Developing and Testing a Smartphone Application to Enhance Adherence to Voice Therapy: A Pilot Study
Abstract
1. Introduction
Study Objective
2. Materials and Methods
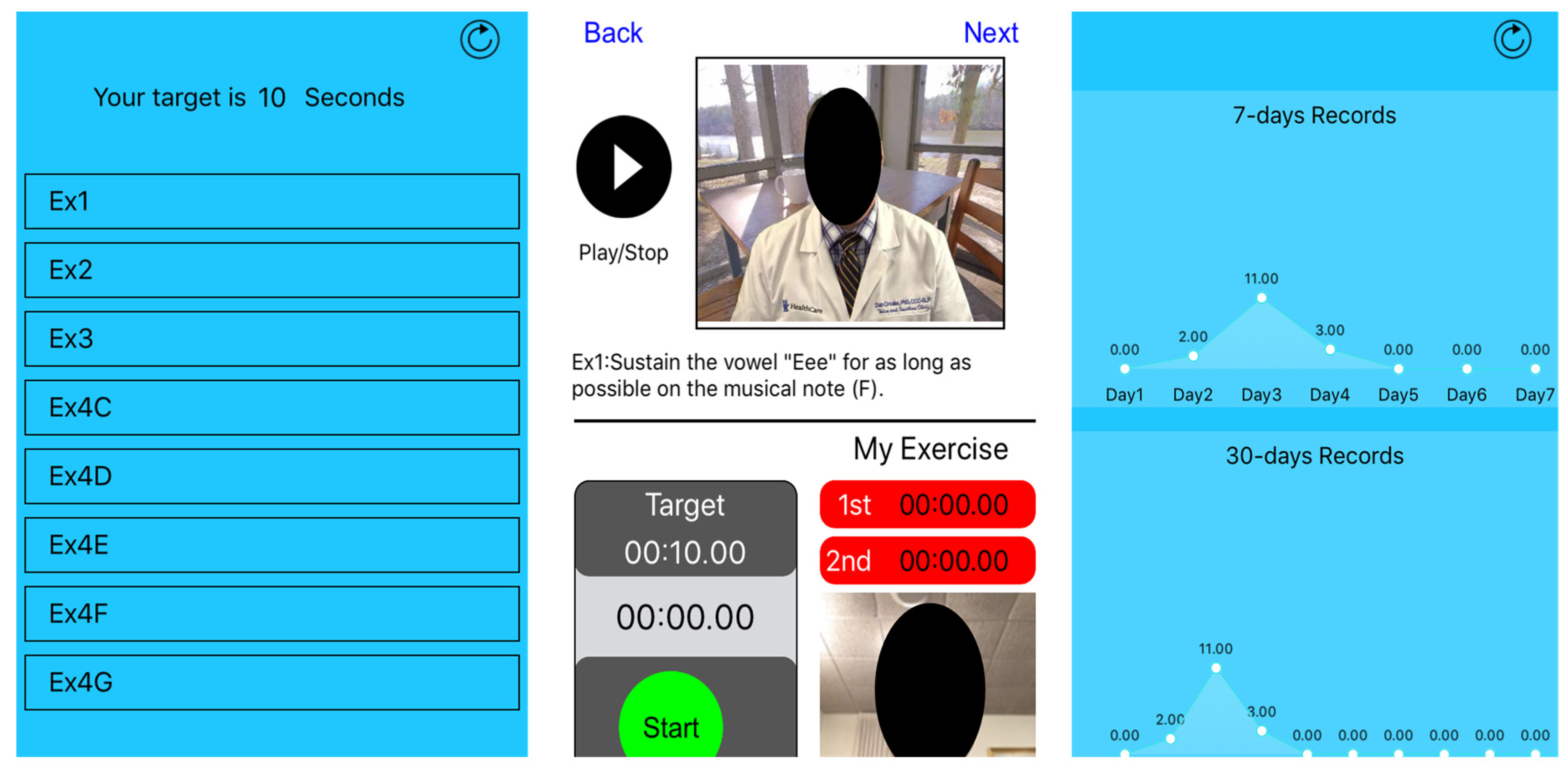
2.1. Study Protocol
2.2. Statistical Analyses
3. Results
3.1. Clinical Trial
3.2. Usability Evaluation
4. Discussion
5. Limitations
6. Future Directions
7. Conclusions and Implications for Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, N.; Merrill, R.M.; Gray, S.D.; Smith, E.M. Voice disorders in the general population: Prevalence, risk factors, and occupational impact. Laryngoscope 2005, 115, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Verdolini, K.; Ramig, L.O. Review: Occupational risks for voice problems. Logop. Phoniatr. Vocol. 2001, 26, 37–46. [Google Scholar] [CrossRef]
- Hseu, A.F.; Spencer, G.; Woodnorth, G.; Kagan, S.; Kawai, K.; Nuss, R.C. Barriers to voice therapy in dysphonic children. J. Voice 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Angadi, V.; Dressler, E.; Stemple, J. A multidimensional study of vocal function following radiation therapy for laryngeal cancers. Ann. Otol. Rhinol. Laryngol. 2017, 126, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hapner, E.R. The changing landscape of vocal needs in the aging baby boomer. Perspect. ASHA Spec. Interest Groups 2017, 2, 24–31. [Google Scholar] [CrossRef]
- Etter, N.M.; Stemple, J.C.; Howell, D.M. Defining the lived experience of older adults with voice disorders. J. Voice 2013, 27, 61–67. [Google Scholar] [CrossRef]
- Speyer, R. Effects of voice therapy: A systematic review. J. Voice 2008, 22, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Angadi, V.; Croake, D.; Stemple, J. Effects of Vocal Function Exercises: A Systematic Review. J. Voice 2019, 33, 124.e13–124.e34. [Google Scholar] [CrossRef]
- van Leer, E.; Connor, N.P. Use of portable digital media players increases patient motivation and practice in voice therapy. J. Voice 2012, 26, 447–453. [Google Scholar] [CrossRef]
- van Leer, E.; Connor, N.P. Patient perceptions of voice therapy adherence. J. Voice 2010, 24, 458–469. [Google Scholar] [CrossRef]
- van Leer, E.; Lewis, B.; Porcaro, N. Effect of an iOS app on voice therapy adherence and motivation. Am. J. Speech-Lang. Pathol. 2021, 30, 210–227. [Google Scholar] [CrossRef]
- Quanbeck, A.; Chih, M.-Y.; Isham, A.; Johnson, R.; Gustafson, D. Mobile delivery of treatment for alcohol use disorders: A review of the literature. Alcohol Res. Curr. Rev. 2014, 36, 111. [Google Scholar]
- Kleinman, N.J.; Shah, A.; Shah, S.; Phatak, S.; Viswanathan, V. Improved Medication Adherence and Frequency of Blood Glucose Self-Testing using an m-Health Platform Versus Usual Care in a Multisite Randomized Clinical Trial Among People with Type 2 Diabetes in India. Telemed. e-Health 2017, 23, 733–740. [Google Scholar] [CrossRef]
- Yoo, W.; Shah, D.V.; Chih, M.Y.; Gustafson, D.H. A smartphone-based support group for alcoholism: Effects of giving and receiving emotional support on coping self-efficacy and risky drinking. Health Inform. J. 2020, 26, 1764–1776. [Google Scholar] [CrossRef]
- Chih, M.Y.; McCowan, A.; Whittaker, S.; Krakow, M.; Ahern, D.K.; Aronoff-Spencer, E.; Hesse, B.W.; Mullett, T.W.; Vanderpool, R.C. The Landscape of Connected Cancer Symptom Management in Rural America: A Narrative Review of Opportunities for Launching Connected Health Interventions. J. Appalach. Health 2020, 2, 64–81. [Google Scholar] [CrossRef]
- Chih, M.-Y.; Patton, T.; McTavish, F.M.; Isham, A.J.; Judkins-Fisher, C.L.; Atwood, A.K.; Gustafson, D.H. Predictive modeling of addiction lapses in a mobile health application. J. Subst. Abus. Treat. 2014, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nahum-Shani, I.; Smith, S.N.; Spring, B.J.; Collins, L.M.; Witkiewitz, K.; Tewari, A.; Murphy, S.A. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Ann. Behav. Med. 2016, 52, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Stemple, J.C.; Hapner, E.R. Voice Therapy: Clinical Case Studies; Plural Publishing: San Diego, CA, USA, 2019. [Google Scholar]
- NIH’s Definition of a Clinical Trial. Available online: https://grants.nih.gov/policy/clinical-trials/definition.htm (accessed on 1 April 2016).
- Rennie, D. CONSORT revised—Improving the reporting of randomized trials. JAMA 2001, 285, 2006–2007. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, G.; Elbourne, D.R.; Pocock, S.J.; Evans, S.J.; Altman, D.G. Reporting of noninferiority and equivalence randomized trials: Extension of the CONSORT 2010 statement. JAMA 2012, 308, 2594–2604. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef]
- Hirano, M. Clinical examination of voice. Disord. Hum. Commun. 1981, 5, 1–99. [Google Scholar]
- Broekhuis, M.; van Velsen, L.; Hermens, H. Assessing usability of eHealth technology: A comparison of usability benchmarking instruments. Int. J. Med. Inform. 2019, 128, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R. Voice training and therapy with a semi-occluded vocal tract: Rationale and scientific underpinnings. J. Speech Lang. Hear. Res. 2006, 49, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Stemple, J.C.; Lee, L.; D’Amico, B.; Pickup, B. Efficacy of vocal function exercises as a method of improving voice production. J. Voice 1994, 8, 271–278. [Google Scholar] [CrossRef]
- Adam, A.; Hellig, J.C.; Perera, M.; Bolton, D.; Lawrentschuk, N. ‘Prostate Cancer Risk Calculator’ mobile applications (Apps): A systematic review and scoring using the validated user version of the Mobile Application Rating Scale (uMARS). World J. Urol. 2018, 36, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R. The system usability scale: Past, present, and future. Int. J. Hum. –Comput. Interact. 2018, 34, 577–590. [Google Scholar] [CrossRef]
- Yen, P.Y.; Wantland, D.; Bakken, S. Development of a Customizable Health IT Usability Evaluation Scale. In Proceedings of the AMIA Annual Symposium Proceedings, Washington, DC, USA, 13–17 November 2010; pp. 917–921. [Google Scholar]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Wilson, H. Development and Validation of the User Version of the Mobile Application Rating Scale (uMARS). JMIR Mhealth Uhealth 2016, 4, e72. [Google Scholar] [CrossRef]
- Sauro, J.; Lewis, J.R. Quantifying the User Experience: Practical Statistics for User Research; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- Perrin, A. Mobile technology and Home Broadband 2021. 2021. Available online: https://www.pewresearch.org/internet/wp-content/uploads/sites/9/2021/06/PI_2021.06.03_Mobile-Broadband_FINAL.pdf (accessed on 1 December 2022).
- Vogels, E. Some Digital Divides Persist between Rural, Urban and Suburban America. 2021. Available online: https://policycommons.net/artifacts/1808201/some-digital-divides-persist-between-rural-urban-and-suburban-america/2543052/ (accessed on 1 December 2022).
- Perrin, A.; Atske, S. Americans with Disabilities Less Likely than Those without to Own Some Digital Devices. 2021. Available online: https://www.pewresearch.org/fact-tank/2021/09/10/americans-with-disabilities-less-likely-than-those-without-to-own-some-digital-devices/ (accessed on 1 December 2022).
- ASHA. 2022 Medicare Part B Final Rule Includes New Remote Monitoring Codes, Significant Payment Cuts. Available online: https://www.asha.org/news/2021/2022-medicare-part-b-final-rule-includes-new-remote-monitoring-codes-significant-payment-cuts/ (accessed on 1 October 2021).
| Group | n | Missed Home Practice (Means and SD) | p-Value (Mann–Whitney U-test) | MPT (Means and SD Percentage of 80% MPT Goal) | p-Value (t-test) |
|---|---|---|---|---|---|
| Traditional | 13 | 409 (274) | 0.041 * | 89.9 (16.8) | 0.729 |
| App | 12 | 202 (79) | 89.54 (15.9) |
| Usability Measures | Mean | Median | SD | Min. | Max. |
|---|---|---|---|---|---|
| System Usability Scale (SUS) | |||||
| Overall Usability | 78.13 | 81.25 | 16.21 | 52.5 | 97.5 |
| Health IT Usability Evaluation Scale (Health-ITUES) | |||||
| Quality of Work Life | 3.91 | 4 | 0.9 | 2.33 | 5 |
| Perceived Usefulness | 4.22 | 4.11 | 0.73 | 2.89 | 5 |
| Perceived Ease of Use | 4.46 | 4.6 | 0.51 | 3.6 | 5 |
| User Control | 3.42 | 3.5 | 0.71 | 1.67 | 4.67 |
| User Version of the Mobile Application Rating Scale (uMARS) | |||||
| uMARS-App Quality | |||||
| Engagement | 2.95 | 2.8 | 0.78 | 1.4 | 4.6 |
| Functionality | 3.77 | 3.87 | 1.18 | 1.25 | 5 |
| Aesthetics | 3.94 | 4.17 | 0.84 | 2.67 | 5 |
| Information | 4.15 | 4.38 | 0.91 | 2.25 | 5 |
| Overall Quality | 3.7 | 3.74 | 0.83 | 2.27 | 4.84 |
| uMARS-Subjective Quality | |||||
| Recommend | 3.91 | 4 | 1.16 | 2 | 5 |
| Future Use | 3.67 | 4 | 1.15 | 1 | 5 |
| Willing to Pay | 2.33 | 2 | 1.15 | 1 | 4 |
| Start Rating | 3.55 | 4 | 0.82 | 2 | 5 |
| uMARS-Perceived Impact | |||||
| Overall Impact | 3.93 | 4 | 1.03 | 2.33 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angadi, V.; Chih, M.-Y.; Stemple, J. Developing and Testing a Smartphone Application to Enhance Adherence to Voice Therapy: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 2436. https://doi.org/10.3390/ijerph20032436
Angadi V, Chih M-Y, Stemple J. Developing and Testing a Smartphone Application to Enhance Adherence to Voice Therapy: A Pilot Study. International Journal of Environmental Research and Public Health. 2023; 20(3):2436. https://doi.org/10.3390/ijerph20032436
Chicago/Turabian StyleAngadi, Vrushali, Ming-Yuan Chih, and Joseph Stemple. 2023. "Developing and Testing a Smartphone Application to Enhance Adherence to Voice Therapy: A Pilot Study" International Journal of Environmental Research and Public Health 20, no. 3: 2436. https://doi.org/10.3390/ijerph20032436
APA StyleAngadi, V., Chih, M.-Y., & Stemple, J. (2023). Developing and Testing a Smartphone Application to Enhance Adherence to Voice Therapy: A Pilot Study. International Journal of Environmental Research and Public Health, 20(3), 2436. https://doi.org/10.3390/ijerph20032436






