Behavioral and Psychological Symptoms of Dementia: Prevalence, Symptom Severity, and Caregiver Distress in South-Western Uganda—A Quantitative Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Participants
2.4. Sample Size Determination, Sampling Procedure, and Recruitment
2.5. Data Collection Tools and Data Collection Methods
2.5.1. Neuropsychiatric Inventory–Questionnaire (NPI-Q)
2.5.2. Demographic Questionnaire
2.6. Data Management and Data Analysis
2.7. Quality Assurance
3. Results
3.1. Socio-Demographic Characteristics of the Study Sample
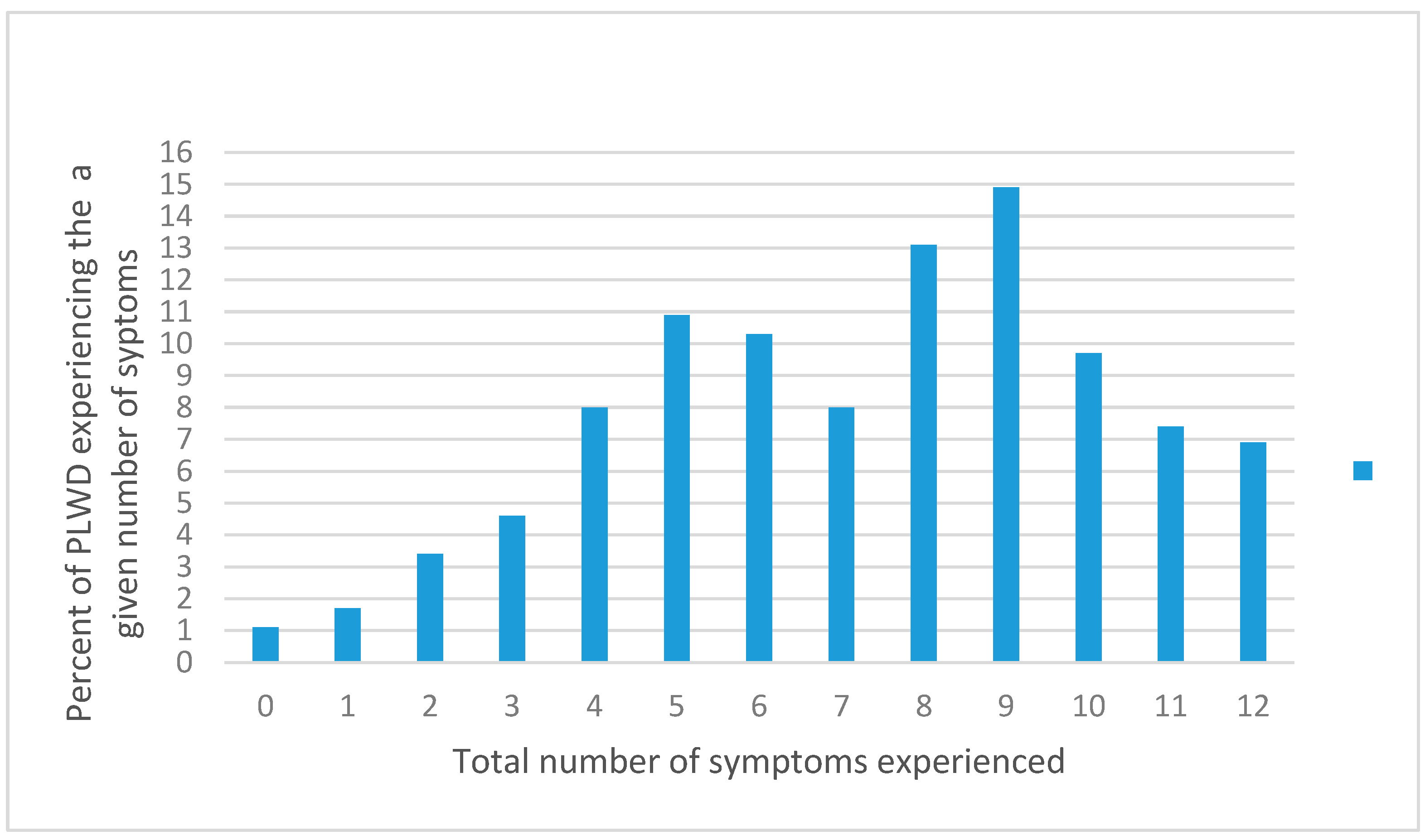
3.2. Prevalence of BPSD
3.3. Correlations between the Total Numbers of BPSD with Other Studied Variables
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPSD | Behavioral and psychological symptoms of dementia |
| CBI | Caregiver Burden Inventory |
| QoL | Quality of life |
| ROTOM | Reach One Touch One Ministries |
| SD | Standard deviation |
| N | Number |
| NPI-Q | Neuropsychiatric Inventory–Questionnaire (NPI-Q) |
| NPI | Standard Neuropsychiatric Inventory (NPI) |
References
- Prince, M.J.; Anders, W.; Maëlenn, G.; Ali, G.; Wu, Y.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International (ADI): London, UK, 2015. [Google Scholar]
- Johnson, R.A.; Karlawish, J. A review of ethical issues in dementia. Int. Psychogeriatr. 2015, 27, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Guerchet, M.M.; Mayston, R.; Lloyd-Sherlock, P.; Prince, M.J.; Aboderin, I.; Akinyemi, R.; Paddick, S.-M.; Wimo, A.; Amoakoh-Coleman, M.; Uwakwe, R. Dementia in Sub-Saharan Africa: Challenges and Opportunities; Alzheimer’s Disease International: London, UK, 2017. [Google Scholar]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- WHO World health Organization, Dementia Fact Sheets 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 April 2022).
- Mubangizi, V.; Maling, S.; Obua, C.; Tsai, A.C. Prevalence and correlates of Alzheimer’s disease and related dementias in rural Uganda: Cross-sectional, population-based study. BMC Geriatr. 2020, 20, 48. [Google Scholar] [CrossRef]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Mangialasche, F.; Camarda, C.; Ercolani, S.; Camarda, R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J. Alzheimer’s Dis. 2009, 18, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Finkel, S.I.; e Silva, J.C.; Cohen, G.; Miller, S.; Sartorius, N. Behavioral and psychological signs and symptoms of dementia: A consensus statement on current knowledge and implications for research and treatment. Int. Psychogeriatr. 1997, 8, 497–500. [Google Scholar] [CrossRef]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Assessment and management of behavioral and psychological symptoms of dementia. BMJ Br. Med. J. 2015, 350, h369. [Google Scholar] [CrossRef]
- Laks, J.; Engelhardt, E. Behavioral and psychological symptoms in dementia is not a unitary concept: A critical review with emphasis on Alzheimer’s disease. Dement. Neuropsychol. 2008, 2, 272–277. [Google Scholar]
- Amoo, G.; Akinyemi, R.; Onofa, L.; Akinyemi, J.; Baiyewu, O.; Ogunlesi, A.; Ogunniyi, A. Profile of clinically-diagnosed dementias in a neuropsychiatric practice in Abeokuta, South-Western Nigeria. Afr. J. Psychiatry 2011, 14, 377–382. [Google Scholar] [CrossRef]
- Yoro-Zohoun, I.; Nubukpo, P.; Houinato, D.; Mbelesso, P.; Ndamba-Bandzouzi, B.; Clément, J.P.; Dartigues, J.F.; Preux, P.M.; Guerchet, M.; EPIDEMCA Group. Neuropsychiatric symptoms among older adults living in two countries in Central Africa (EPIDEMCA study). Int. J. Geriatr. Psychiatry 2019, 34, 169–178. [Google Scholar] [CrossRef]
- Toot, S.; Swinson, T.; Devine, M.; Challis, D.; Orrell, M. Causes of nursing home placement for older people with dementia: A systematic review and meta-analysis. Int. Psychogeriatr. 2017, 29, 195–208. [Google Scholar] [CrossRef]
- Ryu, S.-H.; Ha, J.H.; Park, D.-H.; Yu, J.; Livingston, G. Persistence of neuropsychiatric symptoms over six months in mild cognitive impairment in community-dwelling Korean elderly. Int. Psychogeriatr. 2011, 23, 214–220. [Google Scholar] [CrossRef]
- Karttunen, K.; Karppi, P.; Hiltunen, A.; Vanhanen, M.; Välimäki, T.; Martikainen, J.; Valtonen, H.; Sivenius, J.; Soininen, H.; Hartikainen, S. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2011, 26, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, H.; Nakaaki, S.; Torii, K.; Shinagawa, Y.; Watanabe, N.; Murata, Y.; Sato, J.; Mimura, M.; Furukawa, T.A. Neuropsychiatric symptoms predict change in quality of life of Alzheimer disease patients: A two-year follow-up study. Psychiatry Clin. Neurosci. 2009, 63, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Tun, S.-M.; Murman, D.L.; Long, H.L.; Colenda, C.C.; Von Eye, A. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer disease. Am. J. Geriatr. Psychiatry 2007, 15, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Biswas, A.; Roy, A.; Biswas, S.; Gangopadhyay, G.; Das, S.K.J.D. Behavioural and psychological symptoms of dementia: Correlates and impact on caregiver distress. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 354–365. [Google Scholar] [CrossRef]
- Russ, T.C.; Batty, G.D.; Hearnshaw, G.F.; Fenton, C.; Starr, J.M. Geographical variation in dementia: Systematic review with meta-analysis. Int. J. Epidemiol. 2012, 41, 1012–1032. [Google Scholar] [CrossRef]
- Emanuel, J.E.; Lopez, O.L.; Houck, P.R.; Becker, J.T.; Weamer, E.A.; DeMichele-Sweet, M.A.A.; Kuller, L.; Sweet, R.A. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am. J. Geriatr. Psychiatry 2011, 19, 160–168. [Google Scholar] [CrossRef]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G.; Detroit Expert Panel on the Assessment; Management of the Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. J. Am. Geriatr. Soc. 2014, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Lee, M.-C.; Liao, Y.-C.; Wang, W.-F.; Lai, T.-J. Caregiver burden associated with behavioral and psychological symptoms of dementia (BPSD) in Taiwanese elderly. Arch. Gerontol. Geriatr. 2012, 55, 55–59. [Google Scholar] [CrossRef]
- Feast, A.; Moniz-Cook, E.; Stoner, C.; Charlesworth, G.; Orrell, M. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int. Psychogeriatr. 2016, 28, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.S.; Lee, H.-G.; Xiongwei, Z.; Perry, G.; Smith, M.A.; Castellani, R.J. Current approaches in the treatment of Alzheimer’s disease. Biomed. Pharmacother. 2008, 62, 99–207. [Google Scholar] [CrossRef] [PubMed]
- Olasoji, M.; Maude, P.; McCauley, K. Not sick enough: Experiences of carers of people with mental illness negotiating care for their relatives with mental health services. J. Psychiatr. Ment. Health Nurs. 2017, 24, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Adonteng-Kissi, B.; Moyle, W.; Grealish, L. Informal care of older adults with chronic life-limiting illness in Africa: An integrative review. Int. Soc. Work 2022, 65, 124–138. [Google Scholar] [CrossRef]
- Brodaty, H.; Donkin, M. Family caregivers of people with dementia. Dialogues Clin. Neurosci. 2022, 11, 217–228. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Grossberg, G.T. Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. Handb. Clin. Neurol. 2019, 165, 5–32. [Google Scholar]
- Azermai, M.; Petrovic, M.; Elseviers, M.M.; Bourgeois, J.; Van Bortel, L.M.; Vander Stichele, R.H. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Res. Rev. 2012, 11, 78–86. [Google Scholar] [CrossRef]
- Uganda Bureau of Statistics. The National Population and Housing Census 2014–Main report; Uganda Bureau of Statistics: Kampala, Uganda, 2016. [Google Scholar]
- Kish, L.; Frankel, M.R. Inference from complex samples. J. R. Stat. Soc. Ser. B (Methodol.) 1974, 36, 1–22. [Google Scholar] [CrossRef]
- Paddick, S.-M.; Gray, W.K.; Ogunjimi, L.; Olakehinde, O.; Kisoli, A.; Kissima, J.; Mbowe, G.; Mkenda, S.; Dotchin, C.L.; Walker, R.W. Validation of the identification and intervention for dementia in elderly Africans (IDEA) cognitive screen in Nigeria and Tanzania. BMC Geriatr. 2015, 15, 53. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Tripathi, R.K.; Tiwari, S.C.; Singh, B.; Tripathi, S.M. Caregiver burden and quality of life of key caregivers of patients with dementia. Indian J. Psychol. Med. 2016, 38, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Jathanna, R.P.; Latha, K.; Bhandary, P. Burden and coping in informal caregivers of persons with dementia: A cross sectional study. Online J. Health Allied Sci. 2010, 9, 7. [Google Scholar]
- Aarsland, D.; Brønnick, K.; Ehrt, U.; De Deyn, P.P.; Tekin, S.; Emre, M.; Cummings, J.L. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: Frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry 2007, 78, 36–42. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric Symptoms in Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, 2011; Volume 7, pp. 532–539. [Google Scholar]
- Hsieh, S.W.; Huang, L.C.; Hsieh, T.J.; Lin, C.F.; Hsu, C.C.; Yang, Y.H. Behavioral and psychological symptoms in institutional residents with dementia in Taiwan. Geriatr. Gerontol. Int. 2021, 21, 718–724. [Google Scholar] [CrossRef]
- Rossi, R.; Socci, V.; Talevi, D.; Mensi, S.; Niolu, C.; Pacitti, F.; Di Marco, A.; Rossi, A.; Siracusano, A.; Di Lorenzo, G. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front. Psychiatry 2020, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzato, E.; Zabberoni, S.; Costa, A.; Cona, G. Impact of COVID-19-lockdown and vulnerability factors on cognitive functioning and mental health in Italian population. MedRxiv 2020. [Google Scholar] [CrossRef]
- Cellini, N.; Canale, N.; Mioni, G.; Costa, S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J. Sleep Res. 2020, 29, e13074. [Google Scholar] [CrossRef]
- Bussè, C.; Barnini, T.; Zucca, M.; Rainero, I.; Mozzetta, S.; Zangrossi, A.; Cagnin, A. Depression, Anxiety and Sleep Alterations in Caregivers of Persons With Dementia After 1-Year of COVID-19 Pandemic. Front. Psychiatry 2022, 13, 826371. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. Jama 2002, 288, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-F.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Xu, W.; Li, J.-Q.; Wang, J.; Lai, T.-J. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Baharudin, A.D.; Din, N.C.; Subramaniam, P.; Razali, R. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health 2019, 19, 447. [Google Scholar] [CrossRef]
- Vu, M.; Mangal, R.; Stead, T.; Lopez-Ortiz, C.; Ganti, L. Impact of Alzheimer’s Disease on Caregivers in the United States. Health Psychol. Res. 2022, 10, 37454. [Google Scholar] [CrossRef] [PubMed]
- Peavy, G.; Mayo, A.M.; Avalos, C.; Rodriguez, A.; Shifflett, B.; Edland, S.D. Perceived Stress in Older Dementia Caregivers: Mediation by Loneliness and Depression. Am. J. Alzheimer’s Dis. Other Dement. 2022, 37, 15333175211064756. [Google Scholar] [CrossRef] [PubMed]
- Mayo, A.M.; Siegle, K.; Savell, E.; Bullock, B.; Preston, G.J.; Peavy, G.M. Lay caregivers’ experiences with caring for persons with dementia: A phenomenological study. J. Gerontol. Nurs. 2020, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mausbach, B.T.; Chattillion, E.A.; Roepke, S.K.; Patterson, T.L.; Grant, I. A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. Am. J. Geriatr. Psychiatry 2013, 21, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Manzini, C.S.S.; Vale, F.A.C.d. Emotional disorders evidenced by family caregivers of older people with Alzheimer’s disease. Dement. Neuropsychol. 2020, 14, 56–61. [Google Scholar] [CrossRef] [PubMed]
- del-Pino-Casado, R.; Rodriguez Cardosa, M.; López-Martínez, C.; Orgeta, V. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0217648. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Agosti, C.; Padovani, A. Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): Frequency and relationship with disease severity and motor impairment. Arch. Gerontol. Geriatr. 2008, 46, 101–106. [Google Scholar] [CrossRef]
| Study Variables | Total Sample; n (%) | Mean ± SD of BPSD |
|---|---|---|
| Socio-demographic variables | ||
| District location | ||
| Rubanda District | 131 (74.9) | 7.7 ± 2.9 |
| Rukiga District | 44 (25.1) | 5.9 ± 2.5 |
| Age (in completed years) | ||
| 60 years and below | 148 (84.6) | 7.3 ± 3.0 |
| Above 60 years | 27 (15.4) | 7.3 ± 2.8 |
| Gender | ||
| Female | 132 (75.4) | 7.6 ± 2.9 |
| Male | 43 (24.6) | 6.3 ± 2.9 |
| Marital status | ||
| Separated/Divorced | 14 (8.0) | 7.4 ± 2.4 |
| Married/Cohabiting | 128 (73.1) | 7.2 ± 3.1 |
| Single | 13 (7.4) | 6.6 ± 2.7 |
| Widow/Widower | 20 (11.4) | 7.8 ± 2.6 |
| Level of education | ||
| None | 32 (18.3) | 7.2 ± 2.9 |
| Primary | 114 (65.1) | 7.3 ± 2.9 |
| Secondary | 19 (10.9) | 8.1 ± 3.3 |
| Tertiary | 10 (5.7) | 6.0 ± 2.2 |
| Employment status | ||
| Previously employed, retired, but currently still active | 1 (0.6) | 2.0 ± 0 |
| Previously employed, retired, but currently not active | 10 (5.7) | 6.1 ± 2.0 |
| Previously not employed and currently not active | 49 (28.0) | 7.4 ± 2.9 |
| Previously not employed and currently still active | 115 (65.7) | 7.4 ± 3.0 |
| Presence of chronic illness | ||
| Yes | 67 (38.3) | 7.4 ± 2.7 |
| No | 108 (61.7) | 7.2 ± 3.1 |
| History of alcohol use | ||
| Yes | 40 (22.9) | 7.4 ± 2.8 |
| No | 135 (77.1) | 7.2 ± 3.0 |
| History of cigarette smoking | ||
| Yes | 14 (8.0) | 7.2 ± 3.0 |
| No | 161 (92.0) | 7.9 ± 2.3 |
| BPSD Types | Persons Reporting BPSD N = 175 (%) | Mean Severity ± SD | Severity > 2, n (%) | Mean Distress ± SD | Distress > 3, n (%) |
|---|---|---|---|---|---|
| Delusions | 109 (62.3) | 2.4 ± 0.7 | 95 (54.3) | 2.7 ± 1.7 | 59 (33.7) |
| Hallucinations | 132 (75.4) | 2.5 ± 0.6 | 123 (70.3) | 2.9 ± 1.7 | 76 (43.4) |
| Agitation/Aggression | 90 (51.4) | 2.2 ± 0.7 | 75 (42.9) | 2.7 ± 1.6 | 46 (26.3) |
| Dysphoria/Depression | 142 (81.1) | 2.3 ± 0.7 | 119 (68.0) | 2.6 ± 1.7 | 69 (39.4) |
| Anxiety | 118 (67.4) | 2.2 ± 0.6 | 105 (60.0) | 2.7 ± 1.7 | 61 (34.9) |
| Elation/Euphoria | 94 (53.7) | 2.1 ± 0.7 | 61 (34.9) | 1.6 ± 2.0 | 28 (16.0) |
| Apathy/Indifference | 99 (56.6) | 2.3 ± 0.8 | 82 (46.9) | 2.2 ± 1.8 | 39 (22.3) |
| Disinhibition | 71 (40.6) | 2.2 ± 0.8 | 53 (30.3) | 2.9 ± 1.9 | 39 (22.3) |
| Irritability/Lability | 97 (55.4) | 2.4 ± 0.7 | 87 (49.7) | 2.9 ± 1.7 | 57 (32.6) |
| Aberrant motor | 111 (63.4) | 2.6 ± 0.6 | 105 (60.0) | 2.5 ± 1.7 | 105 (60.0) |
| Nighttime behavior | 90 (51.4) | 2.4 ± 0.8 | 71 (40.6) | 2.6 ± 1.9 | 46 (26.3) |
| Appetite/Eating | 118 (67.4) | 1.8 ± 0.7 | 77 (44.0) | 2.1 ± 1.8 | 49 (28.0) |
| Study Variables | Pearson Correlation Coefficients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Age # (1) | 1 | |||||||||||
| District location (2) | 0.14 | 1 | ||||||||||
| Gender (3) | 0.12 | 0.01 | 1 | |||||||||
| Level of education (4) | −0.33 ** | −0.16 * | 0.24 ** | 1 | ||||||||
| Marital status (5) | 0.12 | 0.09 | −0.1 | −0.09 | 1 | |||||||
| Employment status (6) | −0.05 | 0.07 | −0.20 ** | −0.47 ** | 0.06 | 1 | ||||||
| Presence of chronic illness (7) | 0.33 ** | 0.22 ** | −0.04 | −0.17 * | 0.16 * | −0 | 1 | |||||
| History of cigarette smoking (8) | 0.17 * | −0.07 | 0.08 | −0.1 | 0.08 | 0.1 | −0.06 | 1 | ||||
| History of alcohol use (9) | 0.07 | −0.03 | 0.32 ** | −0.01 | −0.1 | 0 | −0.06 | 0.34 ** | 1 | |||
| Total distress score # (10) | −0.11 | −0.22 ** | −0.18 | 0.03 | −0 | 0.1 | 0.09 | 0.08 | 0.03 | 1 | ||
| Total severity score # (11) | −0.11 | −0.24 ** | −0.23 ** | 0.01 | 0.02 | 0.1 | 0.08 | 0.06 | 0.02 | 0.82 ** | 1 | |
| Cumulative number of BPSD # (12) | −0.07 | −0.28 ** | −0.18 | −0.02 | 0.02 | 0.1 | 0.05 | 0.07 | 0.02 | 0.71 ** | 0.93 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoga, R.; Mubangizi, V.; Owokuhaisa, J.; Muwanguzi, M.; Natakunda, S.; Rukundo, G.Z. Behavioral and Psychological Symptoms of Dementia: Prevalence, Symptom Severity, and Caregiver Distress in South-Western Uganda—A Quantitative Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 2336. https://doi.org/10.3390/ijerph20032336
Kamoga R, Mubangizi V, Owokuhaisa J, Muwanguzi M, Natakunda S, Rukundo GZ. Behavioral and Psychological Symptoms of Dementia: Prevalence, Symptom Severity, and Caregiver Distress in South-Western Uganda—A Quantitative Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(3):2336. https://doi.org/10.3390/ijerph20032336
Chicago/Turabian StyleKamoga, Ronald, Vincent Mubangizi, Judith Owokuhaisa, Moses Muwanguzi, Sylivia Natakunda, and Godfrey Zari Rukundo. 2023. "Behavioral and Psychological Symptoms of Dementia: Prevalence, Symptom Severity, and Caregiver Distress in South-Western Uganda—A Quantitative Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 3: 2336. https://doi.org/10.3390/ijerph20032336
APA StyleKamoga, R., Mubangizi, V., Owokuhaisa, J., Muwanguzi, M., Natakunda, S., & Rukundo, G. Z. (2023). Behavioral and Psychological Symptoms of Dementia: Prevalence, Symptom Severity, and Caregiver Distress in South-Western Uganda—A Quantitative Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(3), 2336. https://doi.org/10.3390/ijerph20032336








