Soil pH and Soluble Organic Matter Shifts Exerted by Heating Affect Microbial Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Experimental Design
2.3. Soil Characterization
2.4. Organic Matter Analysis Composition Measured by Analytical Pyrolysis (Py-GC/MS)
2.5. Microbial Abundance Estimation
2.6. Culture Media Preparation
2.7. Plates Inoculation and Spreading
2.8. Statistical Analyses
3. Results and Discussion
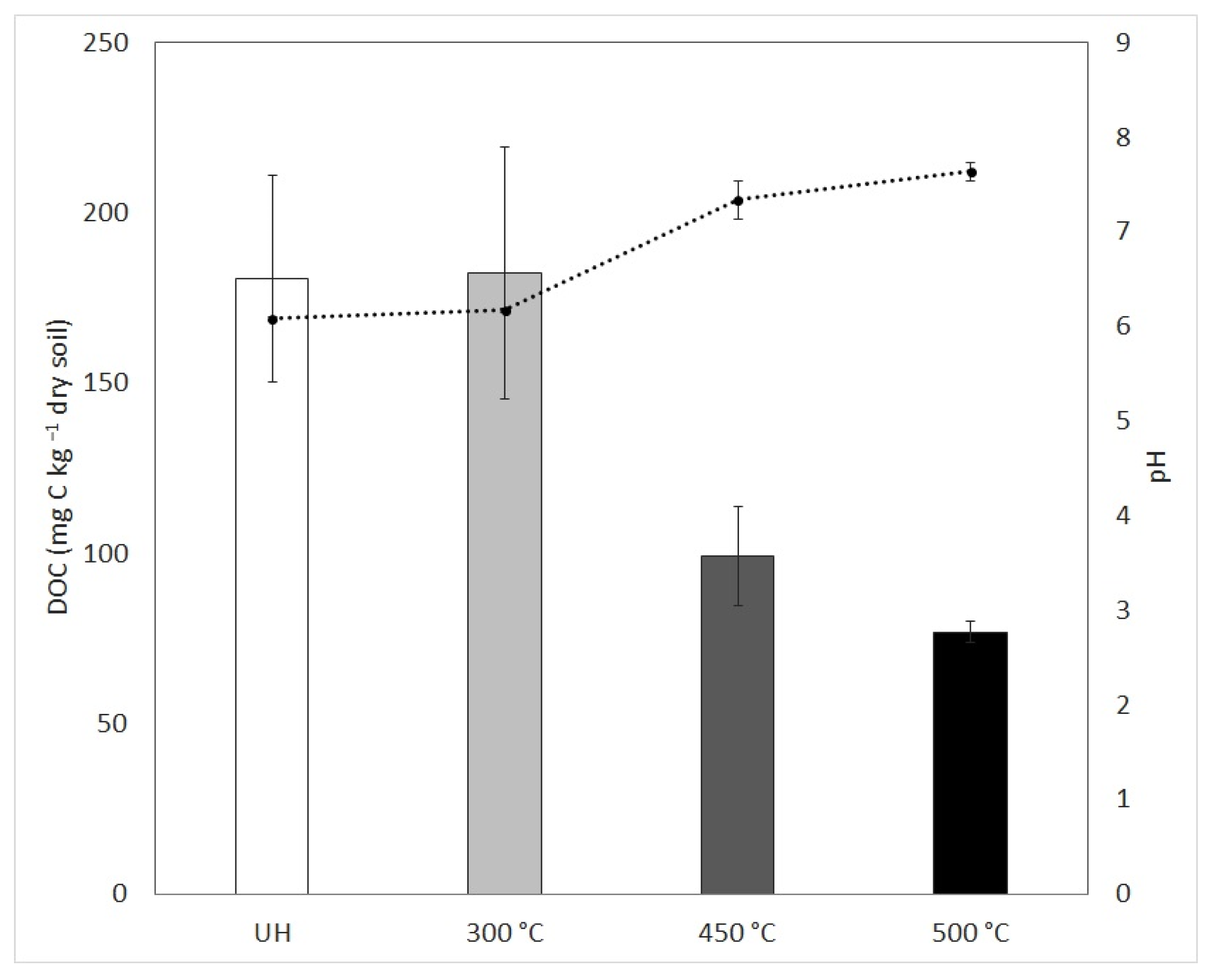
3.1. Soil Characterization
3.2. Characterization of Soil: Water Extract to Culture Media
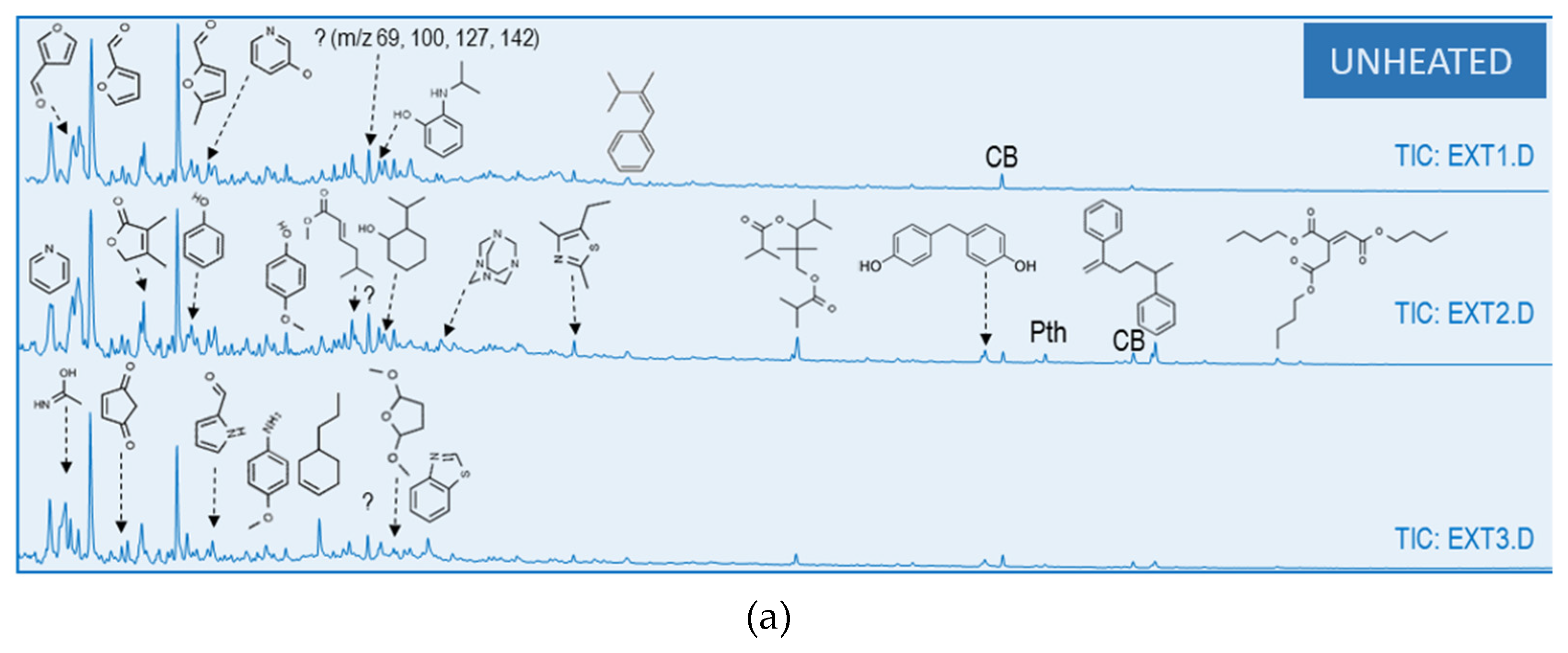
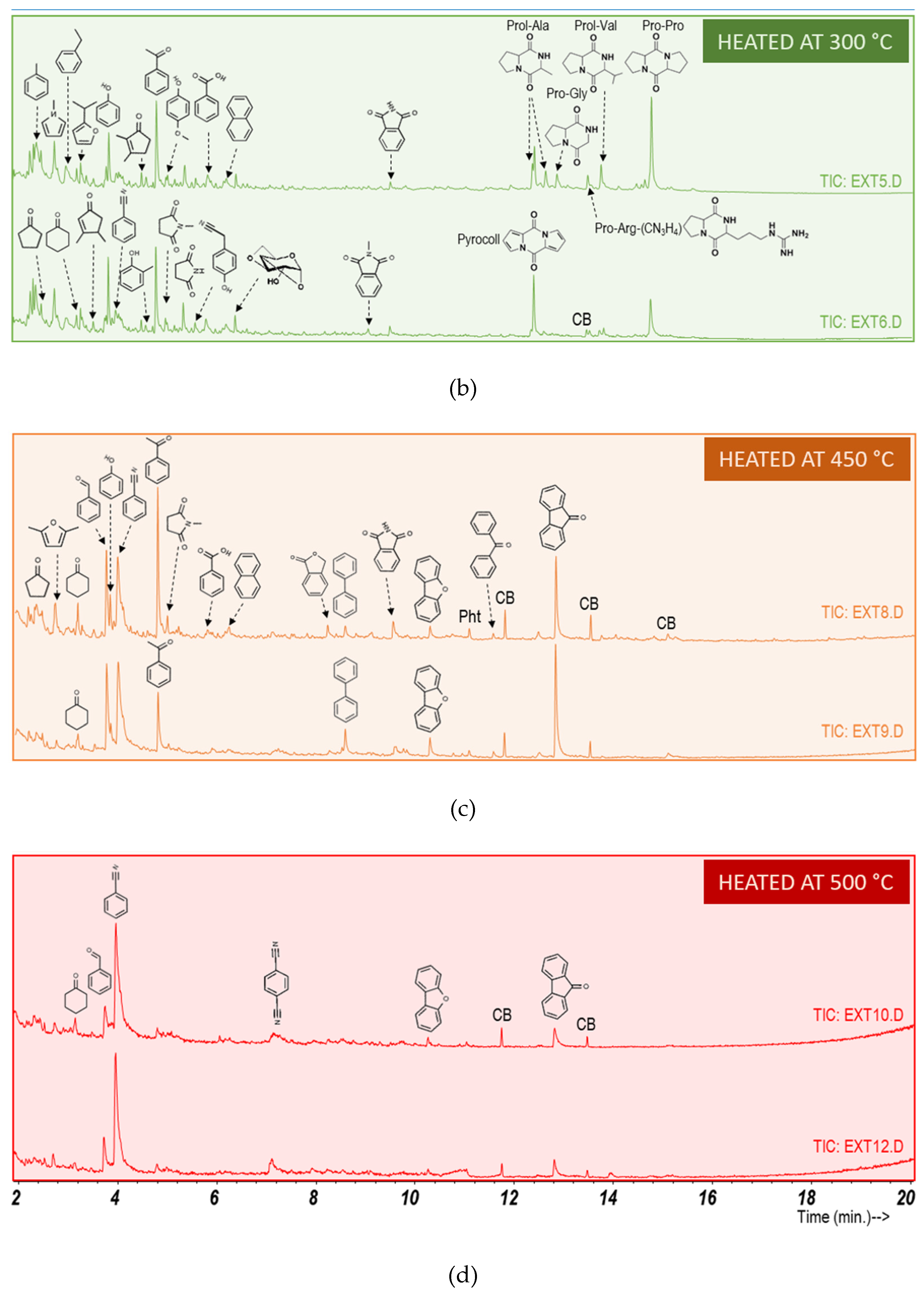
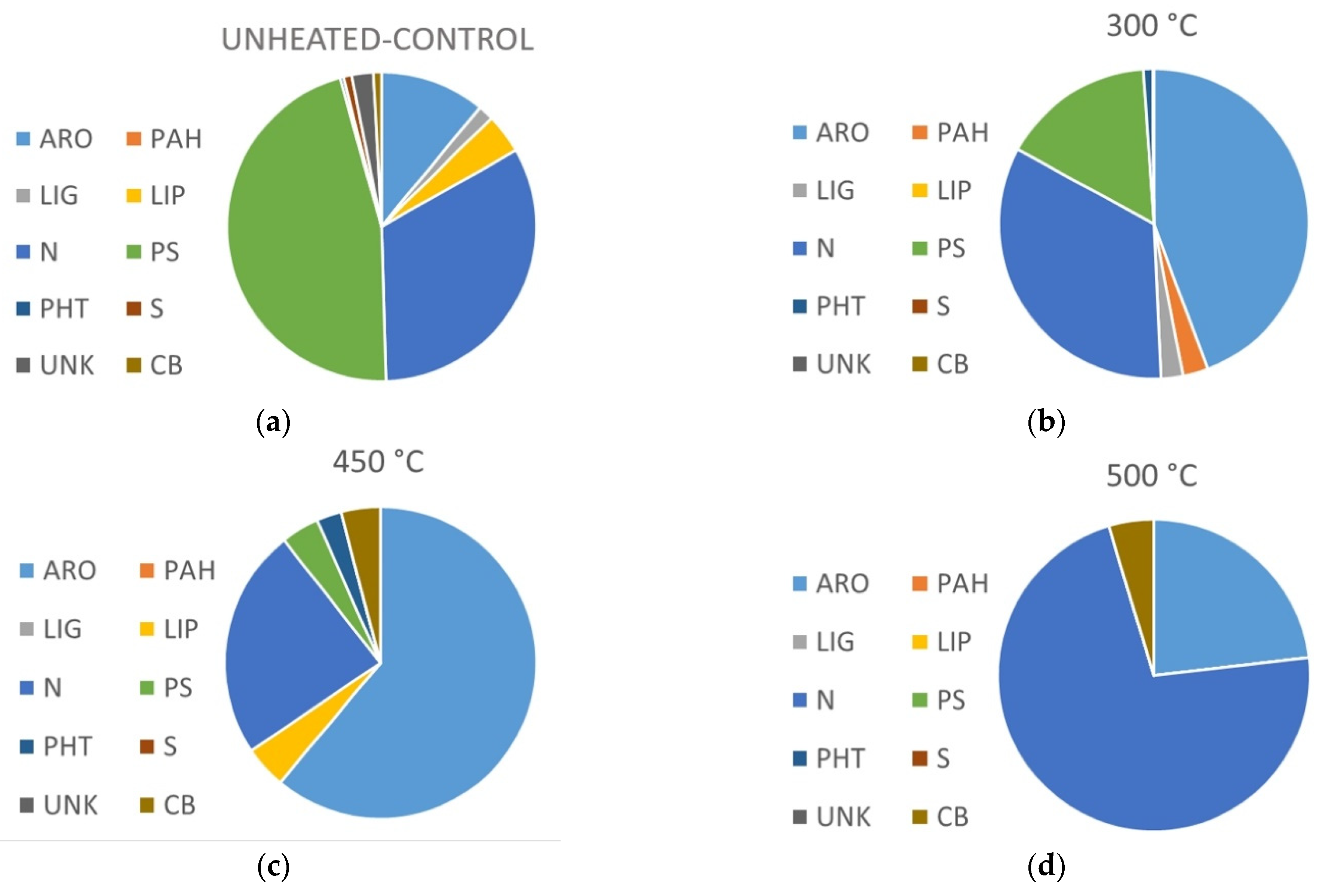
3.3. Heating Effect on Organic Matter Composition
3.4. Van Krevelen Diagrams
3.5. Abundance of Viable and Cultivable Micro-Organisms
3.5.1. General Heating Effect
3.5.2. Compensation of Nutrient Weakening Induced by Heating
3.5.3. Compensation of pH Rise Induced by Heating
3.5.4. Effect of Qualitative Changes in Organic Matter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, L. The Mediterranean Burns: WWF’s Mediterranean Proposal for the Prevention of Rural Fires; WWF (Asociación para la defensa de la naturaleza WWF/ADENA): Madrid, Spain, 2019. [Google Scholar]
- Machado Nunes Romeiro, J.; Eid, T.; Antón-Fernández, C.; Kangas, A.; Trømborg, E. Natural disturbances risks in European Boreal and Temperate forests and their links to climate change—A review of modelling approaches. For. Ecol. Manag. 2022, 509, 120071. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. A Burning Story: The Role of Fire in the History of Life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth-Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Zavala, L.; Granged, A.; Jordan, A.; Barcenas-Moreno, G. Effect of burning temperature on water repellency and aggregate stability in forest soils under laboratory conditions. Geoderma 2010, 158, 366–374. [Google Scholar] [CrossRef]
- Barcenas-Moreno, G.; Garcia-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Baath, E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- Granged, A.J.P.; Jordán, A.; Zavala, L.M.; Bárcenas, G. Fire-induced changes in soil water repellency increased fingered flow and runoff rates following the 2004 Huelva wildfire. Hydrol. Process. 2011, 25, 1614–1629. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Bárcenas-Moreno, G.; Torres, P. Fire effects on soil microbiology. In Restoration Strategies after Forest Fire; Cerdà, A., Robichaud, P., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 2009; pp. 133–175. [Google Scholar]
- Muñoz-Rojas, M.; Bárcenas-Moreno, G. Microbiology. In Fire Effects on Soil Properties; Pereira, P., Mataix-Solera, J., Úbeda, X., Rein, G., Cerdà, A., Eds.; CSIRO Publishing: Clayton, VIC, Autralia, 2019. [Google Scholar]
- Barcenas-Moreno, G.; Baath, E. Bacterial and fungal growth in soil heated at different temperatures to simulate a range of fire intensities. Soil Biol. Biochem. 2009, 41, 2517–2526. [Google Scholar] [CrossRef]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn effects on soil properties associated to heat transfer under contrasting moisture content. Sci. Total Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barcenas-Moreno, G.; Rousk, J.; Baath, E. Fungal and bacterial recolonisation of acid and alkaline forest soils following artificial heat treatments. Soil Biol. Biochem. 2011, 43, 1023–1033. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Barcenas-Moreno, G.; Bååth, E.; Rousk, J. Functional implications of the pH-trait distribution of the microbial community in a re-inoculation experiment across a pH gradient. Soil Biol. Biochem. 2016, 93, 69–78. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Beneyto, J.; Bååth, E. Plant species influence on soil microbial short-term response after fire simulation. Plant Soil 2014, 374, 701–713. [Google Scholar] [CrossRef]
- Guerrero, C.; Mataix-Solera, J.; Gómez, I.; García-Orenes, F.; Jordán, M.M. Microbial recolonization and chemical changes in a soil heated at different temperatures. Int. J. Wildland Fire 2005, 14, 385–400. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; De Pascale, R.A.; Gentile, A.; De Santo, A.V. Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires. Int. J. Wildland Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]
- Widden, P.; Parkinson, D. The effects of a forest fire on soil microfungi. Soil Biol. Biochem. 1975, 7, 125–138. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Prieto, A.; Bååth, E. Bacterial activity in a forest soil after soil heating and organic amendments measured by the thymidine and leucine incorporation techniques. Soil Biol. Biochem. 1996, 28, 419–426. [Google Scholar] [CrossRef]
- Barcenas-Moreno, G.; Garcia-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J. Plant community influence on soil microbial response after a wildfire in Sierra Nevada National Park (Spain). Sci. Total Environ. 2016, 573, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Molero-Mesa, J.; Pérez-Raya, F.; Valle-Tendero, F. Medio natural. In Parque Natural de Sierra Nevada, Paisaje, Fauna, Flora e Itinerarios; Molero-Mesa, J., Pérez-Raya, F., Valle-Tendero, F., Eds.; Editorial Rueda: Madrid, Spain, 1992; pp. 27–58. [Google Scholar]
- Baillie, I.C. Soil Survey Staff 1999, Soil Taxonomy. Soil Use Manag. 2001, 17, 57–60. [Google Scholar] [CrossRef]
- Bouyoucos, G.S. Recalibration of the hydrometer method for making mechanical analysis of soil. Agron. J. 1951, 7, 8. [Google Scholar] [CrossRef]
- Foster, J.C. Soil sampling, handling, storage and analyses. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press Inc.: San Diego, California, USA, 1995; pp. 49–121. [Google Scholar]
- Walkley, A.; Black, I.A. An examinat on of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Miller, A.L., Keeney, R.H., Eds.; American Society of Agronomy: Madison, Wisconsin, USA, 1982; pp. 595–624. [Google Scholar]
- Jiménez-Morillo, N.T.; de la Rosa, J.M.; Waggoner, D.; Almendros, G.; González-Vila, F.J.; González-Pérez, J.A. Fire effects in the molecular structure of soil organic matter fractions under Quercus suber cover. CATENA 2016, 145, 266–273. [Google Scholar] [CrossRef]
- Zuberer, D.A. Recovery and Enumeration of Viable Bacteria. In Methods of Soil Analysis. Part 2-Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1994; Volume 2, pp. 119–144. [Google Scholar]
- Wollum, A.G., II. Cultural Methods for Soil Microorganisms. In Methods of Soil Analysis; Agronomy Monographs; American Society of Agronomy; John Wiley and Sons: Hoboken, NJ, USA, 1983; pp. 781–802. [Google Scholar]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Effects of heating on some chemical parameters related to soil fertility and plant growth. Soil Sci. 1990, 149, 344–350. [Google Scholar] [CrossRef]
- DeBano, L.F.; Neary, D.G.; Ffolliott, P.F. Fire’s Effects on Ecosystems; John Willey and Sons Inc.: New York, NY, USA, 1998. [Google Scholar]
- Choromanska, U.; DeLuca, T.H. Microbial activity and nitrogen mineralization in forest mineral soils following heating: Evaluation of post-fire effects. Soil Biol. Biochem. 2002, 34, 263–271. [Google Scholar] [CrossRef]
- Prokushkin, A.S.; Tokareva, I.V. The influence of heating on organic matter of forest litters and soils under experimental conditions. Eurasian Soil Sci. 2007, 40, 628–635. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; De la Rosa, J.M.; Jiménez-Morillo, N.T.; Almendros, G.; González-Pérez, J.A.; Knicker, H. Post-fire recovery of soil organic matter in a Cambisol from typical Mediterranean forest in Southwestern Spain. Sci. Total Environ. 2016, 572, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, G. Effect of Fire on Soil Quality. In Soil Erosion as a Consequence of Forest Fires; Sala, M., Rubio, J.L., Eds.; Geoforma Ediciones: Logroño, Spain, 1994; pp. 15–27. [Google Scholar]
- Fernández, I.; Cabaneiro, A.; Carballas, T. Organic matter changes immediately after a wildfire in an atlantic forest soil and comparison with laboratory soil heating. Soil Biol. Biochem. 1997, 29, 1–11. [Google Scholar] [CrossRef]
- Fernández, I.; Cabaneiro, A.; Carballas, T. Thermal resistance to high temperatures of different organic fractions from soils under pine forests. Geoderma 2001, 104, 281–298. [Google Scholar] [CrossRef]
- Kiersch, K.; Kruse, J.; Eckhardt, K.-U.; Fendt, A.; Streibel, T.; Zimmermann, R.; Broll, G.; Leinweber, P. Impact of grassland burning on soil organic matter as revealed by a synchrotron- and pyrolysis–mass spectrometry-based multi-methodological approach. Org. Geochem. 2012, 44, 8–20. [Google Scholar] [CrossRef]
- Fabbri, D.; Adamiano, A.; Falini, G.; De Marco, R.; Mancini, I. Analytical pyrolysis of dipeptides containing proline and amino acids with polar side chains. Novel 2,5-diketopiperazine markers in the pyrolysates of proteins. J. Anal. Appl. Pyrolysis 2012, 95, 145–155. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; Bárcenas, G.M.; Jiménez-Morillo, N.T.; Colchero-Asensio, M.; San Emeterio, L.M.; de la Rosa, J.M. Effect of pH and vegetation cover in soil organic matter structure at a high-mountain ecosystem (Sierra Nevada National Park, Granada, Spain). In Proceedings of the EGU General Assembly 2020, On-line, 4–8 May 2020; p. EGU2020-8246. [Google Scholar]
- De la Rosa, J.M.; González-Pérez, J.A.; González-Vázquez, R.; Knicker, H.; López-Capel, E.; Manning, D.A.C.; González-Vila, F.J. Use of pyrolysis/GC–MS combined with thermal analysis to monitor C and N changes in soil organic matter from a Mediterranean fire affected forest. CATENA 2008, 74, 296–303. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Colodette, J.L.; Longue Júnior, D.; Gomes, F.J.B.; Martino, D.C. Preliminary Studies on Furfural Production from Lignocellulosics. J. Wood Chem. Technol. 2014, 34, 178–190. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Lyu, X. Development of a universal method for high yield of furfural and hydrogen from raw lignocellulosic biomass. Bioresour. Technol. Rep. 2022, 18, 101022. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10341, 2(5H)-Furanone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_5H_-Furanone (accessed on 12 August 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1049, Pyridine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pyridine (accessed on 12 August 2022).
- Yates, F.S.; Katritzky, A.R.; Rees, C.W. 2.09—Pyridines and their Benzo Derivatives: (vi) Applications. In Comprehensive Heterocyclic Chemistry; Pergamon: Oxford, UK, 1984; pp. 511–524. [Google Scholar]
- Bush, L.; Hempfling, W.P.; Burton, H.; Gorrod, J.W.; Jacob, P. Chapter 2—Biosynthesis of nicotine and related compounds. In Analytical Determination of Nicotine and Related Compounds and Their Metabolites; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 13–44. [Google Scholar]
- Verma, S.K.; Thareja, S. Chapter 11—An overview on chemistry of natural aldose reductase inhibitors for the management of diabetic complications. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 65, pp. 381–429. [Google Scholar]
- Rodríguez-Rodríguez, C.E.; Jesús García-Galán, M.; Blánquez, P.; Díaz-Cruz, M.S.; Barceló, D.; Caminal, G.; Vicent, T. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J. Hazard. Mater. 2012, 213–214, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, L.; Chen, W. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231, 1163–1171. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Geoffrey Chan, W.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Boon, J.J.; Pastorova, I.; Botto, R.E.; Arisz, P.W. Structural studies on cellulose pyrolysis and cellulose chars by PYMS, PYGCMS, FTIR, NMR and by wet chemical techniques. Biomass Bioenergy 1994, 7, 25–32. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Hajaligol, M.R. Characterization of chars from biomass-derived materials: Pectin chars. Fuel 2001, 80, 1825–1836. [Google Scholar] [CrossRef]
- McGrath, T.E.; Chan, W.G.; Hajaligol, M.R. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2003, 66, 51–70. [Google Scholar] [CrossRef]
- Buurman, P.; Peterse, F.; Almendros Martin, G. Soil organic matter chemistry in allophanic soils: A pyrolysis-GC/MS study of a Costa Rican Andosol catena. Eur. J. Soil Sci. 2007, 58, 1330–1347. [Google Scholar] [CrossRef]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Pyrolysis of polyunsaturated fatty acids. Fuel Process. Technol. 2014, 120, 89–95. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of Organic Molecules, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. i–ii. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Chan, W.G.; Seeman, J.I.; Hajaligol, M.R. Formation of low molecular weight heterocycles and polycyclic aromatic compounds (PACs) in the pyrolysis of α-amino acids. J. Anal. Appl. Pyrolysis 2003, 66, 97–121. [Google Scholar] [CrossRef]
- Coleman, W.M.; Chung, H.L. Pyrolysis GC-MS analysis of Amadori compounds derived from selected amino acids with glucose and rhamnose. J. Anal. Appl. Pyrolysis 2002, 63, 349–366. [Google Scholar] [CrossRef]
- Almendros, G.; González-Vila, F.J.; Martín, F. Fire induced transformation of soil organic matter from an oak forest. An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- Rantuch, P.; Chrebet, T. Thermal decomposition of cellulose insulation. Cellul. Chem. Technol. 2014, 48, 461–467. [Google Scholar]
- Johns, I.B.; McElhill, E.A.; Smith, J.O. Thermal Stability of Organic Compounds. IEC Prod. Res. Dev. 1962, 1, 2–6. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Vance, E.D.; Chapin Iii, F.S. Substrate limitations to microbial activity in taiga forest floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Kamble, P.N.; Rousk, J.; Frey, S.D.; Bååth, E. Bacterial growth and growth-limiting nutrients following chronic nitrogen additions to a hardwood forest soil. Soil Biol. Biochem. 2013, 59, 32–37. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Change Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Kamble, P.N.; Bååth, E. Induced N-limitation of bacterial growth in soil: Effect of carbon loading and N status in soil. Soil Biol. Biochem. 2014, 74, 11–20. [Google Scholar] [CrossRef]
- Zafra, G.; Cortés-Espinosa, D.V. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environ. Sci. Pollut. Res. 2015, 22, 19426–19433. [Google Scholar] [CrossRef] [PubMed]
- Vipotnik, Z.; Michelin, M.; Tavares, T. Ligninolytic enzymes production during polycyclic aromatic hydrocarbons degradation: Effect of soil pH, soil amendments and fungal co-cultivation. Biodegradation 2021, 32, 193–215. [Google Scholar] [CrossRef]
- Pawar, R.M. The effect of soil pH on bioremediation of Polycyclic Aaromatic Hydrocarbons (PAHS). J. Bioremediation Biodegrad. 2015, 6, 291–304. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; Jiménez-Morillo, N.T.; Mataix-Beneyto, J.; Martín-Sánchez, I. Fungal role in post-fire ecosystem recovery in Sierra Nevada National Park (Spain). In Proceedings of the European Geoscience Assembly, Vienna, Austria, 17–22 April 2016; p. EGU2016-17923. [Google Scholar]
- Casellas, M.; Grifoll, M.; Sabaté, J.; Solanas, A.M. Isolation and characterization of a 9-fluorenone-degrading bacterial strain and its role in synergistic degradation of fluorene by a consortium. Can. J. Microbiol. 1998, 44, 734–742. [Google Scholar] [CrossRef]
- Nojiri, H.; Habe, H.; Omori, T. Bacterial degradation of aromatic compounds via angular dioxygenation. J. Gen. Appl. Microbiol. 2001, 47, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Garon, D.; Krivobok, S.; Seigle-Murandi, F. Fungal degradation of fluorene. Chemosphere 2000, 40, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Kristanti, R.A. Fluorene biodegradation and identification of transformation products by white-rot fungus Armillaria sp. F022. Biodegradation 2014, 25, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Weitz, H.J.; Ballard, A.L.; Campbell, C.D.; Killham, K. The effect of culture conditions on the mycelial growth and luminescence of naturally bioluminescent fungi. FEMS Microbiol. Lett. 2001, 202, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Hatcher, P.G.; Kumar, S.; Lee, J.W. Investigation into the Sources of Biochar Water-Soluble Organic Compounds and Their Potential Toxicity on Aquatic Microorganisms. ACS Sustain. Chem. Eng. 2016, 4, 2550–2558. [Google Scholar] [CrossRef]
- Hao, S.; Zhu, X.; Liu, Y.; Qian, F.; Fang, Z.; Shi, Q.; Zhang, S.; Chen, J.; Ren, Z.J. Production Temperature Effects on the Structure of Hydrochar-Derived Dissolved Organic Matter and Associated Toxicity. Environ. Sci. Technol. 2018, 52, 7486–7495. [Google Scholar] [CrossRef] [PubMed]
- Hage, A.; Schoemaker, H.E.; Wever, R.; Zennaro, E.; Heipieper, H.J. Determination of the toxicity of several aromatic carbonylic compounds and their reduced derivatives on Phanerochaete chrysosporium using a Pseudomonas putida test system. Biotechnol. Bioeng. 2001, 73, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, R.; Chang, X.; Li, X.; Wang, G. Toxicity of Aromatic Ketone to Yeast Cell and Improvement of the Asymmetric Reduction of Aromatic Ketone Catalyzed by Yeast Cell with the Introduction of Resin Adsorption. Food Technol. Biotechnol. 2008, 46, 6. [Google Scholar]
- Muhr, E.; Leicht, O.; González Sierra, S.; Thanbichler, M.; Heider, J. A Fluorescent Bioreporter for Acetophenone and 1-Phenylethanol derived from a Specifically Induced Catabolic Operon. Front. Microbiol. 2016, 6, 1561. [Google Scholar] [CrossRef] [PubMed]
- Cserháti, T.; Illés, Z.; Nemes-Kósa, S. Effect of benzonitrile ester herbicides on the growth of some soil bacteria. J. Appl. Bacteriol. 1992, 72, 523–528. [Google Scholar] [CrossRef]
- Ajane, V.H.; Dharmadhikari, S.M. Studies on bacterial biodegradation of benzonitrile. TERI Inf. Dig. Energy Environ. 2011, 10, 6. [Google Scholar]



| Treatment | Heating Temperature (°C) | Nutrient Addition | pH Rectification | Final pH |
|---|---|---|---|---|
| UH N− | Unheated | NO | NO | 6.3 |
| UH N+ | Unheated | YES | NO | 6.1 |
| H300 N− | 300 | NO | NO | 7 |
| H300 N+ | 300 | YES | NO | 7 |
| H300 N+ pH | 300 | YES | YES | 6.2 |
| H450 N− | 450 | NO | NO | 7.3 |
| H450 N+ | 450 | YES | NO | 7.3 |
| H450 N+ pH | 450 | YES | YES | 6.1 |
| H500 N− | 500 | NO | NO | 7.4 |
| H500 N+ | 500 | YES | NO | 7.4 |
| H500 N+ pH | 500 | YES | YES | 6.1 |
| Soil Parameters | Mean ± Standard Error |
|---|---|
| Soil organic carbon (g C kg−1 dry soil) | 70.36 ± 1.25 |
| Kjeldahl Nitrogen (g N kg−1 dry soil) | 2.31 ± 0.27 |
| C:N Ratio | 31.4 ± 0.4 |
| Electrical conductivity (1:2.5, μS cm−1) | 332.5 ± 5.5 |
| Texture | Sandy loam |
| Microbial Biomass-C (mgC kg−1 dry soil) | 275.79 ± 26.34 |
| Respiration rate (μg CO2 h−1 g−1 dry soil) | 9.95 ± 0.55 |
| Bacterial growth (Leu pmol h−1 g−1 dry soil) | 100.96 ± 13.1 |
| Bacterial PLFAs (nmol g−1 drysoil) | 288.69 ± 32.19 |
| Fungal PLFAs (nmol g−1 dry soil) | 73.799 ± 8.54 |
| Cmp | RT | UH | 300 | 450 | 500 | Library/ID | m/z | MW |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.21 | 0.0 | 0.0 | 4.3 | 0.0 | 2-Butene, 2,3-dimethyl- | 69, 84 | 84 |
| 2 | 2.28 | 8.6 | 5.6 | 0.0 | 0.0 | Pyridine | 52, 79 | 79 |
| 3 | 2.39 | 1.7 | 9.5 | 6.5 | 0.0 | Benzene, methyl-(Toluene) | 91, 92 | 92 |
| 4 | 2.46 | 4.1 | 0.0 | 0.0 | 0.0 | Formamide, N-methyl- | 59 | 59 |
| 5 | 2.47 | 0.0 | 6.8 | 2.4 | 0.0 | Cyclopentanone | 55, 84 | 84 |
| 6 | 2.55 | 5.8 | 0.0 | 0.0 | 0.0 | 2H-Pyran, 3,4-dihydro- | 55, 84 | 84 |
| 7 | 2.61 | 7.4 | 0.0 | 0.0 | 0.0 | 3-Furancarboxaldehyde | 67, 94 | 94 |
| 8 | 2.75 | 0.0 | 8.0 | 4.2 | 0.0 | Furan, 2,5-dimethyl- | 53, 81, 96 | 96 |
| 9 | 2.76 | 11.1 | 0.0 | 0.0 | 0.0 | 2-Furancarboxaldehyde (Furfural) | 67, 94 | 94 |
| 10 | 2.98 | 0.0 | 6.6 | 0.0 | 0.0 | Benzene, 1,3-dimethyl- | 91, 106 | 106 |
| 11 | 3.00 | 1.7 | 0.0 | 0.0 | 0.0 | Pyridine, 4-methyl- | 66, 93 | 93 |
| 12 | 3.13 | 2.0 | 0.0 | 0.0 | 0.0 | 4-Cyclopentene-1, 3-dione | 54, 68, 96 | 96 |
| 13 | 3.19 | 0.0 | 3.0 | 3.3 | 3.5 | Cyclohexanone | 55, 69, 98 | 98 |
| 14 | 3.20 | 2.2 | 0.0 | 0.0 | 0.0 | Styrene | 78, 104 | 104 |
| 15 | 3.28 | 0.0 | 2.5 | 0.0 | 0.0 | 2-Cyclopenten, 1-one, 2-methyl- | 53, 67, 96 | 96 |
| 16 | 3.35 | 1.6 | 0.0 | 0.0 | 0.0 | 2,3,4-Trimethylfuran | 81, 95, 110 | 110 |
| 17 | 3.39 | 4.8 | 0.0 | 0.0 | 0.0 | 2(5H)-Furanone | 55, 84 | 84 |
| 18 | 3.39 | 0.0 | 2.2 | 0.0 | 0.0 | 1H-Pyrrole-1-ethyl- | 53, 80, 95 | 95 |
| 19 | 3.53 | 0.0 | 2.1 | 0.0 | 0.0 | 2-Cyclopentene-1-one, 2,3-dimethyl | 67, 95, 110 | 110 |
| 20 | 3.77 | 0.0 | 0.0 | 12.9 | 10.5 | Benzaldehyde | 51, 77, 106 | 106 |
| 21 | 3.79 | 8.6 | 0.0 | 0.0 | 0.0 | 2-Furancarboxaldehyde, 5-methyl | 53, 110 | 110 |
| 22 | 3.85 | 3.8 | 6.6 | 3.7 | 0.0 | Phenol | 66, 94 | 94 |
| 23 | 3.92 | 0.0 | 0.0 | 0.7 | 0.0 | 1H-Pyrrole-2, 5-dione | 54, 69, 97 | 97 |
| 24 | 4.01 | 2.0 | 4.0 | 23.4 | 62.2 | Benzonitrile | 76, 103 | 103 |
| 25 | 4.15 | 2.3 | 0.0 | 0.0 | 0.0 | Pyridine, 3-methoxi | 66, 94, 109 | 109 |
| 26 | 4.23 | 2.8 | 0.0 | 0.0 | 0.0 | 2-Acetylpyrrole | 66, 94, 109 | 109 |
| 27 | 4.50 | 0.0 | 1.8 | 0.0 | 0.0 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 67, 95, 110 | 110 |
| 28 | 4.60 | 0.0 | 1.6 | 0.0 | 0.0 | Phenol, 2-methyl- | 77, 107, 108 | 108 |
| 29 | 4.60 | 2.0 | 0.0 | 0.0 | 0.0 | Benzaldehyde, 2 hydroxy- | 65, 93, 122 | 122 |
| 30 | 4.80 | 0.0 | 7.8 | 13.4 | 0.0 | Acetophenone | 77, 105, 120 | 120 |
| 31 | 4.84 | 2.5 | 0.0 | 0.0 | 0.0 | Benzamine, 2-methoxy | 80, 108, 123 | 123 |
| 32 | 5.00 | 0.0 | 0.0 | 1.6 | 0.0 | 2, 5-Pyrrolidinedione, 1-methyl- | 56, 113 | 113 |
| 33 | 5.07 | 1.7 | 2.3 | 0.0 | 0.0 | Phenol, 2-methoxy- | 81, 109, 124 | 124 |
| 34 | 5.38 | 0.0 | 2.9 | 0.0 | 0.0 | Succinimide | 56, 99 | 99 |
| 35 | 5.49 | 3.2 | 0.0 | 0.0 | 0.0 | 2-Cyclohexen-1-one, 3,5-dimethyl- | 54, 82, 124 | 124 |
| 36 | 5.64 | 1.3 | 1.4 | 0.0 | 0.0 | Benzyl nitrile | 90, 117 | 117 |
| 37 | 5.76 | 2.1 | 0.0 | 0.0 | 0.0 | 2,4(3H, 5H)-Furandione, 3-ethyl | 55, 70, 100, 128 | 128 |
| 38 | 5.84 | 0.0 | 3.0 | 0.0 | 0.0 | Phenol, 3,4-dimethyl- | 77, 91, 107, 122 | 122 |
| 39 | 5.85 | 2.1 | 0.0 | 0.0 | 0.0 | Pentane, 1,5-dimethoxy- | 71, 100, 117,128 | 128 |
| 40 | 6.17 | 2.6 | 0.0 | 0.0 | 0.0 | 6-(tert-butyl)-2(1H)-pyridone | 136, 151 | 151 |
| 41 | 6.22 | 0.0 | 2.6 | 0.0 | 0.0 | Naphtalene | 102, 128 | 128 |
| 42 | 6.24 | 1.8 | 0.0 | 0.0 | 0.0 | Cyclohexanol, 2-(1-methylethyl)- | 57, 69, 85, 98, 144 | 144 |
| 43 | 6.35 | 1.9 | 0.0 | 0.0 | 0.0 | Furan, tetrahydro-2, 5-dimethoxy- | 72, 101, 131 | 131 |
| 44 | 6.40 | 0.0 | 1.7 | 0.0 | 0.0 | 1,4:3,6-Dianhydro-.alpha.-d-glucopyranose | 57, 69, 85, 98, 144 | 144 |
| 45 | 6.55 | 2.6 | 0.0 | 0.0 | 0.0 | Benzenamine, 3-ethoxy- | 80, 109, 137 | 137 |
| 46 | 6.75 | 1.0 | 0.0 | 0.0 | 0.0 | Methenamine | 112, 140 | 140 |
| 47 | 6.86 | 0.3 | 0.0 | 0.0 | 0.0 | Benzenepropanenitrile | 91, 131 | 131 |
| 48 | 6.91 | 1.3 | 0.0 | 0.0 | 0.0 | Gly-Val | 85, 114, 141, 156 | 156 |
| 49 | 7.06 | 0.5 | 0.0 | 0.0 | 0.0 | Caprolactam | 55, 85, 113 | 113 |
| 50 | 7.20 | 0.0 | 0.0 | 0.0 | 13.5 | Dicyanobenzene | 50, 75, 101, 128 | 128 |
| 51 | 7.48 | 1.0 | 0.0 | 0.0 | 0.0 | Benzoic acid, 2,5-dihydroxy | 80, 108, 136, 154 | 154 |
| 52 | 8.22 | 0.0 | 0.0 | 1.0 | 0.0 | 1(3H)-Isobenzofuranone | 77, 105, 134 | 134 |
| 53 | 8.30 | 0.8 | 0.0 | 0.0 | 0.0 | Pro-Gly | 83, 98, 111, 154 | 154 |
| 54 | 8.51 | 0.0 | 0.0 | 0.4 | 0.0 | 1-Tetradecene | 55, 196 | 196 |
| 55 | 8.59 | 0.0 | 0.0 | 2.9 | 0.0 | Byphenyl | 76, 154 | 154 |
| 56 | 9.09 | 0.0 | 0.5 | 0.0 | 0.0 | 1H-Isoindole-1, 3(2H)-dione, 2-methyl- | 76, 104, 117, 132, 161 | 161 |
| 57 | 10.27 | 0.0 | 0.0 | 2.7 | 2.2 | Dibenzofuran | 84, 193, 168 | 168 |
| 58 | 11.55 | 0.0 | 0.0 | 0.7 | 0.0 | Benzophenone | 77, 105, 182 | 182 |
| 59 | 12.27 | 0.0 | 0.5 | 0.0 | 0.0 | Cyclo (Pro-Ala) | 70, 97, 125, 168 | 168 |
| 60 | 12.43 | 0.0 | 5.0 | 0.0 | 0.0 | Pyrocoll | 65, 93, 130, 186 | 186 |
| 61 | 12.66 | 0.0 | 0.9 | 0.0 | 0.0 | Cyclo (Pro-Ala) | 70, 97, 125, 168 | 168 |
| 62 | 12.80 | 0.0 | 0.0 | 15.9 | 8.1 | 9H-Fluoren-9-one | 76, 152, 180 | 180 |
| 63 | 12.89 | 0.0 | 1.0 | 0.0 | 0.0 | Cyclo (Pro-Gly) | 70, 83, 98, 111, 154 | 154 |
| 64 | 13.34 | 0.5 | 0.0 | 0.0 | 0.0 | Phenol, 4,4′-methylenebis- | 107, 183, 200 | 200 |
| 65 | 13.50 | 0.0 | 1.2 | 0.0 | 0.0 | Cyclo (Pro-Ala)-(CN3H4) | 70, 125, 154 | 154 |
| 66 | 13.77 | 0.0 | 2.1 | 0.0 | 0.0 | Cyclo(Pro-Val) | 70, 72, 125, 154, 196 | 196 |
| 67 | 14.79 | 0.0 | 6.7 | 0.0 | 0.0 | Cyclo(Pro-Pro) | 70, 96, 138, 166, 194 | 194 |
| 68 | 15.35 | 0.5 | 0.0 | 0.0 | 0.0 | Hex-1-ene, 2,5-diphenyl- | 91, 118, 236 | 236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárcenas-Moreno, G.; Jiménez-Compán, E.; San Emeterio, L.M.; Jiménez-Morillo, N.T.; González-Pérez, J.A. Soil pH and Soluble Organic Matter Shifts Exerted by Heating Affect Microbial Response. Int. J. Environ. Res. Public Health 2022, 19, 15751. https://doi.org/10.3390/ijerph192315751
Bárcenas-Moreno G, Jiménez-Compán E, San Emeterio LM, Jiménez-Morillo NT, González-Pérez JA. Soil pH and Soluble Organic Matter Shifts Exerted by Heating Affect Microbial Response. International Journal of Environmental Research and Public Health. 2022; 19(23):15751. https://doi.org/10.3390/ijerph192315751
Chicago/Turabian StyleBárcenas-Moreno, Gael, Elizabeth Jiménez-Compán, Layla M. San Emeterio, Nicasio T. Jiménez-Morillo, and José A. González-Pérez. 2022. "Soil pH and Soluble Organic Matter Shifts Exerted by Heating Affect Microbial Response" International Journal of Environmental Research and Public Health 19, no. 23: 15751. https://doi.org/10.3390/ijerph192315751
APA StyleBárcenas-Moreno, G., Jiménez-Compán, E., San Emeterio, L. M., Jiménez-Morillo, N. T., & González-Pérez, J. A. (2022). Soil pH and Soluble Organic Matter Shifts Exerted by Heating Affect Microbial Response. International Journal of Environmental Research and Public Health, 19(23), 15751. https://doi.org/10.3390/ijerph192315751











