Three-Dimensional Carbon Monolith Coated by Nano-TiO2 for Anode Enhancement in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Anodes
2.2. MFC Setup and Operation
2.3. Characterizations and Analysis
3. Results
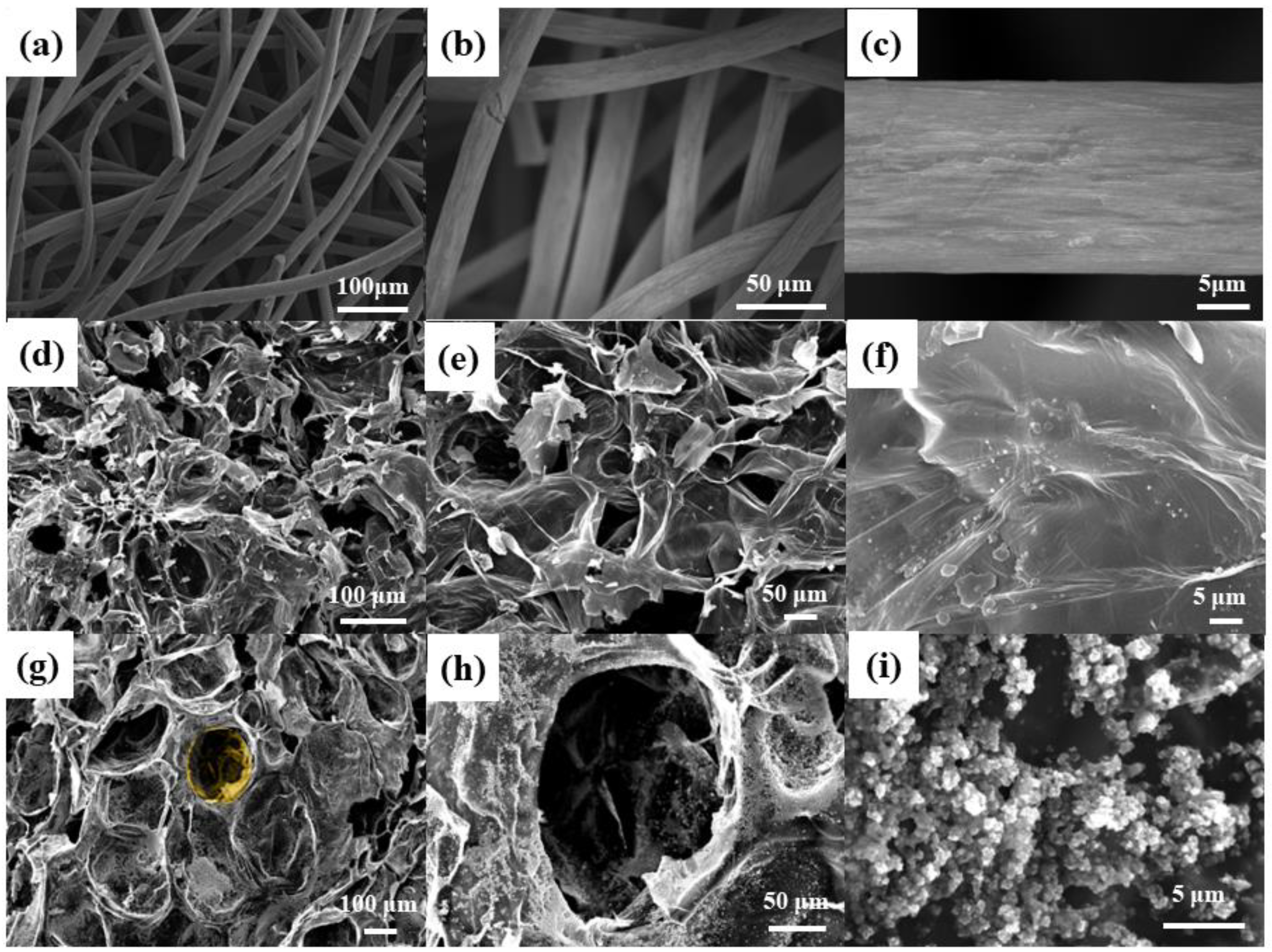
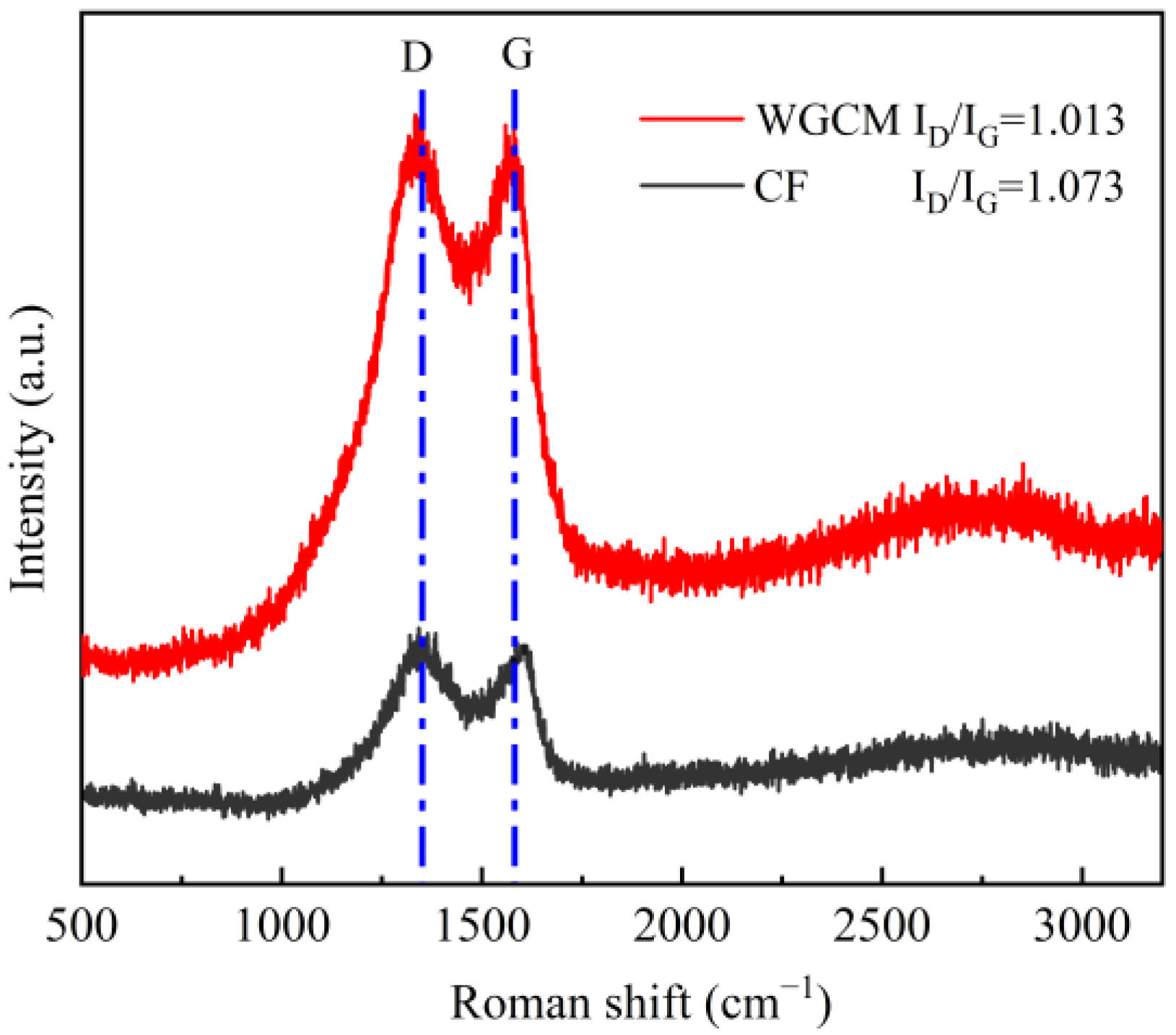
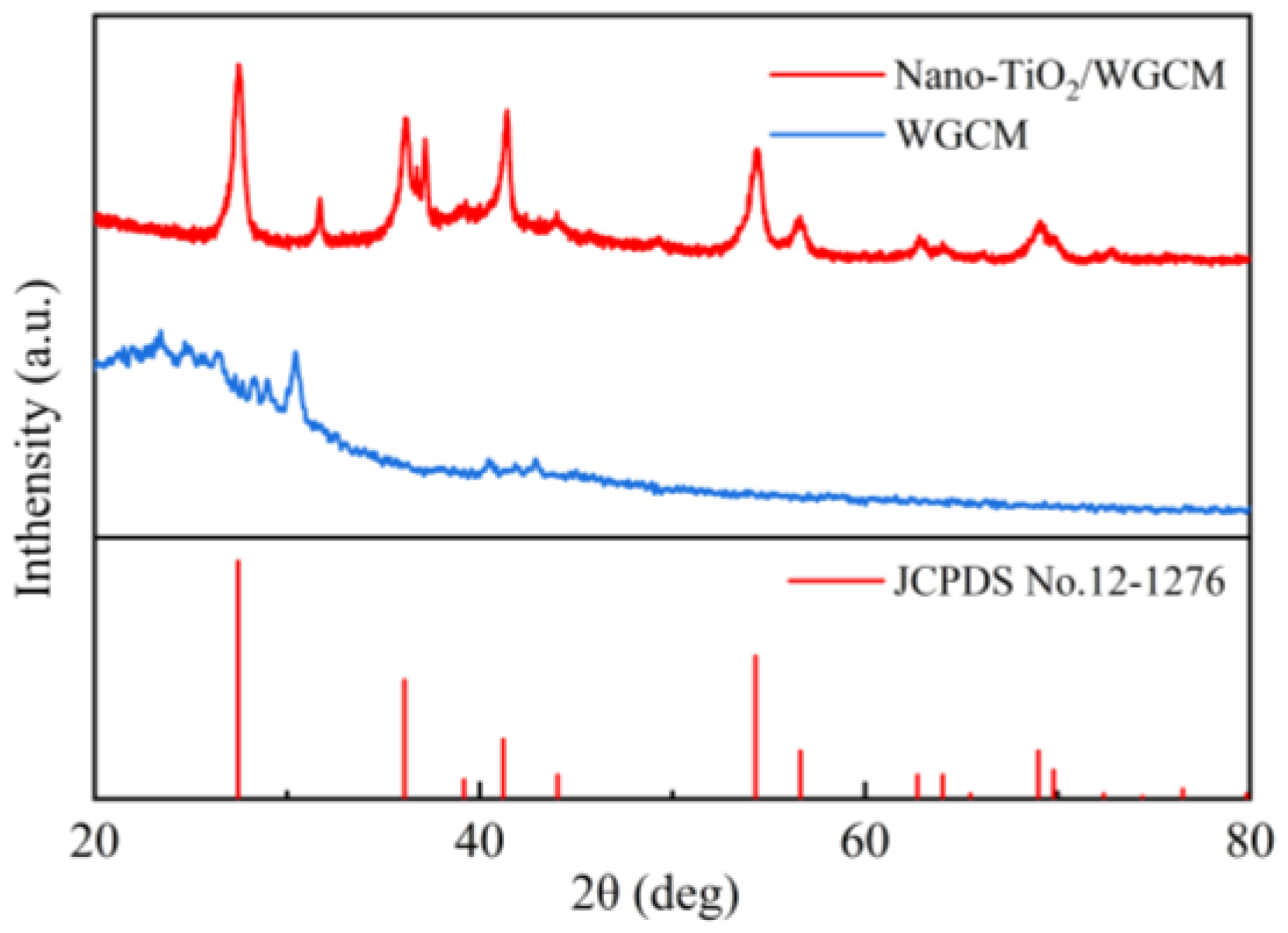
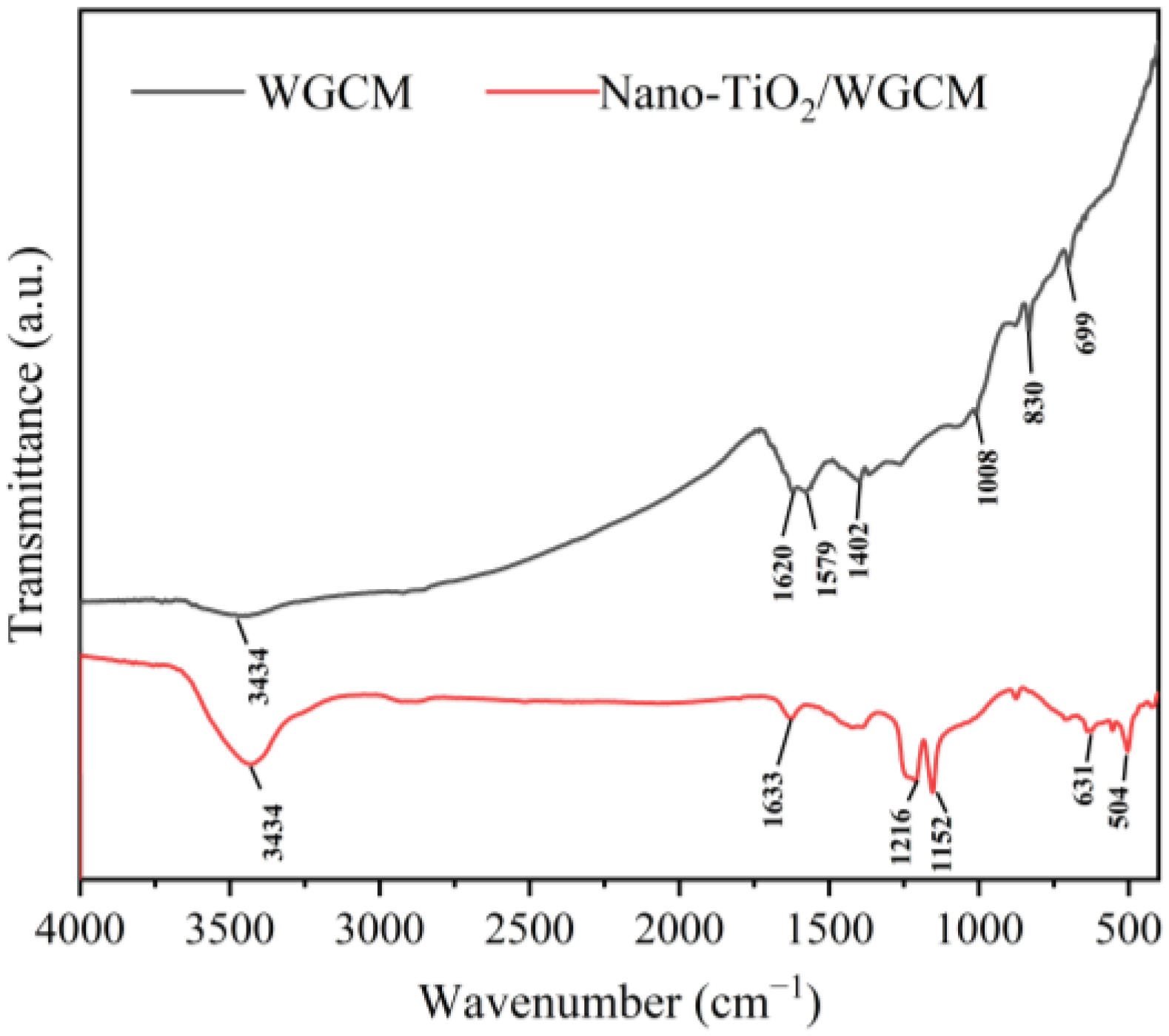
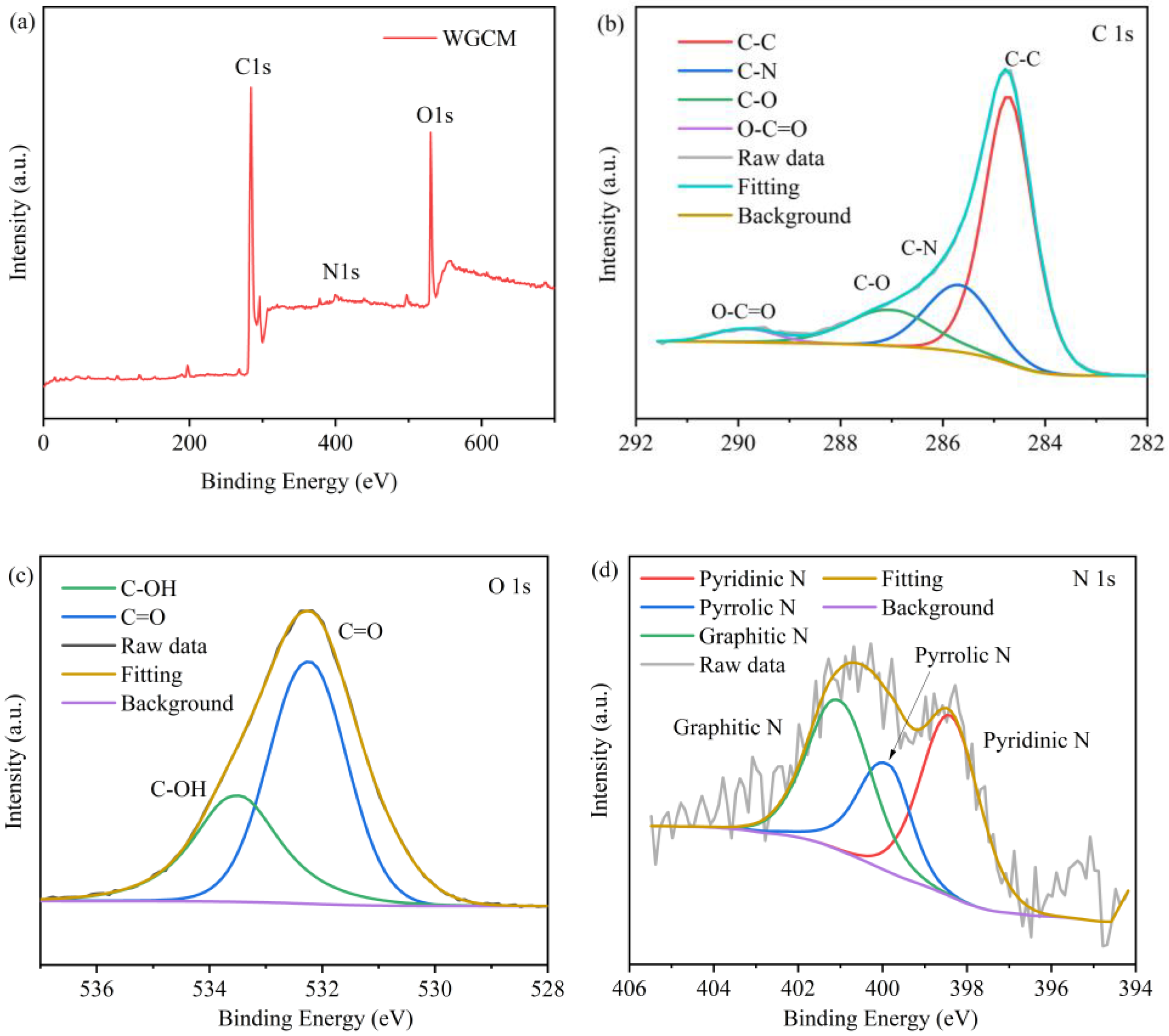
3.1. Characteristics of Anodes
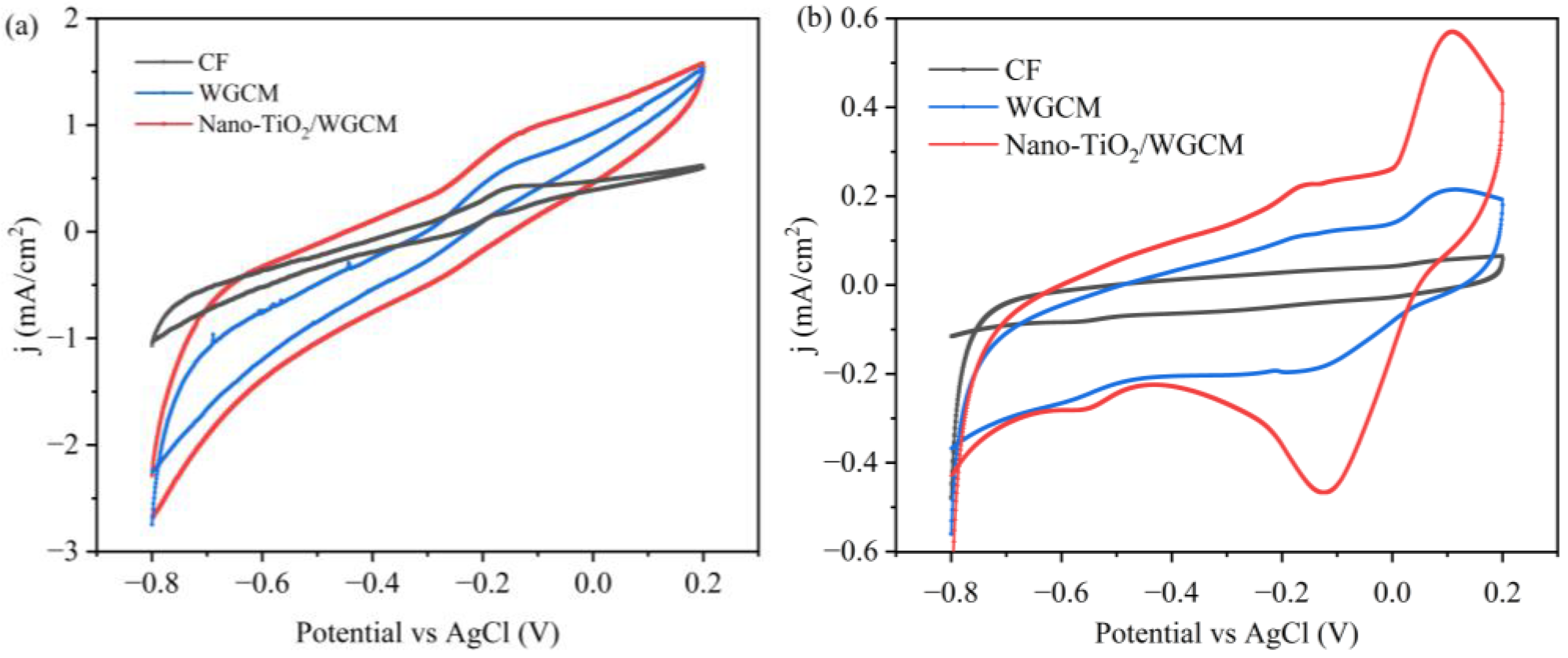
3.2. Electrochemical Analysis
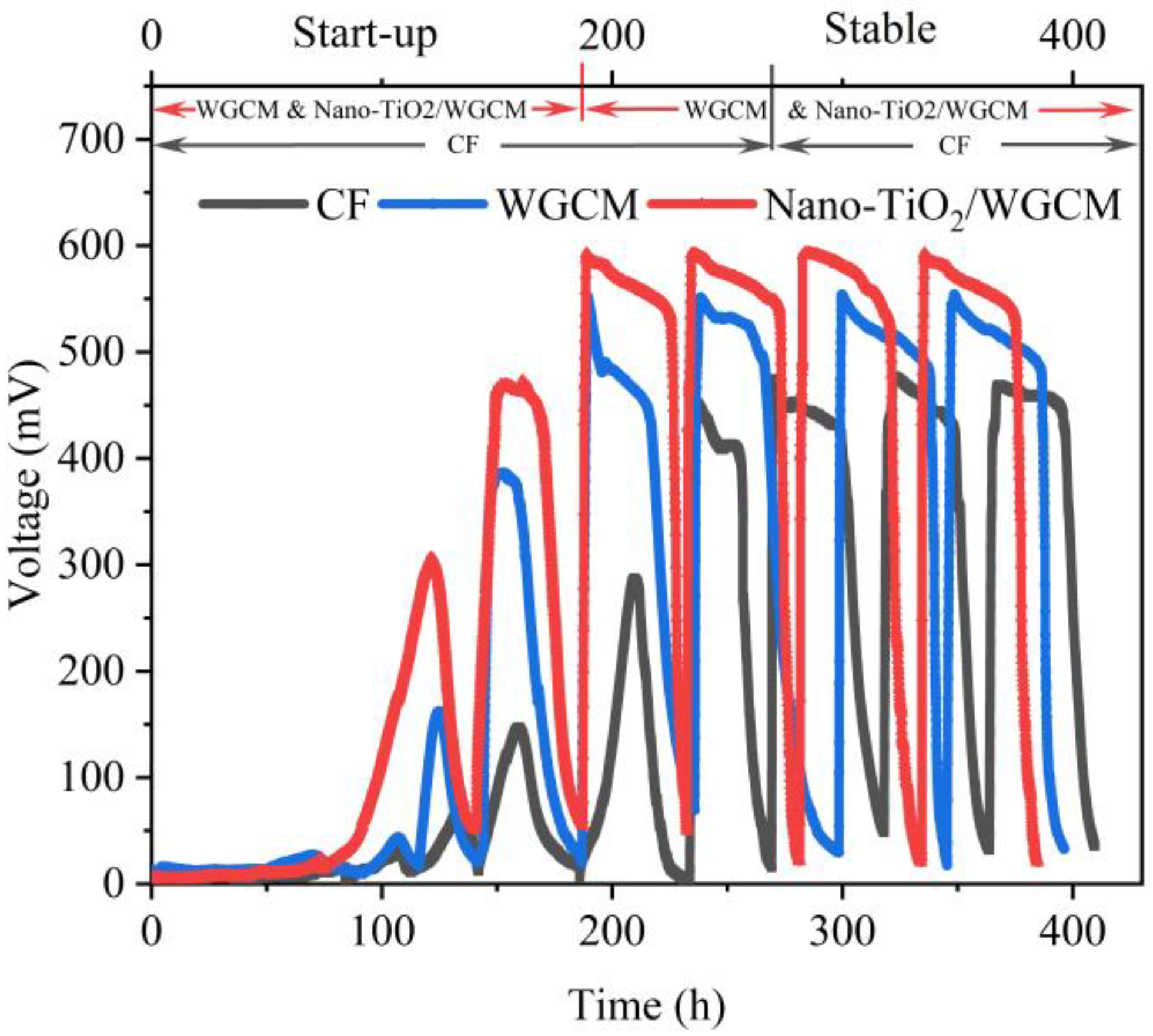
3.3. MFC Performance
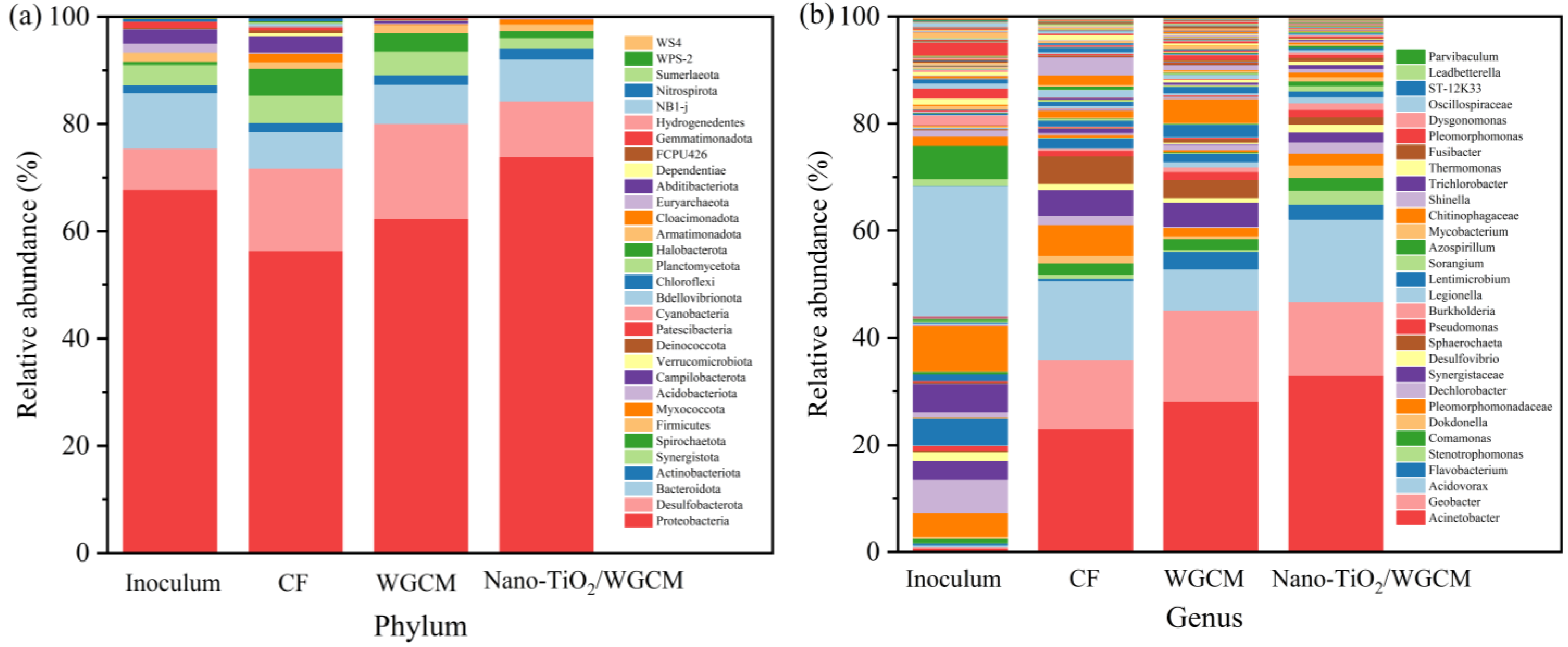
3.4. Biofilm on Anodes
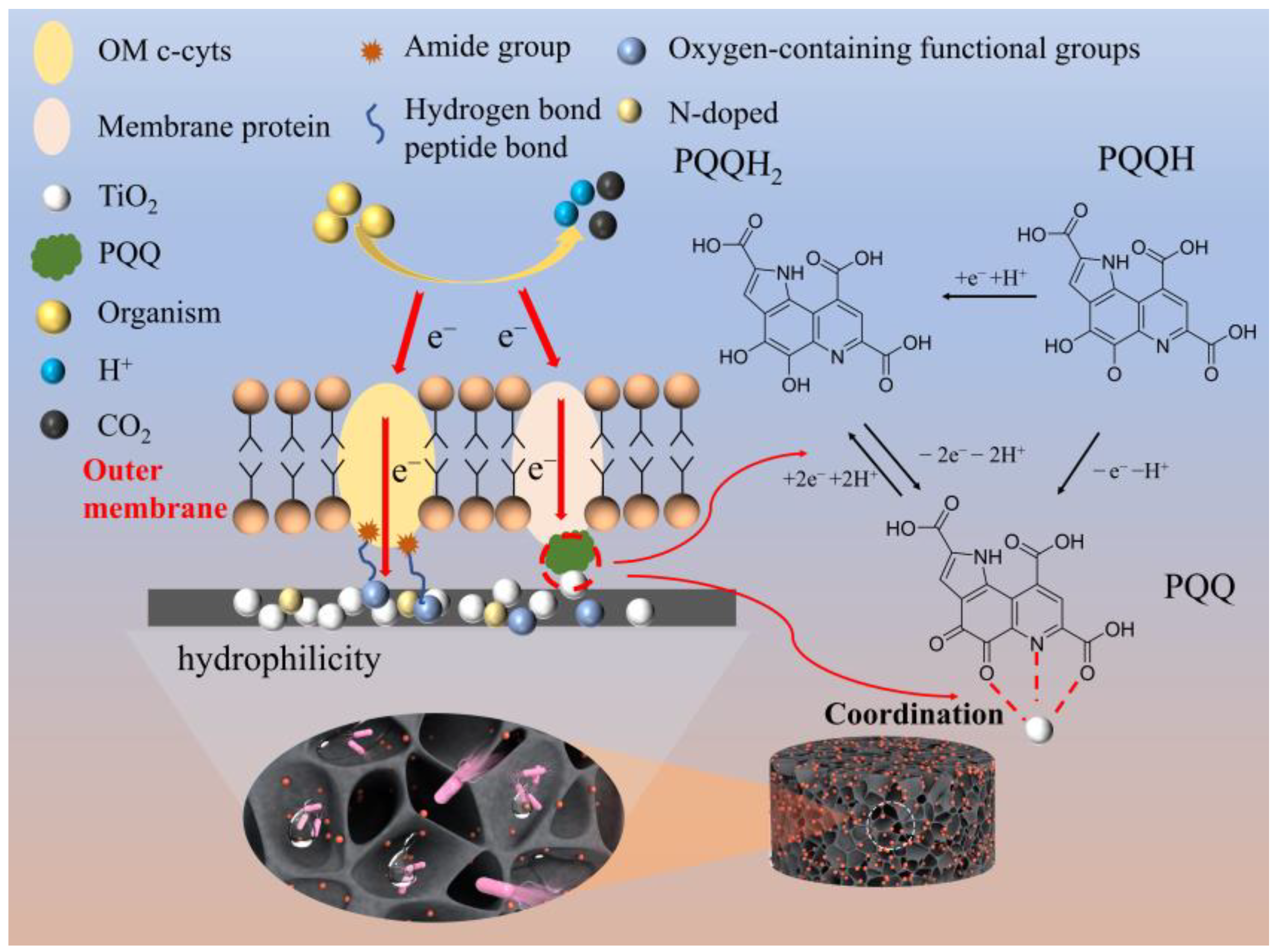
3.5. Mechanisms and Novelties of Anode Enhancement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Pandey, A.; Sharma, P.; Varjani, S.; Nguyen, T.A.H.; Hoang, N.B. A dual chamber microbial fuel cell based biosensor for monitoring copper and arsenic in municipal wastewater. Sci. Total Environ. 2022, 811, 152261. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, F.; Liu, J. Enhancing simultaneous electricity production and reduction of sewage sludge in two-chamber MFC by aerobic sludge digestion and sludge pretreatments. J. Hazard. Mater. 2011, 189, 444–449. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.; Gumel, A. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Cai, H.; Wang, J.; Bu, Y.; Zhong, Q. Treatment of carbon cloth anodes for improving power generation in a dual-chamber microbial fuel cell. J. Chem. Technol. Biotechnol. 2012, 88, 623–628. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Siara, S.; Elvis, C.; Harishkumar, R.; Chellam, P.V. ZnAl2O4 supported on lychee-biochar applied to ibuprofen photodegradation. Mater. Res. Bull. 2021, 145, 111530. [Google Scholar] [CrossRef]
- Chaijak, P.; Sato, C.; Lertworapreecha, M.; Sukkasem, C.; Boonsawang, P.; Paucar, N. Potential of Biochar-Anode in a Ceramic-Separator Microbial Fuel Cell (CMFC) with a Laccase-Based Air Cathode. Pol. J. Environ. Stud. 2019, 29, 499–503. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Xu, H.; Huang, J.; Lin, C.; Zheng, J.; Qiu, Z.; Wen, Q.; Chen, Y.; Qi, L.; Wang, Y. Bio-functional metal organic framework composite as bioanode for enhanced electricity generation by a microbial fuel cell. Electrochim. Acta 2020, 368, 137622. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: Effect of different anodic operating conditions. Bioresour. Technol. 2014, 166, 451–457. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Yue, X.; Feng, C.; Wei, C. Ruthenium oxide-coated carbon felt electrode: A highly active anode for microbial fuel cell applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Guo, W.; Qiu, J.; Mou, X.; Li, A.; Liu, H. Construction of titanium dioxide nanorod/graphite microfiber hybrid electrodes for a high performance electrochemical glucose biosensor. Nanoscale 2016, 8, 9382–9389. [Google Scholar] [CrossRef]
- Taşkan, B.; Taşkan, E.; Hasar, H. Electricity generation potential of sewage sludge in sediment microbial fuel cell using Ti–TiO2 electrode. Environ. Prog. Sustain. Energy 2020, 39, 13407. [Google Scholar] [CrossRef]
- Tang, X.; Liu, L.; Cui, Y. β-FeOOH modified carbon monolith anode derived from wax gourd for microbial fuel cells. Int. J. Hydrogen Energy 2021, 47, 4838–4845. [Google Scholar] [CrossRef]
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power Generation Using Different Cation, Anion, and Ultrafiltration Membranes in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Du, Z.; Ng, H.Y. Spontaneous modification of graphite anode by anthraquinone-2-sulfonic acid for microbial fuel cells. Bioresour. Technol. 2014, 164, 184–188. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Estimation of Cell Number by Hemocytometry Counting. Cold Spring Harb. Protoc. 2006, 2006, 4454. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Chen, H.; Yan, S.; Zhu, H.; Zhang, H.; Sun, H.; Zhang, S.; Zhang, S.; Wu, Y. Roles of graphitization degree and surface functional groups of N-doped activated biochar for phenol adsorption. J. Anal. Appl. Pyrolysis 2022, 167, 105700. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Li, J.; Cao, L.; Kajiyoshi, K. Two C-S bonds derived from carbons with different IG/ID values promote high sulfur loads and stable capacity storage. Appl. Surf. Sci. 2022, 584, 152620. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef]
- Han, T.H.; Sawant, S.Y.; Hwang, S.-J.; Cho, M.H. Three-dimensional, highly porous N-doped carbon foam as microorganism propitious, efficient anode for high performance microbial fuel cell. RSC Adv. 2016, 6, 25799–25807. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Li, W.-Q.; He, C.-S.; Zhao, H.-Q.; Han, J.-C.; Liu, X.-C.; Mu, Y. Active N dopant states of electrodes regulate extracellular electron transfer of Shewanella oneidensis MR-1 for bioelectricity generation: Experimental and theoretical investigations. Biosens. Bioelectron. 2020, 160, 112231. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Aziz, A.; Pannipara, M.; Alphonsa, A.T.; Al-Sehemi, A.G.; Balasubramani, A.; Kumar, G.G. Waste paper derived three-dimensional carbon aerogel integrated with ceria/nitrogen-doped reduced graphene oxide as freestanding anode for high performance and durable microbial fuel cells. Bioprocess Biosyst. Eng. 2019, 43, 97–109. [Google Scholar] [CrossRef]
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S. FeS2Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, e1800618. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-Dimensional Carbon Nanotube−Textile Anode for High-Performance Microbial Fuel Cells. Nano Lett. 2010, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef]
- Wang, R.; Liu, D.; Yan, M.; Zhang, L.; Chang, W.; Sun, Z.; Liu, S.; Guo, C. Three-dimensional high performance free-standing anode by one-step carbonization of pinecone in microbial fuel cells. Bioresour. Technol. 2019, 292, 121956. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, W.; Hou, H.; Hu, J.; Liu, B.; Li, J.; Cheng, K.; Huang, L.; Yuan, X.; Yang, C.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef]
- Li, D.; Deng, L.; Yuan, H.; Dong, G.; Chen, J.; Zhang, X.; Chen, Y.; Yuan, Y. N, P-doped mesoporous carbon from onion as trifunctional metal-free electrode modifier for enhanced power performance and capacitive manner of microbial fuel cells. Electrochim. Acta 2018, 262, 297–305. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, X.; Shi, Y.; Moradian, J.M.; Wang, Y.; Sun, G.; Du, J.; Ye, X.; Yong, Y. Surface activated natural wood biomass electrode for efficient microbial electrocatalysis: Performance and mechanism. Int. J. Energy Res. 2022, 46, 8480–8490. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Pannipara, M.; Al-Sehemi, A.G.; Kumar, G.G. PEDOT/NiFe2O4 nanocomposites on biochar as a free-standing anode for high-performance and durable microbial fuel cells. New J. Chem. 2019, 43, 7743–7750. [Google Scholar] [CrossRef]
- Yuan, H.; Dong, G.; Li, D.; Deng, L.; Cheng, P.; Chen, Y. Steamed cake-derived 3D carbon foam with surface anchored carbon nanoparticles as freestanding anodes for high-performance microbial fuel cells. Sci. Total Environ. 2018, 636, 1081–1088. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Genet. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Shahi, A.; Chellam, P.V.; Verma, A.; Singh, R. A comparative study on the performance of microbial fuel cell for the treatment of reactive orange 16 dye using mixed and pure bacterial species and its optimization using response surface methodology. Sustain. Energy Technol. Assess. 2021, 48, 101667. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Chen, D.; Liu, G.; Li, D.; Lee, H.-S.; Feng, Y. Hydrophilic graphene aerogel anodes enhance the performance of microbial electrochemical systems. Bioresour. Technol. 2020, 304, 122907. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Zhang, E.-H.; Chen, Z.; Liang, P.; Huang, X.; Yang, Z.-H.; Ng, I.-S.; Chen, B.-Y.; et al. Pyrosequencing Reveals a Core Community of Anodic Bacterial Biofilms in Bioelectrochemical Systems from China. Front. Microbiol. 2015, 6, 1410. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Benites, S.M.; Delfín-Narciso, D.; Luis, A.-S.; Díaz, F.; Luis, C.-C.; Moises, G.C. Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules 2022, 27, 5198. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, X.; Wang, L.; Guo, Q.; Wu, J. Study on the mechanism of anaerobic fluidized bed microbial fuel cell for coal chemical wastewater treatment. Bioprocess Biosyst. Eng. 2022, 45, 481–492. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Ganesh, V.; Berchmans, S. Bio-electrocatalysis of Acetobacter aceti through direct electron transfer using a template deposited nickel anode. Catal. Sci. Technol. 2012, 2, 1234–1241. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Poluektov, O.G.; Saponjic, Z.V.; Rajh, T. Complex and Charge Transfer between TiO2 and Pyrroloquinoline Quinone. J. Phys. Chem. B 2006, 110, 25392–25398. [Google Scholar] [CrossRef]




| Anode Materials | Surface Area of Electrodes (cm2) | Size of Electrodes | Inoculum | Power Density (mW/m2) | References |
|---|---|---|---|---|---|
| KOH-activated chestnut shells | 125.65 | 0.3 × 66.4 cm | Anaerobic mixed sludge | 850 | [35] |
| N–P-doped onion peels | 7 | 1.0 × 2.0 × 0.5 cm | Mix sludge | 742 | [36] |
| Basswood | 4 | 1.0 × 2.0 × 0.1 cm | Shewanella oneidensis MR-1 | 483 | [37] |
| PEDOT/NiFe2O4/ Neem wood | 1 | 1 × 1 cm | Pre-acclimated wastewater | 1200 | [38] |
| PANI/ Steamed cake | 18.70 | 7 cm2 × 0.5 cm | Effluent from another stable operation of MFCs | 1307 | [39] |
| WGCM | 12.17 | Φ 2.5 × 0.3 cm | Effluent from active acetate-fed MFCs | 957.6 | This study |
| Nano-TiO2/WGCM | 12.17 | Φ 2.5 × 0.3 cm | Same as above | 1396.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Chen, Y.; Zhang, S.; Li, M.; Tang, X. Three-Dimensional Carbon Monolith Coated by Nano-TiO2 for Anode Enhancement in Microbial Fuel Cells. Int. J. Environ. Res. Public Health 2023, 20, 3437. https://doi.org/10.3390/ijerph20043437
Zhao F, Chen Y, Zhang S, Li M, Tang X. Three-Dimensional Carbon Monolith Coated by Nano-TiO2 for Anode Enhancement in Microbial Fuel Cells. International Journal of Environmental Research and Public Health. 2023; 20(4):3437. https://doi.org/10.3390/ijerph20043437
Chicago/Turabian StyleZhao, Fan, Yini Chen, Shiyang Zhang, Meng Li, and Xinhua Tang. 2023. "Three-Dimensional Carbon Monolith Coated by Nano-TiO2 for Anode Enhancement in Microbial Fuel Cells" International Journal of Environmental Research and Public Health 20, no. 4: 3437. https://doi.org/10.3390/ijerph20043437
APA StyleZhao, F., Chen, Y., Zhang, S., Li, M., & Tang, X. (2023). Three-Dimensional Carbon Monolith Coated by Nano-TiO2 for Anode Enhancement in Microbial Fuel Cells. International Journal of Environmental Research and Public Health, 20(4), 3437. https://doi.org/10.3390/ijerph20043437







