Catastrophic Costs among Tuberculosis-Affected Households in Egypt: Magnitude, Cost Drivers, and Coping Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size and Sampling Technique
2.2. WHO Survey Instrument
2.3. Calculation of Catastrophic Health Expenditure
2.4. Sensitivity Analysis
2.5. Operational Definitions
2.6. Data Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Active case finding |
| PCF | Passive case finding |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease 2019 |
| DTU | Diagnostic and treatment units |
| EPTB | Extrapulmonary tuberculosis |
| HIV | Human immunodeficiency virus |
| LTBI | Latent TB infection |
| MDR | Multi-drug resistance |
| NTP | National Tuberculosis Program |
| ODK | Open data kit |
| OR | Odds ratio |
| PTB | Pulmonary tuberculosis |
| SES | Socioeconomic status |
| TB | Tuberculosis |
| WHO | World Health Organization |
| XDR | Extended drug resistant |
References
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.; et al. Global Tuberculosis Report 2020—Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, J.; Wang, P.; Lan, J.-H.; Lian, W.-Q.; Fan, Y.-Y.; Xu, B.-N.; Yin, J.-P.; Feng, Z.-H.; Zhou, J.; et al. Burden of tuberculosis and its association with socio-economic development status in 204 countries and territories, 1990–2019. Front. Med. 2022, 9, 905245. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Maddison, E.R.; Henry, N.J.; Mumford, J.E.; Barber, R.; Shields, C.; Brown, J.C.; Nguyen, G.; Carter, A.; Wolock, T.M.; et al. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2018, 18, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Chakaya, J.; Petersen, E.; Nantanda, R.; Mungai, B.N.; Migliori, G.B.; Amanullah, F.; Lungu, P.; Ntoumi, F.; Kumarasamy, N.; Maeurer, M. The WHO Global Tuberculosis 2021 Report–not so good news and turning the tide back to End TB. Int. J. Infect. Dis. 2022, 124, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Incidence of Tuberculosis (per 100,000 People). Available online: https://data.worldbank.org/indicator/SH.TBS.INCD (accessed on 20 January 2022).
- Bhardwaj, A.K.; Kumar, D.; Raina, S.K.; Sharma, S.; Chander, V. Assessment of extra pulmonary tuberculosis (EPTB) cases from selected tuberculosis units (TUs) of Himachal Pradesh, India. Int. J. Health 2015, 3, 29–33. [Google Scholar] [CrossRef]
- Lee, J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc. Respir. Dis. 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Centers for Diseases Control and Prevention. The Difference between Latent TB Infection and TB Disease. Available online: https://www.cdc.gov/tb/publications/factsheets/general/ltbiandactivetb.htm (accessed on 20 January 2022).
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R., Jr. Management of drug-resistant tuberculosis. Lancet 2019, 394, 953–966. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Fox, W.; Ellard, G.A.; Mitchison, D.A. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int. J. Tuberc. Lung Dis. 1999, 3, S231–S279. [Google Scholar]
- Lee, M.; Lee, J.; Carroll, M.W.; Choi, H.; Min, S.; Song, T.; Via, L.E.; Goldfeder, L.C.; Kang, E.; Jin, B. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 2012, 367, 1508–1518. [Google Scholar] [CrossRef]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lönnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef]
- Laurence, Y.V.; Griffiths, U.K.; Vassall, A. Costs to Health Services and the Patient of Treating Tuberculosis: A Systematic Literature Review. Pharmacoeconomics 2015, 33, 939–955. [Google Scholar] [CrossRef]
- Balinda, I.G.; Sugrue, D.D.; Ivers, L.C. More Than Malnutrition: A Review of the Relationship Between Food Insecurity and Tuberculosis. Open Forum Infect. Dis. 2019, 6, ofz102. [Google Scholar] [CrossRef]
- Viney, K.; Islam, T.; Hoa, N.B.; Morishita, F.; Lönnroth, K. The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region. Trop. Med. Infect. Dis. 2019, 4, 94. [Google Scholar] [CrossRef]
- Mauch, V.; Bonsu, F.; Gyapong, M.; Awini, E.; Suarez, P.; Marcelino, B.; Melgen, R.E.; Lönnroth, K.; Nhung, N.V.; Hoa, N.B.; et al. Free tuberculosis diagnosis and treatment are not enough: Patient cost evidence from three continents. Int. J. Tuberc. Lung Dis. 2013, 17, 381–387. [Google Scholar] [CrossRef]
- Wingfield, T.; Boccia, D.; Tovar, M.; Gavino, A.; Zevallos, K.; Montoya, R.; Lönnroth, K.; Evans, C.A. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: A prospective cohort study, Peru. PLoS Med. 2014, 11, e1001675. [Google Scholar] [CrossRef]
- Fuady, A.; Houweling, T.A.J.; Mansyur, M.; Burhan, E.; Richardus, J.H. Catastrophic costs due to tuberculosis worsen treatment outcomes: A prospective cohort study in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 666–673. [Google Scholar] [CrossRef]
- Ukwaja, K.N.; Alobu, I.; Gidado, M.; Onazi, O.; Oshi, D.C. Economic support intervention improves tuberculosis treatment outcomes in rural Nigeria. Int. J. Tuberc. Lung Dis. 2017, 21, 564–570. [Google Scholar] [CrossRef]
- Ghazy, R.M.; El Saeh, H.M.; Abdulaziz, S.; Hammouda, E.A.; Elzorkany, A.M.; Khidr, H.; Zarif, N.; Elrewany, E.; Abd ElHafeez, S. A systematic review and meta-analysis of the catastrophic costs incurred by tuberculosis patients. Sci. Rep. 2022, 12, 558. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report. 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 30 December 2022).
- World Health Organization. Global Tuberculosis Report. 2019. Available online: https://www.who.int/publications/i/item/9789241565714 (accessed on 30 December 2022).
- Pedrazzoli, D.; Borghi, J.; Viney, K.; Houben, R.; Lönnroth, K. Measuring the economic burden for TB patients in the End TB Strategy and Universal Health Coverage frameworks. Int. J. Tuberc. Lung Dis. 2019, 23, 5–11. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis Patient Cost Surveys: A Handbook. Available online: https://www.who.int/publications/i/item/9789241513524 (accessed on 30 December 2022).
- Lu, L.; Jiang, Q.; Hong, J.; Jin, X.; Gao, Q.; Bang, H.; DeRiemer, K.; Yang, C. Catastrophic costs of tuberculosis care in a population with internal migrants in China. BMC Health Serv. Res. 2020, 20, 832. [Google Scholar] [CrossRef] [PubMed]
- Kirubi, B.; Ong'ang'o, J.; Nguhiu, P.; Lönnroth, K.; Rono, A.; Sidney-Annerstedt, K. Determinants of household catastrophic costs for drug sensitive tuberculosis patients in Kenya. Infect. Dis. Poverty 2021, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Stracker, N.; Hanrahan, C.; Mmolawa, L.; Nonyane, B.; Tampi, R.; Tucker, A.; West, N.; Lebina, L.; Martinson, N.; Dowdy, D. Risk factors for catastrophic costs associated with tuberculosis in rural South Africa. Int. J. Tuberc. Lung Dis. 2019, 23, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, T.; Jeyashree, K.; Chinnakali, P.; Bahurupi, Y.; Vasudevan, K.; Das, M. Catastrophic costs of tuberculosis care: A mixed methods study from Puducherry, India. Glob. Health Action 2018, 11, 1477493. [Google Scholar] [CrossRef] [PubMed]
- Muttamba, W.; Tumwebaze, R.; Mugenyi, L.; Batte, C.; Sekibira, R.; Nkolo, A.; Katamba, A.; Kasasa, S.; Majwala, R.K.; Turyahabwe, S.; et al. Households experiencing catastrophic costs due to tuberculosis in Uganda: Magnitude and cost drivers. BMC Public Health 2020, 20, 1409. [Google Scholar] [CrossRef]
- Aung, S.T.; Thu, A.; Aung, H.L.; Thu, M. Measuring Catastrophic Costs Due to Tuberculosis in Myanmar. Trop. Med. Infect. Dis. 2021, 6, 130. [Google Scholar] [CrossRef]
- Egyptian Ministry of Health and Population. National Tuberculosis Control Program: Egypt. Available online: http://www.ccs.gov.eg/ntp/NTP_introd.htm (accessed on 21 February 2022).
- Ghazy, R.; Ashmawy, R.; Reyad, O.; Abd ElHafeez, S.; El-Shishtawy, M.; Khedr, H.; El-Rewiny, E.; El-Saeh, H.; Yacoub, M.; Ali, N.; et al. Translation and cultural adaptation of the WHO generic tuberculosis patient cost survey to an Egyptian context. East Mediterr. Health J. 2022, 28, 649–657. [Google Scholar] [CrossRef]
- El-Gilany, A.; El-Wehady, A.; El-Wasify, M. Updating and validation of the socioeconomic status scale for health research in Egypt. East Mediterr. Health J. 2012, 18, 962–968. [Google Scholar] [CrossRef]
- World Health Organization. Protocol for Survey to Determine Direct and Indirect Costs Due to TB and to Estimate Proportion of TB-Affected Households Experiencing Catastrophic Total Costs Due to TB. Available online: https://www.who.int/publications/m/item/protocol-for-survey-to-determine-direct-and-indirect-costs-due-to-tb-and-to-estimate-proportion-of-tb-affected-households-experiencing-catastrophic-total-costs-due-to-tb (accessed on 1 January 2023).
- Jo, C. Cost-of-illness studies: Concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef]
- Grosh, M.; Glewwe, P. Designing Household Survey Questionnaires for Developing Countries; World Bank: Washington, DC, USA, 2000. [Google Scholar]
- Fahmy, S.I.; El Sherbini, A.F. Determining Simple Parameters for Social Classification for Health Research. Bull. High Inst. Public Health 1983, 13, 95–107. [Google Scholar]
- Ellaban, M.M.; Basyoni, N.I.; Boulos, D.N.K.; Rady, M.; Gadallah, M. Assessment of Household Catastrophic Total Cost of Tuberculosis and Its Determinants in Cairo: Prospective Cohort Study. Tuberc. Respir. Dis. 2022, 85, 165–174. [Google Scholar] [CrossRef]
- Timire, C.; Ngwenya, M.; Chirenda, J.; Metcalfe, J.Z.; Kranzer, K.; Pedrazzoli, D.; Takarinda, K.C.; Nguhiu, P.; Madzingaidzo, G.; Ndlovu, K.; et al. Catastrophic costs among tuberculosis-affected households in Zimbabwe: A national health facility-based survey. Trop. Med. Int. Health 2021, 26, 1248–1255. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Siroka, A.; Boccia, D.; Bonsu, F.; Nartey, K.; Houben, R.; Borghi, J. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop. Med. Int. Health 2018, 23, 870–878. [Google Scholar] [CrossRef]
- Asres, A.; Jerene, D.; Deressa, W. Pre- and post-diagnosis costs of tuberculosis to patients on Directly Observed Treatment Short course in districts of southwestern Ethiopia: A longitudinal study. J. Health Popul. Nutr. 2018, 37, 15. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, R.; Kant, S.; Parthasarathy, R.; Krishnan, A. Direct and indirect patient costs of tuberculosis care in India. Trop. Med. Int. Health 2020, 25, 803–812. [Google Scholar] [CrossRef]
- Ministry of Health and Population/Egypt. National Tuberculosis Control Program—Egypt. Available online: http://ccs.gov.eg/ntp/publications_guidelines_physicians_defin.htm#5 (accessed on 13 April 2022).
- Fuady, A.; Houweling, T.A.J.; Mansyur, M.; Richardus, J.H. Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infect. Dis. Poverty 2018, 7, 3. [Google Scholar] [CrossRef]
- Tomeny, E.M.; Mendoza, V.L.; Marcelo, D.B.; Barrameda, A.J.D.; Langley, I.; Abong, J.M.; Dalay, V.B.; Yu, C.Y.; Squire, S.B. Patient-cost survey for tuberculosis in the context of patient-pathway modelling. Int. J. Tuberc. Lung Dis. 2020, 24, 420–427. [Google Scholar] [CrossRef]
- Rudgard, W.E.; Evans, C.A.; Sweeney, S.; Wingfield, T.; Lönnroth, K.; Barreira, D.; Boccia, D. Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study. PLOS Med. 2017, 14, e1002418. [Google Scholar] [CrossRef]
- Aamir, S.; Aisha. Co-morbid anxiety and depression among pulmonary tuberculosis patients. J. Coll. Physicians Surg. Pak. 2010, 20, 703–704. [Google Scholar] [CrossRef]
- Rouf, A.; Masoodi, M.A.; Dar, M.M.; Khan, S.M.S.; Bilquise, R. Depression among Tuberculosis patients and its association with treatment outcomes in district Srinagar. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 25, 100281. [Google Scholar] [CrossRef]
- Tola, H.H.; Shojaeizadeh, D.; Garmaroudi, G.; Tol, A.; Yekaninejad, M.S.; Ejeta, L.T.; Kebede, A.; Karimi, M.; Kassa, D. Psychological distress and its effect on tuberculosis treatment outcomes in Ethiopia. Glob. Health Action 2015, 8, 29019. [Google Scholar] [CrossRef] [PubMed]
- Orusa, T.; Borgogno Mondino, E. Exploring Short-Term Climate Change Effects on Rangelands and Broad-Leaved Forests by Free Satellite Data in Aosta Valley (Northwest Italy). Climate 2021, 9, 47. [Google Scholar] [CrossRef]
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Borgogno Mondino, E. Geomatics and EO data to support wildlife diseases assessment at landscape level: A pilot experience to map infectious keratoconjunctivitis in chamois and phenological trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542. [Google Scholar] [CrossRef]

| Variable | Number (N = 276) | Percentage |
|---|---|---|
| Gender (male) | 211 | 76.40% |
| Age (years) | ||
| Mean (SD) | 36.1 (13.5) | |
| <18 | 9 | 3.30% |
| 18–35 | 138 | 50.00% |
| 36–60 | 116 | 42.00% |
| >60 | 13 | 4.70% |
| MDR-TB (Yes) | 23 | 8.30% |
| TB-treatment phase | ||
| Continuation phase | 154 | 55.80% |
| Intensive phase | 122 | 44.20% |
| HIV status | ||
| Negative | 216 | 78.30% |
| Positive | 5 | 1.80% |
| Unknown | 55 | 19.90% |
| Patient currently hospitalized (yes) | 13 | 4.70% |
| Delay in diagnosis * | 80 | 28.99% |
| Socioeconomic score | ||
| Median (range) | 31.0 (15.0–46.0) | |
| Socioeconomic status | ||
| Very low | 28 | 10.10% |
| Low | 243 | 88.00% |
| Middle | 5 | 1.80% |
| High | 0 | 0.00% |
| Education level | ||
| Below education age | 2 | 0.70% |
| Illiterate | 50 | 18.10% |
| Read and write | 17 | 6.20% |
| Primary | 46 | 16.70% |
| Secondary | 41 | 14.90% |
| Higher | 120 | 43.50% |
| Occupation | ||
| Unemployed/housewife | 49 | 17.80% |
| Employee | 95 | 34.40% |
| Hand worker | 118 | 42.80% |
| Trades/business | 14 | 5.10% |
| Residence | ||
| Rural | 33 | 12.00% |
| Slum rural | 12 | 4.30% |
| Urban | 231 | 83.70% |
| Insurance status | ||
| No insurance | 221 | 80.10% |
| Governmental insurance | 34 | 12.30% |
| Private insurance | 11 | 4.00% |
| Donors | 10 | 3.60% |
| Household size median, (range) | 4.0 (0–10.0) Person | |
| Number of rooms median, (range) | 3.0 (1.0–8.0) Room | |
| Pre Diagnosis | Post Diagnosis | Total | p Value | |
|---|---|---|---|---|
| Household yearly income, Mean ± SD | 3029.8 ± 161.39 | 1178 ± 88.57 | - | <0.001 |
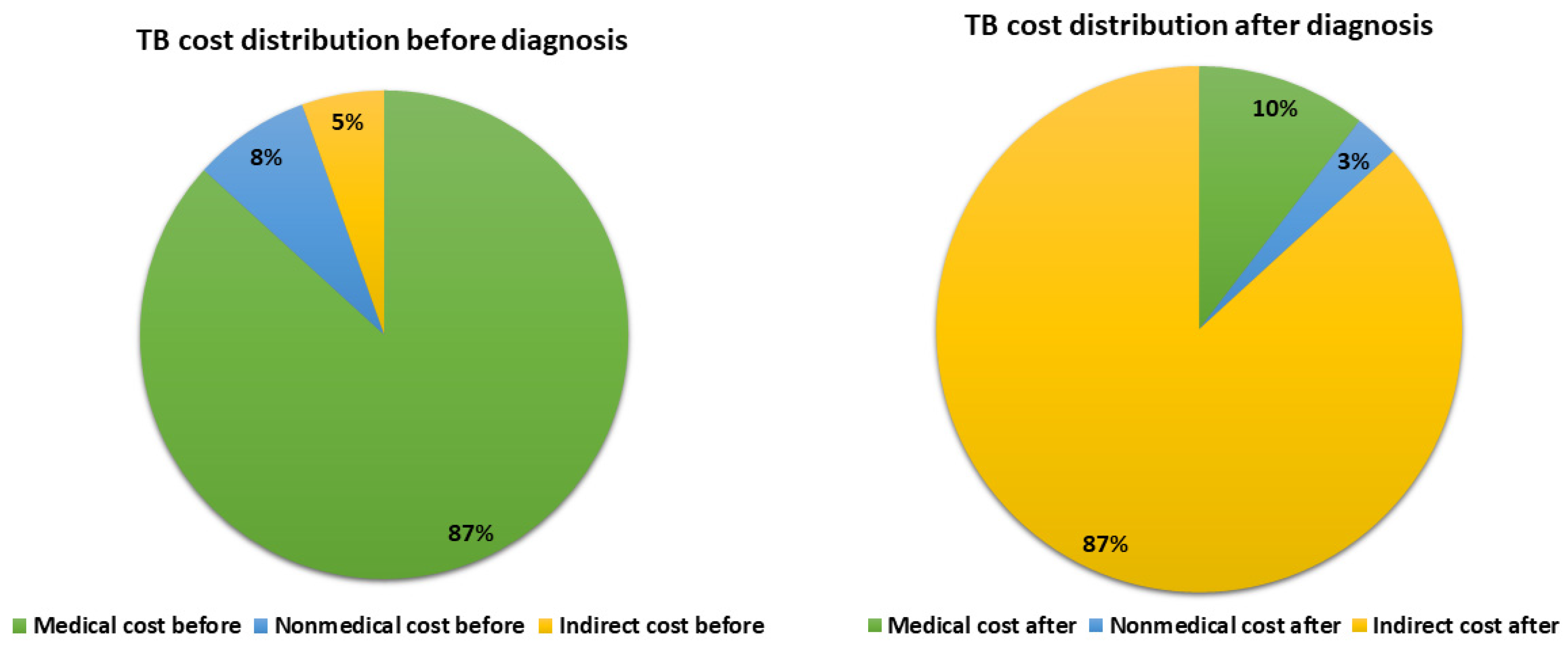
| Total TB direct medical cost, Mean ± SD | 150.23± 26.89 | 77.25± 9.91 | 121.37 ±15.2 | 0.013 |
| Hospitalization | 6.35 ± 0.001 | 154.7± 59.7o | 121.89 ± 47.71 | <0.001 |
| Lab tests cost | 29.72 ± 7.17 | 12.36 ± 2.78 | 49.36 ± 7.62 | <0.001 |
| Radiological cost | 53.91 ± 13.61 | 25.84 ± 4.85 | 76.3 ± 10.53 | <0.001 |
| Consultation | 12.41 ± 1.64 | 4.50± 0.77 | 15.9 ± 1.60 | <0.001 |
| Medication | 53.76 ± 12.03 | 9.63± 2.94 | 62.6 ± 10.71 | <0.001 |
| Other medical costs | 6.04 ± 0.83 | 11.55 ± 4.26 | 50.4 ± 17.31 | <0.001 |
| Total TB direct non-medical cost, Mean ± SD | 14.11 ± 2.42 | 19.77 ± 1.08 | 24.1± 1.31 | 0.050 |
| Accommodation | 3.86 ± 0.67 | 0.28 ± 0.14 | 4.7 ± 1.72 | 0.002 |
| Food | 16.54 ± 4.28 | 31.75 ± 5.92 | 39.49 ± 7.01 | 0.040 |
| Other nonmedical | 13.17 ± 2.28 | 4.37 ± 1.38 | 21.32± 3.60 | 0.001 |
| Total direct cost | 152.34 ± 59.73 | 48.7 ± 4.82 | 103 ± 10.91 | <0.001 |
| Total indirect cost (lost productivity) * | 4.68 ± 1.18 | 192.84 ± 25.32 | 194.15 ±25.52 | <0.001 |
| Total TB costs | 149.63 ± 24.6 | 238.04 ± 26.74 | 280.28 ± 29.9 | <0.001 |
| Coping Strategy * | Total (N = 276) | |
|---|---|---|
| N | % | |
| Dissaving due to TB illness | 64 | 23.2 |
| Borrowing due to TB illness | 184 | 66.7 |
| Sold any item (one or more) | 96 | 34.8 |
| Sold vehicle | 6 | 2.2 |
| Sold household property or land | 70 | 25.4 |
| Sold gold | 25 | 9.1 |
| Use livestock | 3 | 1.1 |
| The social and psychological effect * | ||
| Psychological | 61 | 22.1 |
| Socially excluded | 38 | 13.8 |
| Loss of job | 183 | 66.3 |
| Decreased food | 171 | 62.0 |
| Divorce | 10 | 3.6 |
| Predictor | OR | 95% CI | p Value |
|---|---|---|---|
| Intercept | 14.605 | 0.019 * | |
| Age | 0.971 | (0.939–1.004) | 0.083 |
| Sex Female® Male | 1 0.352 | Ref (0.117–1.065) | 0.064 |
| MDR-TB No® Yes | 1 12.89 | Ref (0.42–19.82) | 0.279 |
| Diagnosis place Chest clinic ® Chest hospital Private lab Other | 1 0.829 1.59 0.646 | Ref (0.278–2.47) (0.11–22.96) (0.197–2.118) | 0.736 0.731 0.471 |
| TB type Extra-pulmonary TB® Pulmonary TB | 1 1.06 | Ref (0.43–5.95) | 0.481 |
| Delay in diagnosis No ® Yes | 1 19.6 | Ref (6.443–59.647) | <0.0001 * |
| Treatment phase Continuation® Intensive | 1 0.108 | Ref (0.029–0.403) | 0.001 * |
| Total TB cost | 0.99 | (0.99–1.00) | 0.487 |
| Household income lost due to TB | 0.99 | (0.99–1.00) | 0.359 |
| Total annual household income before TB LE | 0.99 | (0.99–1.00) | 0.352 |
| p < 0.001, R2 = 41.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazy, R.M.; Sallam, M.; Ashmawy, R.; Elzorkany, A.M.; Reyad, O.A.; Hamdy, N.A.; Khedr, H.; Mosallam, R.A. Catastrophic Costs among Tuberculosis-Affected Households in Egypt: Magnitude, Cost Drivers, and Coping Strategies. Int. J. Environ. Res. Public Health 2023, 20, 2640. https://doi.org/10.3390/ijerph20032640
Ghazy RM, Sallam M, Ashmawy R, Elzorkany AM, Reyad OA, Hamdy NA, Khedr H, Mosallam RA. Catastrophic Costs among Tuberculosis-Affected Households in Egypt: Magnitude, Cost Drivers, and Coping Strategies. International Journal of Environmental Research and Public Health. 2023; 20(3):2640. https://doi.org/10.3390/ijerph20032640
Chicago/Turabian StyleGhazy, Ramy Mohamed, Malik Sallam, Rasha Ashmawy, Amira Mohamed Elzorkany, Omar Ahmed Reyad, Noha Alaa Hamdy, Heba Khedr, and Rasha Ali Mosallam. 2023. "Catastrophic Costs among Tuberculosis-Affected Households in Egypt: Magnitude, Cost Drivers, and Coping Strategies" International Journal of Environmental Research and Public Health 20, no. 3: 2640. https://doi.org/10.3390/ijerph20032640
APA StyleGhazy, R. M., Sallam, M., Ashmawy, R., Elzorkany, A. M., Reyad, O. A., Hamdy, N. A., Khedr, H., & Mosallam, R. A. (2023). Catastrophic Costs among Tuberculosis-Affected Households in Egypt: Magnitude, Cost Drivers, and Coping Strategies. International Journal of Environmental Research and Public Health, 20(3), 2640. https://doi.org/10.3390/ijerph20032640









