Feasibility of Using a Mobile App Supported Executive Function Intervention in Military Service Members and Veterans with mTBI and Co-Occurring Psychological Conditions
Abstract
1. Introduction
2. Materials and Methods
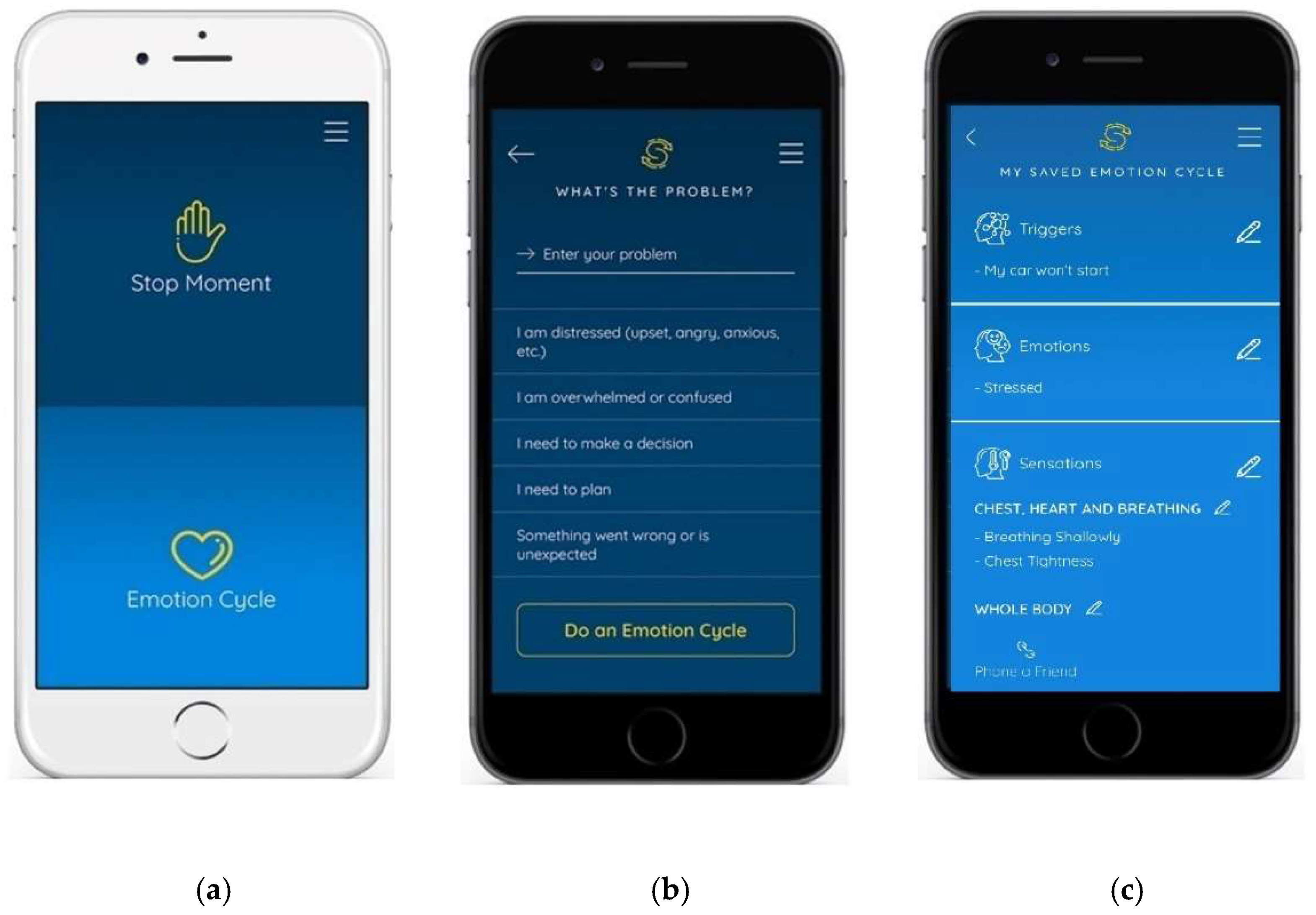
2.1. Intervention
2.2. Participants and Procedures
- Diagnosis of mTBI at least 6 months post-onset;
- Identified by SHARE behavioral health (BH) provider or speech-language pathologist (SLP) as appropriate for Executive Plus/STEP interventions;
- 18 years of age or older;
- Functional hearing and vision;
- Able to follow 2-step directions;
- English fluency;
- Functional reading;
- iOS or Android smartphone user; and
- Speaks or types functionally for smartphone use.
- Participation in SHARE for less than 6 weeks;
- Other conditions that may impact cognitive functioning (e.g., stroke, psychosis).
2.3. Measures
2.4. Data Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DOD TBI Worldwide Numbers. Available online: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers (accessed on 26 November 2022).
- Leddy, J.J.; Sandhu, H.; Sodhi, V.; Baker, J.G.; Willer, B. Rehabilitation of Concussion and Post-Concussion Syndrome. Sport. Health 2012, 4, 147–154. [Google Scholar] [CrossRef]
- Quinn, D.K.; Mayer, A.R.; Master, C.L.; Fann, J.R. Prolonged Postconcussive Symptoms. Am. J. Psychiatry 2018, 175, 103–111. [Google Scholar] [CrossRef]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (MTBI) and Chronic Cognitive Impairment: A Scoping Review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef]
- Madhok, D.Y.; Rodriguez, R.M.; Barber, J.; Temkin, N.R.; Markowitz, A.J.; Kreitzer, N.; Manley, G.T. TRACK-TBI Investigators Outcomes in Patients with Mild Traumatic Brain Injury Without Acute Intracranial Traumatic Injury. JAMA Netw. Open 2022, 5, e2223245. [Google Scholar] [CrossRef]
- Schneider, A.L.C.; Huie, J.R.; Boscardin, W.J.; Nelson, L.; Barber, J.K.; Yaffe, K.; Diaz-Arrastia, R.; Ferguson, A.R.; Kramer, J.; Jain, S.; et al. Cognitive Outcome 1 Year After Mild Traumatic Brain Injury: Results From the TRACK-TBI Study. Neurology 2022, 98, e1248–e1261. [Google Scholar] [CrossRef]
- McMahon, P.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T.; et al. Symptomatology and Functional Outcome in Mild Traumatic Brain Injury: Results from the Prospective TRACK-TBI Study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- Izzy, S.; Tahir, Z.; Grashow, R.; Cote, D.J.; Jarrah, A.A.; Dhand, A.; Taylor, H.; Whalen, M.; Nathan, D.M.; Miller, K.K.; et al. Concussion and Risk of Chronic Medical and Behavioral Health Comorbidities. J. Neurotrauma. 2021, 38, 1834–1841. [Google Scholar] [CrossRef]
- Cooper, D.B.; Kennedy, J.E.; Cullen, M.A.; Critchfield, E.; Amador, R.R.; Bowles, A.O. Association between Combat Stress and Post-Concussive Symptom Reporting in OEF/OIF Service Members with Mild Traumatic Brain Injuries. Brain Inj. 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature from 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef]
- Tate, R.; Kennedy, M.; Ponsford, J.; Douglas, J.; Velikonja, D.; Bayley, M.; Stergiou-Kita, M. INCOG Recommendations for Management of Cognition Following Traumatic Brain Injury, Part III: Executive Function and Self-Awareness. J. Head Trauma Rehabil. 2014, 29, 338–352. [Google Scholar] [CrossRef]
- Shoulson, I.; Wilhelm, E.E.; Koehler, R. Cognitive Rehabilitation Therapy for Traumatic Brain Injury: Evaluating the Evidence. FOC 2013, 11, 387–395. [Google Scholar] [CrossRef]
- Eapen, B.C.; Bowles, A.O.; Sall, J.; Lang, A.E.; Hoppes, C.W.; Stout, K.C.; Kretzmer, T.; Cifu, D.X. The Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury. Brain Inj. 2022, 36, 693–702. [Google Scholar] [CrossRef]
- Rytter, H.M.; Westenbaek, K.; Henriksen, H.; Christiansen, P.; Humle, F. Specialized Interdisciplinary Rehabilitation Reduces Persistent Post-Concussive Symptoms: A Randomized Clinical Trial. Brain Inj. 2019, 33, 266–281. [Google Scholar] [CrossRef]
- Cantor, J.; Ashman, T.; Dams-O’Connor, K.; Dijkers, M.P.; Gordon, W.; Spielman, L.; Tsaousides, T.; Allen, H.; Nguyen, M.; Oswald, J. Evaluation of the Short-Term Executive plus Intervention for Executive Dysfunction after Traumatic Brain Injury: A Randomized Controlled Trial with Minimization. Arch. Phys. Med. Rehabil. 2014, 95, 1–9.e3. [Google Scholar] [CrossRef]
- Tsaousides, T.; Spielman, L.; Kajankova, M.; Guetta, G.; Gordon, W.; Dams-O’Connor, K. Improving Emotion Regulation Following Web-Based Group Intervention for Individuals with Traumatic Brain Injury. J. Head Trauma Rehabil. 2017, 32, 354–365. [Google Scholar] [CrossRef]
- Wong, D.; Sinclair, K.; Seabrook, E.; McKay, A.; Ponsford, J. Smartphones as Assistive Technology Following Traumatic Brain Injury: A Preliminary Study of What Helps and What Hinders. Disabil. Rehabil. 2017, 39, 2387–2394. [Google Scholar] [CrossRef]
- de Joode, E.; van Heugten, C.; Verhey, F.; van Boxtel, M. Efficacy and Usability of Assistive Technology for Patients with Cognitive Deficits: A Systematic Review. Clin. Rehabil. 2010, 24, 701–714. [Google Scholar] [CrossRef]
- LoPresti, E.F.; Bodine, C.; Lewis, C. Assistive Technology for Cognition. IEEE Eng. Med. Biol. Mag. 2008, 27, 29–39. [Google Scholar] [CrossRef]
- Wallace, T.D.; Morris, J.T. SwapMyMood: User-Centered Design and Development of a Mobile App to Support Executive Function. In Computers Helping People with Special Needs; Miesenberger, K., Manduchi, R., Covarrubias Rodriguez, M., Peňáz, P., Eds.; ICCHP 2020; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2020; Volume 12377, pp. 259–265. [Google Scholar] [CrossRef]
- Pearson, N.; Naylor, P.J.; Ashe, M.C.; Fernandez, M.; Yoong, S.L.; Wolfenden, L. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020, 6, 167. [Google Scholar] [CrossRef]
- Arain, M.; Campbell, M.J.; Cooper, C.L.; Lancaster, G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Luna, D.; Quispe, M.; Gonzalez, Z.; Alemrares, A.; Risk, M.; Garcia Aurelio, M.; Otero, C. User-Centered Design to Develop Clinical Applications. Literature Review. Stud. Health Technol. Inform. 2015, 216, 967. [Google Scholar] [PubMed]
- Malec, J.F. Goal Attainment Scaling in Rehabilitation. Neuropsychol. Rehabil. 1999, 9, 253–275. [Google Scholar] [CrossRef]
- Wallace, T.D.; McCauley, K.L.; Hodge, A.T.; Moran, T.P.; Porter, S.T.; Whaley, M.C.; Gore, R.K. Use of person-centered goals to direct interdisciplinary care for military service members and Veterans with chronic mTBI and co-occurring psychological conditions. Front. Neurol. 2022, 13, 1015591. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A); Psychological Assessment Resources: Lutz, FL, USA, 2005. [Google Scholar]
- Heppner, P.P.; Petersen, C.H. The Development and Implications of a Personal Problem-Solving Inventory. J. Couns. Psychol. 1982, 29, 66–75. [Google Scholar] [CrossRef]
- Gratz, K.L.; Roemer, L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Waid-Ebbs, J.K.; Wen, P.S.; Heaton, S.C.; Donovan, N.J.; Velozo, C. The item level psychometrics of the behaviour rating inventory of executive function-adult (BRIEF-A) in a TBI sample. Brain Inj. 2012, 26, 1646–1657. [Google Scholar] [CrossRef]
- Bergersen, K.; Halvorsen, J.Ø.; Tryti, E.A.; Taylor, S.I.; Olsen, A. A systematic literature review of psychotherapeutic treatment of prolonged symptoms after mild traumatic brain injury. Brain Inj. 2017, 31, 279–289. [Google Scholar] [CrossRef]
- Brooke, J. SUS: A “Quick and Dirty” Usability Scale. In Usability Evaluation in Industry; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-429-15701-1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 26 October 2022).
- Powell, L.E.; Wild, M.R.; Glang, A.; Ibarra, S.; Gau, J.M.; Perez, A.; Albin, R.W.; O’Neil Pirozzi, T.M.; Wade, S.L.; Keating, T.; et al. The development and evaluation of a web-based programme to support problem-solving skills following brain injury. Disabil. Rehabil. Assist. Technololgy. 2019, 14, 21–32. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Collier, G.; Vaccaro, M.; Wingfield, R. Development of RehaBot—A Conversational Agent for Promoting Rewarding Activities in Users with Traumatic Brain Injury. J. Head Trauma Rehab 2022, 37, 144–151. [Google Scholar] [CrossRef]
- Wallace, T.; Morris, J.T.; Glickstein, R.; Anderson, R.K.; Gore, R.K. Implementation of a Mobile Technology-Supported Diaphragmatic Breathing Intervention in Military mTBI with PTSD. J. Head Trauma Rehab 2002, 37, 152–161. [Google Scholar] [CrossRef]
- Scherer, M.J.; Federici, S. Why people use and don’t use technologies: Introduction to the special issue on assistive technologies for cognition/cognitive support technologies. NeuroRehabilitation 2015, 37, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, M.; Patil, C.G.; Mukherjee, D.; Ugiliweneza, B.; Nuño, M.; Lad, S.P.; Boakye, M. Effect of Insurance and Racial Disparities on Outcomes in Traumatic Brain Injury. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2015, 76, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Ketchum, J.M.; Gary, K.; Hart, T.; Corrigan, J.; Forster, L.; Mascialino, G. Race/Ethnicity Differences in Satisfaction with Life among Persons with Traumatic Brain Injury. NeuroRehabilitation 2009, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
| Abbreviations | Meaning |
|---|---|
| admin | administration |
| BRIEF-A | Behavioral Rating Inventory of Executive Function—Adult Version |
| DERS | Difficulties in Emotion Regulation Scale |
| GAS | goal attainment scaling |
| mTBI | mild traumatic brain injury |
| PSI | Problem-Solving Inventory |
| PTSD | post-traumatic stress disorder |
| SM/Vs | service members and Veterans |
| SUS | System Usability Scale |
| Assessment | Objective/Description | Scoring/Assessment Type |
|---|---|---|
| Goal Attainment Scaling (GAS) | Individualized outcome measure involving goal selection and scaling, standardized to calculate level of goal attainment over the course of an intervention. Goals were individually identified to suit the participant. | 5-point scale ranging from −2 to +2 set according to participant’s current and expected levels of performance; a score of 0 or greater indicates meaningful improvement on repeated measures |
| Behavioral Rating Inventory of Executive Function-Adult Version (BRIEF-A) | Assess executive function | 75-item self-report questionnaire with three response options: “Never”, “Sometimes”, “Often”; decreased scores indicate improvement on repeated measures |
| Problem-Solving Inventory (PSI) | Assess confidence in problem-solving, whether individuals tend to approach or avoid problems, and elements of self-control | 32-item self-report using a 6-point rating scale from “Strongly Agree” to “Strongly Disagree”; decreased scores indicate improvement on repeated measures |
| Difficulties in Emotion Regulation Scale (DERS) | Assess emotion regulation | 36-item self-report questionnaire with five response options from “Almost Never” to “Almost Always”; decreased scores indicate improvement on repeated measures |
| Likert scale | Assess participant knowledge of how to use the Executive Plus/STEP system | Informal 8-item self-report inventory with varied response options; increased scores indicate improvement on repeated measures |
| System Usability Scale (SUS) | Evaluate usability of technology products and services | 10-item questionnaire with five response options from “Strongly Agree” to “Strongly Disagree”; higher scores indicate greater usability |
| Group (Participant Number) | Age | Gender | Race | Branch | Highest Level of Education | Personal Phone |
|---|---|---|---|---|---|---|
| CCG (1) | 37 | Non-binary | White | Army | Graduate degree | Android |
| CCG (2) | 35 | Female | White | Navy | Graduate degree | iOS |
| CCG (3) | 22 | Male | White | Army | Some college | Android |
| CCG (4) | 45 | Male | White | Army | Some college | iOS |
| EG (5) | 35 | Male | White | Army | High school | iOS |
| EG (6) | 38 | Female | Hispanic | Marines | Some college | iOS |
| EG (7) | 54 | Male | White | Army | College | iOS |
| EG (8) | 33 | Male | White | Army | College | iOS |
| Group (Participant Number) | Hours of Group Treatment | Treatment Delivery | Years Post Index mTBI | Number of mTBI a | Mechanism of Brain Injury | Psychological Conditions |
|---|---|---|---|---|---|---|
| CCG (1) | 9 | Hybrid b | 5 | 3+ | Blast | AUD, MDD, UAD |
| CCG (2) | 8 | In-person | 2.5 | 1 | Fall | AUD, UAD, UMD |
| CCG (3) | 9 | Hybrid | 0.5 | 1 | MVA | PDD, PTSD, UAD |
| CCG (4) | 12 | In-person | 5 | 2 | BA, Blast | UDD |
| EG (5) | 10 | In-person | 3 | 2 | SBO, Blast | MDD, PTSD |
| EG (6) | 8 | Hybrid | 9 | 1 | Sports | MDD, PTSD |
| EG (7) | 6 | Hybrid | 15 | 1 | Blast | ADU |
| EG (8) | 6 | In-person | 3 | 3+ | Blast, MVA, SBO | MDD, PTSD |
| Overall a | CCG a | EG a | Analysis | ||
|---|---|---|---|---|---|
| n = 8 | n = 4 | n = 4 | p | p-adj | |
| Goal Attainment (GAS) | |||||
| Post | 1 | 1 | |||
| 0 | 2 (25) | 1 (25) | 1 (25) | ||
| 1 | 4 (50) | 2 (50) | 2 (50) | ||
| 2 | 2 (25) | 1 (25) | 1 (25) | ||
| BRIEF-A (GEC) | |||||
| Pre | 78.25 (13.12) | 80.75 (8.96) | 75.75 (17.46) | – | – |
| Post | 62.75 (16.03) | 60.75 (14.29) | 64.75 (19.62) | 0.69 | 0.81 |
| Change | −15.50 (14.11) | −20.00 (17.36) | −11.00 (10.46) | 0.69 | 0.94 |
| BRIEF-A (BRI) | |||||
| Pre | 75.75 (11.51) | 76.00 (9.06) | 75.50 (15.07) | – | – |
| Post | 59.13 (14.15) | 56.75 (16.09) | 61.50 (13.89) | 0.49 | 0.88 |
| Change | −16.63 (12.93) | −19.25 (15.46) | −14.00 (11.52) | 1 | 0.81 |
| BRIEF-A (MCI) | |||||
| Pre | 75.88 (13.58) | 79.75 (8.26) | 72.00 (17.94) | – | – |
| Post | 64.25 (16.16) | 63.00 (11.92) | 65.50 (21.52) | 1 | 0.91 |
| Change | −11.62 (13.16) | −16.75 (15.63) | −6.50 (9.47) | 0.48 | 0.78 |
| PSI | |||||
| Pre | 116.88 (12.11) | 117.25 (14.57) | 116.50 (11.39) | – | – |
| Post | 104.50 (16.25) | 102.50 (16.60) | 106.50 (18.16) | 1 | 0.56 |
| Change | −12.37 (19.63) | −14.75 (13.94) | −10.00 (26.27) | 0.89 | 0.53 |
| DERS | |||||
| Pre | 129.88 (33.43) | 131.00 (37.07) | 128.75 (35.08) | – | – |
| Post | 81.13 (28.92) | 79.50 (31.84) | 82.75 (30.51) | 0.69 | 0.98 |
| Change | −48.75 (51.15) | −51.50 (49.16) | −46.00 (60.55) | 1 | 0.77 |
| Know how to use SWAPS | |||||
| Pre | 2.38 (0.74) | 2.25 (0.50) | 2.50 (1.00) | – | – |
| Post | 3.50 (0.54) | 3.50 (0.58) | 3.50 (0.58) | 1 | 0.84 |
| Change | 1.13 (1.13) | 1.25 (0.96) | 1.00 (1.41) | 0.89 | 0.96 |
| Know how to use Emotional Cycle | |||||
| Pre | 1.75 (1.04) | 2.00 (1.16) | 1.50 (1.00) | – | – |
| Post | 3.63 (0.52) | 3.50 (0.58) | 3.75 (0.50) | 0.69 | 0.98 |
| Change | 1.88 (1.13) | 1.50 (1.29) | 2.25 (0.96) | 0.49 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartell, R.; Morris, J.; Wallace, T. Feasibility of Using a Mobile App Supported Executive Function Intervention in Military Service Members and Veterans with mTBI and Co-Occurring Psychological Conditions. Int. J. Environ. Res. Public Health 2023, 20, 2457. https://doi.org/10.3390/ijerph20032457
Gartell R, Morris J, Wallace T. Feasibility of Using a Mobile App Supported Executive Function Intervention in Military Service Members and Veterans with mTBI and Co-Occurring Psychological Conditions. International Journal of Environmental Research and Public Health. 2023; 20(3):2457. https://doi.org/10.3390/ijerph20032457
Chicago/Turabian StyleGartell, Rebecca, John Morris, and Tracey Wallace. 2023. "Feasibility of Using a Mobile App Supported Executive Function Intervention in Military Service Members and Veterans with mTBI and Co-Occurring Psychological Conditions" International Journal of Environmental Research and Public Health 20, no. 3: 2457. https://doi.org/10.3390/ijerph20032457
APA StyleGartell, R., Morris, J., & Wallace, T. (2023). Feasibility of Using a Mobile App Supported Executive Function Intervention in Military Service Members and Veterans with mTBI and Co-Occurring Psychological Conditions. International Journal of Environmental Research and Public Health, 20(3), 2457. https://doi.org/10.3390/ijerph20032457






