystems. A review. Spanish J. Soil Sci. 2012, 2, 8–33. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; González-Pérez, J.A.; Jordán, A.; Zavala, L.M.; De la Rosa, J.M.; Jiménez-González, M.A.; González-Vila, F.J. Organic matter fractions controlling soil water repellency in sandy soils from the Doñana National Park (Southwestern Spain). Land Degrad. Dev. 2014, 27, 1413–1423. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J. Black carbon and the carbon cycle. Science 1998, 280, 1903–1904. [Google Scholar] [CrossRef]
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. Detailed source-specific molecular composition of ambient aerosol organic matter using ultrahigh resolution mass spectrometry and 1H NMR. Atmosphere 2016, 7, 79. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quat. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- Faria, S.R.; De la Rosa, J.M.; Knicker, H.; González-Pérez, J.A.; Keizer, J.J. Molecular characterization of wildfire impacts on organic matter in eroded sediments and topsoil in Mediterranean eucalypt stands. Catena 2015, 135, 29–37. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Jiménez-Morillo, N.T.; González-Pérez, J.A.; Almendros, G.; Vieira, D.; Knicker, H.; Keizer, J.J. Mulching-induced preservation of soil organic matter quality in a burnt eucalypt plantation in Central Portugal. J. Environ. Manag. 2019, 231, 1135–1144. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; De la Rosa, J.M.; Jiménez-Morillo, N.T.; Almendros, G.; González-Pérez, J.A.; Knicker, H. Post-fire recovery of soil organic matter in a Cambisol from typical Mediterranean forest in Southwestern Spain. Sci. Total Environ. 2016, 573, 1414–1421. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; González-Vázquez, R.; Arias, M.E.; Knicker, H. Use of multiple biogeochemical parameters to monitor the recovery of soils after forest fires. Org. Geochem. 2008, 39, 940–944. [Google Scholar] [CrossRef]
- Mutimura, M.; Ebong, C.; Rao, I.M.; Nsahlai, I.V. Effects of supplementation of Brachiaria brizantha cv. Piatá and Napier grass with Desmodium distortum on feed intake, digesta kinetics and milk production in crossbred dairy cows. Anim. Nutr. 2018, 4, 222–227. [Google Scholar] [CrossRef]
- Bliedtner, M.; Schäfer, I.K.; Zech, R.; von Suchodoletz, H. Leaf wax n-alkanes in modern plants and topsoils from eastern Georgia (Caucasus)—Implications for reconstructing regional paleovegetation. Biogeosciences 2018, 15, 3927–3936. [Google Scholar] [CrossRef]
- van Bergen, P.F.; Bull, I.D.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted classical experiments: I. Total lipid extracts, solvent insoluble residues and humic acids from Broadbalk Wilderness. Org. Geochem. 1997, 26, 117–135. [Google Scholar] [CrossRef]
- Velasco-Molina, M.; Berns, A.E.; Macías, F.; Knicker, H. Biochemically altered charcoal residues as an important source of soil organic matter in subsoils of fire-affected subtropical regions. Geoderma 2016, 262, 62–70. [Google Scholar] [CrossRef]
- Dittmar, T.; de Rezende, C.E.; Manecki, M.; Niggemann, J.; Coelho Ovalle, A.R.; Stubbins, A.; Bernardes, M.C. Continuous flux of dissolved black carbon from a vanished tropical forest biome. Nat. Geosci. 2012, 5, 618–622. [Google Scholar] [CrossRef]
- Turcios, M.M.; Jaramillo, M.M.A.; do Vale, J.F.; Fearnside, P.M.; Barbosa, R.I. Soil charcoal as long-term pyrogenic carbon storage in Amazonian seasonal forests. Glob. Chang. Biol. 2016, 22, 190. [Google Scholar] [CrossRef] [PubMed]
- De Blas, E.; Almendros, G.; Sanz, J. Molecular characterization of lipid fractions from extremely water–repellent pine and eucalyptus forest soils. Geoderma 2013, 206, 75–84. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; Spangenberg, J.E.; Miller, A.Z.; Jordán, A.; Zavala, L.M.; González-Vila, F.J.; González-Pérez, J.A. Wildfire effects on lipid composition and hydrophobicity of bulk soil and soil size fractions under Quercus suber cover (SW-Spain). Environ. Res. 2017, 159, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Abney, R.B.; Berhe, A.A. Pyrogenic Carbon Erosion: Implications for Stock and Persistence of Pyrogenic Carbon in Soil. Front. Earth Sci. 2018, 6, 26. [Google Scholar] [CrossRef]
- Brodowski, S.; Rodionov, A.; Haumaier, L.; Glaser, B.; Amelung, W. Revised black carbon assessment using benzene polycarboxylic acids. Org. Geochem. 2005, 36, 1299–1310. [Google Scholar] [CrossRef]
- Almendros, G.; González-Vila, F.J.; Martín, F. Fire–induced transformation of soil organic matter from an oak forest. An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R.P.D. Soil hydrophobicity variations with depth and particle size fraction in burned and unburnt Eucalyptus globulus and Pinus pinaster forest terrain in Aqueda Basin, Portugal. Catena 1996, 27, 25–47. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Tinoco, P.; Almendros, G.; Martín, F. Py-CG-MS analysis of the formation and degradation stages of charred residues from lignocellulosic biomass. J. Agric. Food Chem. 2001, 49, 1128–1131. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; Almendros, G.; De la Rosa, J.M.; González-Vila, F.J. Appraisal of polycyclic aromatic hydrocarbons (PAHs) in environmental matrices by analytical pyrolysis (Py–GC/MS). J. Anal. Appl. Pyrolysis 2014, 109, 1–8. [Google Scholar] [CrossRef]


| Site | pH | P | K+ | Ca2+ | Mg2+ | Al3+ | Al+H | CECe | TOC * |
|---|---|---|---|---|---|---|---|---|---|
| (mg dm−3) | (cmolc dm−3) | g kg−1 | |||||||
| 0–5 cm | |||||||||
| NAF | 3.8 | 4.1 | 26.0 | 0.1 | 0.1 | 2.2 | 15.4 | 2.5 | 14.5 |
| BAF | 4.7 | 3.1 | 53.0 | 1.5 | 0.5 | 1.3 | 7.7 | 3.4 | 17.9 |
| BRA | 4.7 | 2.1 | 47.0 | 0.8 | 0.4 | 0.5 | 3.5 | 1.8 | 10.1 |
| 5–10 cm | |||||||||
| NAF | 4.0 | 2.1 | 17.0 | 0.1 | 0.1 | 2.5 | 15.4 | 2.7 | 10.4 |
| BAF | 4.3 | 3.1 | 32.0 | 0.5 | 0.2 | 2.6 | 9.7 | 3.4 | 10.3 |
| BRA | 4.9 | 1.6 | 25.0 | 1.0 | 0.2 | 0.8 | 3.5 | 2.1 | 8.0 |
| 40–50 cm | |||||||||
| NAF | 4.6 | 1.1 | 11.0 | 0.1 | 0.1 | 4.5 | 17.3 | 4.7 | 3.4 |
| BAF | 4.4 | 0.8 | 39.0 | 0.1 | 0.1 | 3.7 | 12.3 | 4.0 | 4.6 |
| BRA | 4.5 | 0.4 | 22.0 | 0.1 | 0.1 | 3.4 | 7.7 | 3.7 | 2.9 |
| Chemical Family * | NAF | BAF | BRA | NAF | BAF | BRA | NAF | BAF | BRA |
|---|---|---|---|---|---|---|---|---|---|
| g/kg OC | |||||||||
| 0–5 cm | 5–10 cm | 40–50 cm | |||||||
| Lip | 4.10 | 5.26 | 1.54 | 1.59 | 2.44 | 1.44 | 0.30 | 0.00 | 0.62 |
| UACs | 3.21 | 6.03 | 2.26 | 4.46 | 3.66 | 1.86 | 1.47 | 3.45 | 0.70 |
| Lig | 0.65 | 0.37 | 0.31 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 |
| N-comp | 0.09 | 0.28 | 0.29 | 0.63 | 0.07 | 0.16 | 0.00 | 0.00 | 0.00 |
| PAHs | 1.16 | 1.57 | 0.42 | 1.07 | 0.93 | 0.74 | 0.00 | 0.00 | 0.04 |
| Pep | 2.15 | 2.45 | 1.74 | 0.63 | 1.63 | 1.56 | 0.84 | 0.38 | 1.54 |
| Pol | 3.14 | 1.95 | 3.52 | 2.03 | 1.57 | 2.12 | 0.79 | 0.77 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, O.d.A.; Jiménez-Morillo, N.T.; González-Pérez, J.A.; Knicker, H.; de Souza Costa, F.; Jiménez-Morillo, P.N.; de Carvalho Júnior, J.A.; dos Santos, J.C.; Pinheiro Dick, D. Soil Organic Matter Molecular Composition Shifts Driven by Forest Regrowth or Pasture after Slash-and-Burn of Amazon Forest. Int. J. Environ. Res. Public Health 2023, 20, 3485. https://doi.org/10.3390/ijerph20043485
Leal OdA, Jiménez-Morillo NT, González-Pérez JA, Knicker H, de Souza Costa F, Jiménez-Morillo PN, de Carvalho Júnior JA, dos Santos JC, Pinheiro Dick D. Soil Organic Matter Molecular Composition Shifts Driven by Forest Regrowth or Pasture after Slash-and-Burn of Amazon Forest. International Journal of Environmental Research and Public Health. 2023; 20(4):3485. https://doi.org/10.3390/ijerph20043485
Chicago/Turabian StyleLeal, Otávio dos Anjos, Nicasio T. Jiménez-Morillo, José A. González-Pérez, Heike Knicker, Falberni de Souza Costa, Pedro N. Jiménez-Morillo, João Andrade de Carvalho Júnior, José Carlos dos Santos, and Deborah Pinheiro Dick. 2023. "Soil Organic Matter Molecular Composition Shifts Driven by Forest Regrowth or Pasture after Slash-and-Burn of Amazon Forest" International Journal of Environmental Research and Public Health 20, no. 4: 3485. https://doi.org/10.3390/ijerph20043485
APA StyleLeal, O. d. A., Jiménez-Morillo, N. T., González-Pérez, J. A., Knicker, H., de Souza Costa, F., Jiménez-Morillo, P. N., de Carvalho Júnior, J. A., dos Santos, J. C., & Pinheiro Dick, D. (2023). Soil Organic Matter Molecular Composition Shifts Driven by Forest Regrowth or Pasture after Slash-and-Burn of Amazon Forest. International Journal of Environmental Research and Public Health, 20(4), 3485. https://doi.org/10.3390/ijerph20043485










