Effect of Menstrual Cycle Phase on Fuel Oxidation Post HIT in Women Reproductive Age: A Pilot Study
Abstract
1. Introduction
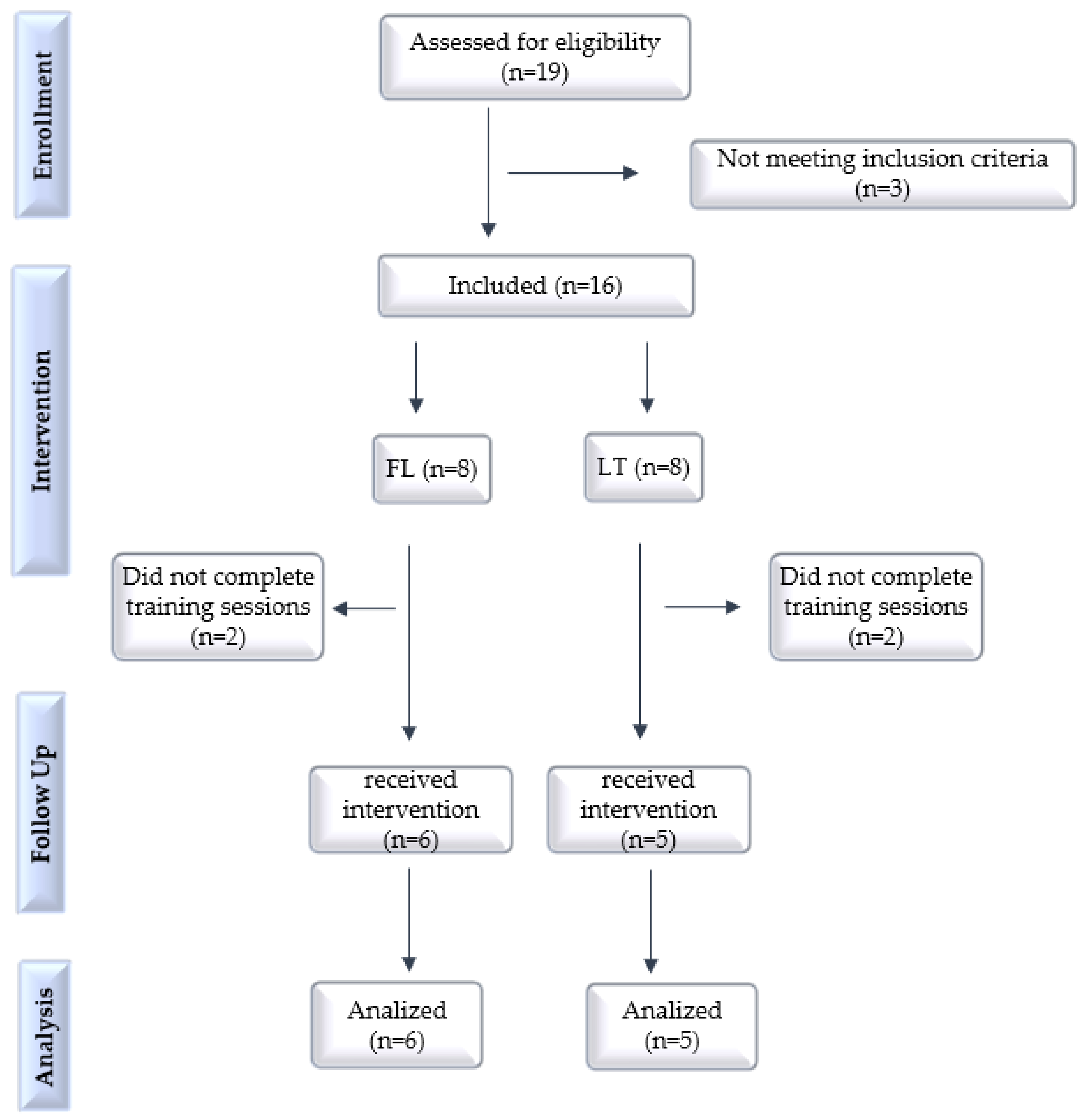
2. Materials and Methods
2.1. Trial Design, Setting and Ethics
2.2. Inclusion and Exclusion Criteria
2.3. Outcomes Evaluations
2.4. Body Composition
2.5. Cardiopulmonary Test
2.6. Indirect Calorimetry (SWRE)
2.7. Training Sessions
2.8. Statistical Analysis
3. Results
3.1. Characterization of Participants
3.2. Max Tests and Exercise Intensity
3.3. Training Sessions
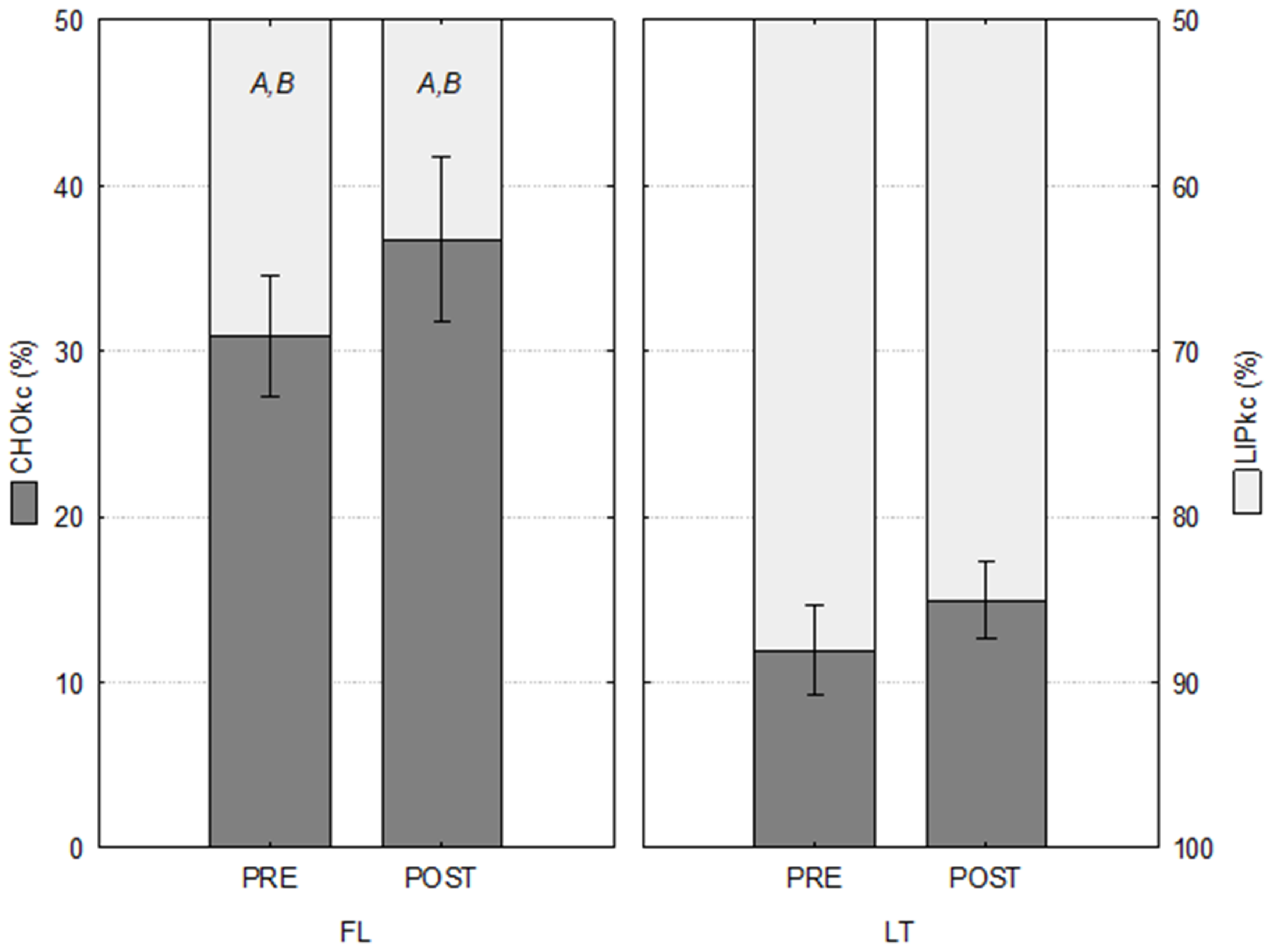
3.4. Oxidation of Substrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHO: | Carbohydrates |
| LIP: | Lipids |
| AA: | Amino acids |
| CHOox: | Carbohydrate oxidation rates |
| LIPox: | Lipid oxidation rates |
| CHOkc: | Relative amount of energy derived from carbohydrate oxidized |
| LIPkc: | Relative amount of energy derived from lipid oxidized |
| TTkc: | Total caloric expenditure |
| O2: | Oxygen |
| VO2max: | Maximal oxygen consumption |
| VO2peak: | Peak oxygen consumption |
| VATs: | Ventilatory anaerobic thresholds |
| VAT1: | First ventilatory threshold |
| VAT2: | Second ventilatory threshold |
| HIT: | High-intensity interval training |
| MICT: | Moderate-intensity continuous training |
| Vpeak: | Peak velocity |
| HR: | Heart rate |
| ECs: | Cardiorespiratory exercise |
| IPAQ: | International physical activity questionnaire |
| SWRE: | Submaximal work rate running exercise (indirect calorimetry) |
| FAS: | Fatty acids |
| BMI: | Body mass index |
| %BF: | Body fat percentage |
| FM: | Fat mass |
| FFM: | Fat free mass |
| BMR: | Basal metabolic rate |
| VE: | Ventilatory equivalents |
| FE: | Expired fractions |
| RQ: | Respiratory quotient |
| FL: | Follicular phase group |
| LT: | Luteal phase group |
| GH: | Growth hormone |
| LH: | Luteinizing hormone |
| FSH: | Follicle stimulating hormone |
| OCs: | Oral contraceptives |
References
- Carstens, M.B.; Goedecke, J.H.; Duga, L.; Evans, J.; Kroff, J.; Levitt, N.S. Fasting substrate oxidation in relation to habitual dietary fat intake and insulin resistance in nondiabetic women: A case for metabolic flexibility? Nutr. Metab. 2013, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Isacco, L. Substrate metabolism during exercise: Sexual dimorphism and women’s specificities. Eur. J. Sport Sci. 2021, 22, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef]
- Wang, N.F.; Skouby, S.O.; Humaidan, P.; Andersen, C.Y. Responseto ovulation trigger is correlated to late follicular phase progesterone levels: A hypothesis explaining reduced reproductive outcomes caused by increased late follicular progesterone rise. Hum. Reprod. 2019, 34, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrheic women. Sport Med. 2010, 40, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Rector, R.S.; Noland, R.C. Metabolic inflexibility in skeletal muscle: A prelude to the cardiometabolic syndrome? J. Cardiometab. Syndr. 2006, 1, 184–189. [Google Scholar] [CrossRef]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Exercise-Induced Inflammation during Different Phases of the Menstrual Cycle. J. Physiother. Phys. Rehabil. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. An acute bout of high-intensity interval training in-creases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 303–310. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolf, D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef]
- Broskey, N.T.; Tam, C.S.; Sutton, E.F.; Altazan, A.D.; Burton, J.H.; Ravussin, E.; Redman, L.M. Metabolic inflexibility in women with PCOS is similar to women with type 2 diabetes. Nutr. Metab. 2018, 15, 75. [Google Scholar] [CrossRef]
- Marquezi, M.L.; Uzunian, M.A.; Gimenez, R.; Souza, M.T. Substrate oxidation patterns during cardiorespiratory exercise prescribed by direct and indirect methods. Rev. Bras. Educ. Fis. Esporte 2018, 31, 373–383. [Google Scholar] [CrossRef]
- Maturana, F.M.; Martus, P.; Zipfel, S.; Nieb, A.M. Effectiveness of HIIE versus MICT in Improving Cardiometabolic Risk Factors in Health and Disease: A Meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 559–573. [Google Scholar] [CrossRef]
- Paz, C.L.; Fraga, A.; Tenório, M. Effect of high-intensity interval training versus continuous training on body composition: A systematic review with meta-analysis. Rev. Bras. Ativ. Fís. Saúde. 2018, 22, 512–522. [Google Scholar] [CrossRef]
- Lu, M.; Li, M.; Yi, L.; Li, F.; Lin, F.; Jin, T.; Zang, Y.; Thu, J. Effects of 8-week high-intensity interval training and moderate-intensity continuous training on bone metabolism in sedentary young females. J. Exerc. Sci. Fit. 2022, 20, 77–83. [Google Scholar] [CrossRef]
- Wetter, A.C.; Economos, C.D. Relationship between quantitative ultrasound, anthropometry, and sports participation in college aged adults. Osteoporos. Int. 2004, 15, 799–806. [Google Scholar] [CrossRef]
- Golestani, F.; Mogharnasi, M.; Rfani-Far, M.; Abtahi-Eivari, S.H. The effects of spirulina under high-intensity interval training on levels of nestafin-1, omentin-1, and lipid profiles in overweight and obese females: A randomized, controlled, single-blind trial. J. Res. Med. Sci. 2021, 26, 10. [Google Scholar] [CrossRef]
- Matsudo, S.; Araujo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. International Physical Activity Questionnaire (IPAQ): Validity and reproducibility in Brazil. J. Phys. Act. Health 2001, 6, 5–18. [Google Scholar] [CrossRef]
- Silva, M.M.; Carvalho, R.S.M.; Freitas, M.B. Bioimpedance for body composition assessment: A didactic-experimental proposal for health students. Braz. J. Phys. Teach. 2019, 41, 2018-271. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a World Health Organization Expert Committee; World Health Organization: Geneva, Switzerland, 1995; p. 854. Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 9 September 2022).
- Kaercher, P.L.K.; Glânzel, M.H.; da Rocha, G.G.; Schmidt, L.M.; Nepomuceno, P.; Stroschöen, L.; Pohl, H.H.; Reckziegel, M.B. The Borg Subjective Perception Scale as a Tool for Monitoring the Physical Effort Intensity. Braz. J. Exerc. Prescr. Physiol. 2019, 12, 1180–1185. Available online: http://www.rbpfex.com.br/index.php/rbpfex/article/view/1603 (accessed on 11 September 2022).
- Marquezi, M.L.; Augustine, C.F.M.; Lima, F.R.; Aparecido, J.M.L.; Cascapera, M.S. Six hit treadmill sessions improve lipid oxidation and ventilatory threshold intensities. Braz. J. Sports Med. 2019, 25, 328–332. [Google Scholar] [CrossRef]
- Riddell, M.C.; Partington, S.L.; Stupka, N.; Armstrong, D.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance trained athletes. Int. J. Sports Nutr. Exerc. Metab. 2003, 13, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; McCracken-Compton, M.A.; Ainsworth, B. Substrate Responses to Submaximal Exercise in the Midfollicular and Midluteal Phases of the Menstrual Cycle. Int. J. Sport Nutr. 1994, 4, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wenz, M.; Berend, J.Z.; Lynch, N.A.; Chappell, S.; Hackney, A.C. Substrate oxidation at rest and during exercise: Effects of menstrual cycle phase and diet composition. J. Physiol. Pharmacol. 1997, 48, 851–860. Available online: https://pubmed.ncbi.nlm.nih.gov/9444630/ (accessed on 1 January 2023). [PubMed]
- Aparecido, J.M.L.; Frientes, C.S.; Martins, G.L.; Santos, G.C.; Silva, J.D.A.; Rogeri, P.S.; Pires, R.S.; Amorim, T.S.; da Silva, T.D.O.; Santo, T.E.; et al. Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile adaptations in Reproductive Age women: A systematic Review and Meta-Analysis. Obesities 2022, 2, 222–235. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef]
- Paravidino, A.B.; Portella, E.S.; Soares, E.A. Energy metabolism in endurance athletes is different between the sexes. Rev. Nutr. Camp. 2007, 20, 317–325. [Google Scholar] [CrossRef]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; Mcgee, S.l. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Suh, S.H.; Casazza, G.A.; Horning, M.A.; Miller, B.F.; Brooks, G.A. Luteal and follicular glucose fluxes during rest and exercise in 3-h postabsorptive women. J. Appl. Physiol. 2002, 93, 42–50. [Google Scholar] [CrossRef]
- Horton, T.J.; Miller, E.K.; Glueck, D.; Tench, K. No effect of menstrual cycle phase on glucose kinetics and fuel oxidation during moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E752–E762. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef]
- Chu, L.; Morrison, K.M.; Riddell, M.C.; Raha, S.; Timmons, B.W. Effect of 7 days of exercise on exogenous carbohydrate oxidation and insulin resistance in children with obesity. Appl. Physiol. Nutr. Metab. 2018, 43, 677–683. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval trainingin health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No effect of menstrual cycle phase on fuel oxidation during exercise in rowers. Eur. J. Appl. Physiol. 2010, 111, 1027–1034. [Google Scholar] [CrossRef]
- Polak, J.; Moro, C.; Klimcakova, E.; Hejnova, J.; Majercik, M.; Viguerie, N.; Langin, D.; Lafontan, M.; Stich, V.; Berlan, M. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2A and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia 2005, 48, 2631–2640. [Google Scholar] [CrossRef]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef]

| FL (n = 6) | LT (n = 5) | |
|---|---|---|
| Age (years) | 22.50 ± 0.81 | 21.20 ± 1.07 |
| Height (m) | 1.60 ± 0.02 | 1.57 ± 0.03 |
| Body mass (kg) | 54.67 ± 3.60 | 55.04 ± 2.04 |
| Body fat (%) | 22.42 ± 2.97 | 26.24 ± 0.85 |
| Resting blood glucose (mg/dL) | 83.05 ± 5.06 | 87.60 ± 5.64 |
| VO2peakPRE (L/min) | 1.93 ± 0.17 | 1.80 ± 0.09 |
| VO2peak/kg PRE (mL/kg/min) | 34.83 ± 2.42 | 32.92 ± 1.84 |
| VO2peak POST (L/min) | 2.16 ± 0.19 | 1.97 ± 0.07 |
| VO2peak/kg POST (mL/kg/min) | 39.22 ± 1.45 | 35.77 ± 1.75 |
| Body mass index (kg/m2) | 21.36 ± 1.64 | 22.44 ± 0.63 |
| VO2 (mL/kg/min) | HR (bpm) | VEL (km/h) | |||
|---|---|---|---|---|---|
| FL (n = 6) | VAT1 | PRE | 14.29 ± 1.24 | 140.00 ± 4.91 | 6.83 ± 0.17 |
| POST | 17.22 ± 1.23 | 130.83 ± 7.61 | 7.00 ± 0.00 | ||
| VAT2 | PRE | 25.65 ± 1.20 | 164.00 ± 5.20 | 8.83 ± 0.17 A | |
| POST | 30.38 ± 1.12 | 169.50 ± 5.31 | 10.33 ± 0.21 | ||
| PEAK | PRE | 34.83 ± 2.42 | 186.33 ± 2.29 | 12.50 ± 0.34 | |
| POST | 39.22 ± 1.45 | 188.67 ± 3.04 | 13.83 ± 0.40 | ||
| LT (n = 5) | VAT1 | PRE | 13.19 ± 0.71 | 140.20 ± 11.96 | 7.00 ± 0.32 |
| POST | 14.23 ± 1.94 | 136.00 ± 7.57 | 7.00 ± 0.00 | ||
| VAT2 | PRE | 25.13 ± 1.93 | 169.40 ± 5.97 | 9.20 ± 0.37 | |
| POST | 27.13 ± 1.11 | 170.40 ± 1.83 | 10.20 ± 0.37 | ||
| PEAK | PRE | 32.92 ± 1.84 | 187.20 ± 4.36 | 11.80 ± 0.49 | |
| POST | 35.74 ± 1.76 | 187.60 ± 1.50 | 13.00 ± 0.45 |
| O2 (%) | HR (%) | VEL (%) | |||
|---|---|---|---|---|---|
| FL (n = 6) | VAT1 | PRE | 47.42 ± 4.30 | 70.83 ± 3.74 | 54.94 ± 2.37 |
| POST | 44.7 ± 3.06 | 69.76 ± 3.56 | 52.20 ± 1.89 | ||
| VAT2 | PRE | 74.26 ± 2.41 | 87.98 ± 2.27 | 72.29 ± 2.13 | |
| POST | 77.67 ± 2.12 | 89.48 ± 1.85 | 75.58 ± 2.38 | ||
| LT (n = 5) | VAT1 | PRE | 40.49 ± 2.93 | 74.52 ± 5.03 | 59.44 ± 2.18 |
| POST | 39.98 ± 5.29 | 72.5 ± 3.99 | 54.10 ± 1.86 | ||
| VAT2 | PRE | 76.02 ± 2.05 | 90.44 ± 1.98 | 78.04 ± 1.72 | |
| POST | 76.18 ± 2.29 | 90.88 ± 1.60 | 78.52 ± 1.38 |
| O2 (%) | HR (%) | |
|---|---|---|
| FL (n = 6) | 89.17 ± 1.82 | 95.59 ± 0.83 A |
| LT (n = 5) | 89.84 ± 1.93 | 91.60 ± 1.02 |
| TTkc (kcal) | CHOkc (%) | LIPkc (%) | ||
|---|---|---|---|---|
| FL (n = 6) | PRE | 4.25 ± 0.29 | 30.89 ± 3.62 A,B | 69.11 ± 3.62 A,B |
| POST | 4.54 ± 0.28 | 36.73 ± 5.01 A,B | 63.27 ± 5.01 A,B | |
| LT (n = 5) | PRE | 4.05 ± 0.23 | 11.92 ± 2.72 | 88.08 ± 2.72 |
| POST | 3.58 ± 0.09 | 14.96 ± 2.30 | 85.04 ± 2.30 |
| CHOkc (%) | LIPkc (%) | ||
|---|---|---|---|
| FL × LT | PRE | −61.42 A | 27.46 A |
| POST | −59.26 | 34.41 | |
| PRE × POST | FL | 18.89 B | −8.45 B |
| LT | 25.50 | −3.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frientes, C.S.; Marquezi, M.L.; Aparecido, J.M.L.; Cascapera, M.S.; Rogeri, P.S.; Lancha Junior, A.H. Effect of Menstrual Cycle Phase on Fuel Oxidation Post HIT in Women Reproductive Age: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 3148. https://doi.org/10.3390/ijerph20043148
Frientes CS, Marquezi ML, Aparecido JML, Cascapera MS, Rogeri PS, Lancha Junior AH. Effect of Menstrual Cycle Phase on Fuel Oxidation Post HIT in Women Reproductive Age: A Pilot Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3148. https://doi.org/10.3390/ijerph20043148
Chicago/Turabian StyleFrientes, Caroline Santana, Marcelo Luis Marquezi, Juliana Monique Lino Aparecido, Marcelo Santin Cascapera, Patrícia Soares Rogeri, and Antônio Herbert Lancha Junior. 2023. "Effect of Menstrual Cycle Phase on Fuel Oxidation Post HIT in Women Reproductive Age: A Pilot Study" International Journal of Environmental Research and Public Health 20, no. 4: 3148. https://doi.org/10.3390/ijerph20043148
APA StyleFrientes, C. S., Marquezi, M. L., Aparecido, J. M. L., Cascapera, M. S., Rogeri, P. S., & Lancha Junior, A. H. (2023). Effect of Menstrual Cycle Phase on Fuel Oxidation Post HIT in Women Reproductive Age: A Pilot Study. International Journal of Environmental Research and Public Health, 20(4), 3148. https://doi.org/10.3390/ijerph20043148







